Abstract
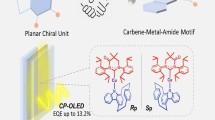
Bright and efficient chiral coinage metal clusters show promise for use in emerging circularly polarized light-emitting materials and diodes. To date, highly efficient circularly polarized organic light-emitting diodes (CP-OLEDs) with enantiopure metal clusters have not been reported. Herein, through rational design of a multidentate chiral N-heterocyclic carbene (NHC) ligand and a modular building strategy, we synthesize a series of enantiopure Au(I)-Cu(I) clusters with exceptional stability. Modulation of the ligands stabilize the chiral excited states of clusters to allow thermally activated delayed fluorescence, resulting in the highest orange-red photoluminescence quantum yields over 93.0% in the solid state, which is accompanied by circularly polarized luminescence. Based on the solution process, a prototypical orange-red CP-OLED with a considerably high external quantum efficiency of 20.8% is prepared. These results demonstrate the extensive designability of chiral NHC ligands to stabilize polymetallic clusters for high performance in chiroptical applications.
Similar content being viewed by others
Introduction
Organic light-emitting diodes (OLEDs)1,2,3 that emit circularly polarized luminescence (CPL)4,5,6 are promising candidates for next-generation solid-state display and sensing applications. Compared with the widely studied expensive heavy metal-containing compounds, such as Ir(III) and Pt(II) complexes7,8, abundant coinage metal-based emitters are more suitable for mass production. However, enantiopure emitters that contain coinage metals have not been developed for circularly polarized OLEDs (CP-OLEDs). Cyclometalated Au(III)9 that emits phosphorescence and cyclic carbene-coordinated Cu(I)10,11,12 that emits thermally activated delayed fluorescence (TADF)13 are capable of utilizing triplet excitons for light generation in OLEDs. Unfortunately, the complicated synthesis of chiral ligands makes the development of new emitters for highly efficient CP-OLEDs challenging.
Metal-based clusters serving as a bridge between single atoms and metal particles have attracted widespread attention14,15,16,17,18,19,20,21. Luminescent coinage metal-based clusters22,23,24,25,26 have been applied in OLEDs27,28, All the chemicals for synthesis were obtained from commercial sources and used without any further purification. The organic chiral ligands R/S-NHCpy-H PF6 and R/S-NHCql-H PF6 used were synthesized with modification according to the literature49,50. Synthesis of R/S-[Au(NHCpy)2]PF6 and R/S-[Au(NHCql)2]PF6. R/S-NHCql-H PF6 (0.65 g, 1 mmol) or R/S-NHCpy-H PF6 (0.54 g, 1 mmol), Ag2O (66 mg, 0.28 mmol), and about 40 mg of nBu4PF6 in 40 mL of CH2Cl2 was added. The mixture was protected from light and stirred for 10 min at room temperature. NaOH (1 M, 3 mL) was then added, and stirring was continued for 4 h. The mixture was filtered through Celite, and the clear filtrate was reduced to the minimum volume under vacuum. R/S-[Ag(NHCql)2]PF6 or R/S-[Ag(NHCpy)2]PF6 was precipitated as a white powder by the addition of diethyl ether. Next, a 25 mL round-bottom flask was charged with R/S-[Ag(NHCql)2]PF6 (0.38 mg, 0.3 mmol) or R/S-[Ag(NHCpy)2]PF6 (0.32 mg, 0.3 mmol) in 30 mL of CH2Cl2. Me2SAuCl (0.096 g, 0.3 mmol) in 10 mL of CH2Cl2 was added dropwise. The mixture was protected from light and stirred for 30 min during which time a precipitate formed. The solution was filtered through Celite removing the precipitated AgCl. The clear filtrate was subsequently reduced to 2 mL, and a white powder was precipitated with diethyl ether. ESI-MS: 1005.3745 for R-[Au(NHCpy)2]PF6, 1205.4320 for R-[Au(NHCql)2]PF6. R/S-[Au(NHCql)2]PF6 or R/S-[Au(NHCpy)2]PF6 (0.05 mmol) was dissolved in 6 mL of CH2Cl2. To this was added CuX (0.2 mmol) (X = Cl, Br, and I) suspended in 6 mL of CH2Cl2. The solution was stirred for an additional 3 h during which a yellow solution formed. The mixture was filtered through Celite, and the clear filtrate was reduced to 2 mL, then a yellow powder was precipitated with diethyl ether. The yellow powder was collected by filtration yielding. The yellow powder was recrystallized from CH2Cl2 and diethyl ether to produce yellow crystals. R/S-py-X (X = Br and I) and R/S-ql-X (X = Cl, Br, and I) were measured by single-crystal X-ray diffraction (SCXRD) with a Bruker diffractometer at 200 K, using Mo-Kα radiation (λ = 0.71073 Å). R-ql-I was also measured at different temperatures (100, 150, 180, 200, 250, and 300 K). The intensities were corrected for absorption using the empirical method implemented in SCALE3 ABSPACK scaling algorithm. The structures were solved with intrinsic phasing methods (SHELXT-2015), and refined by full-matrix least-squares on F2 using OLEX2, which utilizes the SHELXL-2015 module. The least-squares refinement of the structural model was performed under hard geometry restraints and displacement parameter restraints due to the weak diffraction and serious disorder of PF6−, Et2O and CH2Cl2 molecules in the lattice, such as ISOR, SADI, SIMU, and DFIX. Solvent molecules of all clusters have been identified and further confirmed by thermogravimetric and elemental analysis. All host molecular atoms were refined anisotropically, and the hydrogen atoms were included in idealized positions. The crystallographic data were listed in Supplementary Tables 1–6 and 11. In this study, the PL origin of a series of ligand-protected Au(I)-Cu(I) alloy clusters (R-py-X (X = Br and I) and R-ql-X (X = Cl, Br, and I)) has been studied by the DFT and TD-DFT calculations. The DFT and TD-DFT calculations were carried out using the Gaussian 16 program62. The hybrid PBE0 functional in conjunction with the Def2-SVP basis set was used for geometric optimization of the ground state and excited state configuration of ligand-protected Au(I)-Cu(I) alloy clusters. Hole and electron pair distribution analyses were performed using the Multiwfn code63,64. Kohn–Sham (KS) orbital energy levels were calculated by the Amsterdam density functional (ADF 2016) software package65. SOCME were calculated by ORCA 5.0.0 software package66 based on the PBE0 functional and the DKH-def2-TZVP(-f) basis set (SARC-DKH-TZVP for Au and I atoms). The radiative rate of fluorescence (kf) and phosphorescence (kp) were evaluated by Einstein spontaneous emission relationship. The ISC rate constant (kISC), RISC rate constant (kRISC), and the internal conversion rate constant (kIC) were obtained by the semiclassical Marcus theory expression. The MECP structure has been calculated by TD-DFT using sobMECP Program67 and Gaussian 16 program. The specific calculation details are present in Supplementary Information.Methods
Materials and reagents
Synthesis of R/S-ql-X (X = Cl, Br, and I), R/S-py-X (X = Br and I)
Crystallographic data collection and refinement of the structure
Quantum chemical calculations
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data for Figs. 2, 3, 5, 7, and 8 are provided in the figshare (https://doi.org/10.6084/m9.figshare.23559900). The X-ray crystallographic coordinates for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC S-ql-Cl (2225237), R-ql-Cl (2225238), R-py-Br (2225239), S-py-Br (2225240), S-ql-Br (2225243), R-ql-I-100 K (2225244), R-ql-I-180 K (2225245), R-ql-I-150 K (2225246), R-ql-I-200 K (2225247), R-ql-I-250 K (2225248), R-ql-I-300 K (2225249), R-ql-Br (2225250), S-py-I (2225251), R-py-I (2225252), S-ql-I-200 K (2225253). These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Li, L.-K. et al. Strategies towards rational design of gold(III) complexes for high-performance organic light-emitting devices. Nat. Photon. 13, 185–191 (2019).
Song, Y.-H. et al. Planar defect–free pure red perovskite light-emitting diodes via metastable phase crystallization. Sci. Adv. 8, eabq2321 (2022).
Zhang, D.-W., Li, M. & Chen, C.-F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 49, 1331–1343 (2020).
Lu, J.-J. et al. Pyridinylphosphorothioate-based blue Iridium(III) complex with double chiral centers for circularly polarized electroluminescence. J. Mater. Chem. C 9, 5244–5249 (2021).
Zhang, Y. P. et al. Circularly polarized white organic light‐emitting diodes based on spiro‐type thermally activated delayed fluorescence materials. Angew. Chem. Int. Ed. 61, e202200290 (2022).
Hudson, Z. M., Helander, M. G., Lu, Z.-H. & Wang, S. Highly efficient orange electrophosphorescence from a trifunctional organoboron–Pt(II) complex. Chem. Commun. 47, 755–757 (2011).
Li, T.-Y. et al. Rational design of phosphorescent iridium(III) complexes for emission color tunability and their applications in OLEDs. Coord. Chem. Rev. 374, 55–92 (2018).
To, W.-P. et al. Highly luminescent pincer gold(III) aryl emitters: thermally activated delayed fluorescence and solution-processed OLEDs. Angew. Chem. Int. Ed. 56, 14036–14041 (2017).
Jazzar, R., Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)- and (aryl)-(amino)carbene coinage metal complexes and their applications. Chem. Rev. 120, 4141–4168 (2020).
Di, D. et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 356, 159–163 (2017).
Hamze, R. et al. Eliminating nonradiative decay in Cu(I) emitters: > 99% quantum efficiency and microsecond lifetime. Science 363, 601–606 (2019).
Han, Z., Dong, X.-Y. & Zang, S.-Q. Crystalline metal‐organic materials with thermally activated delayed fluorescence. Adv. Opt. Mater. 9, 2100081 (2021).
**, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
**, Y., Zhang, C., Dong, X.-Y., Zang, S.-Q. & Mak, T. C. W. Shell engineering to achieve modification and assembly of atomically-precise silver clusters. Chem. Soc. Rev. 50, 2297–2319 (2021).
Kang, X., Li, Y., Zhu, M. & **, R. Atomically precise alloy nanoclusters: syntheses, structures, and properties. Chem. Soc. Rev. 49, 6443–6514 (2020).
Kong, X.-J. et al. A four-shell, nesting doll-like 3d–4f cluster containing 108 metal ions. Angew. Chem. Int. Ed. 47, 2398–2401 (2008).
Kong, X.-J., Wu, Y., Long, L.-S., Zheng, L.-S. & Zheng, Z. A chiral 60-metal sodalite cage featuring 24 vertex-sharing [Er4(μ3-OH)4] cubanes. J. Am. Chem. Soc 131, 6918–6919 (2009).
Luo, Z. et al. From aggregation-induced emission of Au(I)–thiolate complexes to ultrabright Au(0)@Au(I)–thiolate core–shell nanoclusters. J. Am. Chem. Soc. 134, 16662–16670 (2012).
Shen, H. et al. N-heterocyclic carbene coordinated metal nanoparticles and nanoclusters. Coord. Chem. Rev. 458, 214425 (2022).
Song, Y. et al. Ultrabright Au@Cu14 nanoclusters: 71.3% phosphorescence quantum yield in non-degassed solution at room temperature. Sci. Adv. 7, eabd2091 (2021).
Huang, J.-H. et al. Symmetry breaking of atomically precise fullerene-like metal nanoclusters. J. Am. Chem. Soc. 143, 12439–12444 (2021).
Kong, Y.-J. et al. Achiral-core-metal change in isomorphic enantiomeric Ag12Ag32 and Au12Ag32 clusters triggers circularly polarized phosphorescence. J. Am. Chem. Soc. 144, 19739–19747 (2022).
Li, Q. et al. Structural distortion and electron redistribution in dual-emitting gold nanoclusters. Nat. Commun. 11, 2897 (2020).
Li, Q. et al. A mono-cuboctahedral series of gold nanoclusters: photoluminescence origin, large enhancement, wide tunability, and structure–property correlation. J. Am. Chem. Soc. 141, 5314–5325 (2019).
Zhang, M.-M. et al. Alkynyl-stabilized superatomic silver clusters showing circularly polarized luminescence. J. Am. Chem. Soc. 143, 6048–6053 (2021).
Jiao, Z. et al. High-efficiency solution-processed light-emitting diode based on a phosphorescent Ag3Cu5 cluster complex. J. Mater. Chem. C 9, 5528–5534 (2021).
Olaru, M. et al. A small cationic organo–copper cluster as thermally robust highly photo- and electroluminescent material. J. Am. Chem. Soc. 142, 373–381 (2019).
**e, M. et al. Highly efficient sky blue electroluminescence from ligand-activated copper iodide clusters: overcoming the limitations of cluster light-emitting diodes. Sci. Adv. 5, eaav9857 (2019).
Zhang, N. et al. Overcoming efficiency limitation of cluster light-emitting diodes with asymmetrically functionalized biphosphine Cu4I4 cubes. J. Am. Chem. Soc. 144, 6551–6557 (2022).
Wang, J.-J. et al. Chiral phosphine–copper iodide hybrid cluster assemblies for circularly polarized luminescence. J. Am. Chem. Soc. 143, 10860–10864 (2021).
Feng, L.-Z. et al. Biomimetic non-classical crystallization drives hierarchical structuring of efficient circularly polarized phosphors. Nat. Commun. 13, 4288 (2022).
Han, Z. et al. Ultrastable atomically precise chiral silver clusters with more than 95% quantum efficiency. Sci. Adv. 6, eaay0107 (2020).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Huynh, H. V. Electronic properties of N-heterocyclic carbenes and their experimental determination. Chem. Rev. 118, 9457–9492 (2018).
Visbal, R. & Gimeno, M. C. N-heterocyclic carbene metal complexes: photoluminescence and applications. Chem. Soc. Rev. 43, 3551–3574 (2014).
Wang, Y. et al. N-heterocyclic carbenes and their precursors in functionalised porous materials. Chem. Soc. Rev. 50, 13559–13586 (2021).
Ying, A., Ai, Y., Yang, C. & Gong, S. Aggregation‐dependent circularly polarized luminescence and thermally activated delayed fluorescence from chiral carbene-CuI-amide enantiomers. Angew. Chem. Int. Ed. 61, e202210490 (2022).
Narouz, M. R. et al. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat. Chem. 11, 419–425 (2019).
Lei, Z. et al. N-heterocyclic carbene-based c-centered Au(I)-Ag(I) clusters with intense phosphorescence and organelle-selective translocation in cells. Nat. Commun. 13, 4288 (2022).
Narouz, M. R. et al. Robust, highly luminescent Au13 superatoms protected by N-heterocyclic carbenes. J. Am. Chem. Soc. 141, 14997–15002 (2019).
Polgar, A. M., Weigend, F., Zhang, A., Stillman, M. J. & Corrigan, J. F. A N-heterocyclic carbene-stabilized coinage metal-chalcogenide framework with tunable optical properties. J. Am. Chem. Soc. 139, 14045–14048 (2017).
Shen, H. et al. Copper-hydride nanoclusters with enhanced stability by N-heterocyclic carbenes. Nano Res. 14, 3303–3308 (2021).
Strasser, C. E. & Catalano, V. J. “On−off” Au(I)···Cu(I) interactions in a Au(NHC)2 luminescent vapochromic sensor. J. Am. Chem. Soc. 132, 10009–10011 (2010).
Ube, H., Zhang, Q. & Shionoya, M. A carbon-centered hexagold(I) cluster supported by n-heterocyclic carbene ligands. Organometallics 37, 2007–2009 (2018).
Yi, H. et al. Synthesis and enantioseparation of chiral Au13 nanoclusters protected by bis-N-heterocyclic carbene ligands. Chem. Sci. 12, 10436–10440 (2021).
Man, R. W. Y. et al. Synthesis and characterization of enantiopure chiral bis NHC-stabilized edge-shared Au10 nanocluster with unique prolate shape. J. Am. Chem. Soc. 144, 2056–2061 (2022).
Pei, X.-L. et al. Asymmetric twisting of C-centered octahedral gold(I) clusters by chiral N-heterocyclic carbene ligation. J. Am. Chem. Soc. 144, 2156–2163 (2022).
Magill, A. M. et al. Palladium(II) complexes containing mono-, bi- and tridentate carbene ligands. Synthesis, characterisation and application as catalysts in C-C coupling reactions. J. Organomet. Chem. 617-618, 546–560 (2001).
He, W., Ge, Y.-C. & Tan, C.-H. Halogen-bonding-induced hydrogen transfer to C=N bond with hantzsch ester. Org. Lett. 16, 3244–3247 (2014).
Nitsch, J. et al. Cuprophilic interactions in highly luminescent dicopper(I)–NHC–picolyl complexes – fast phosphorescence or TADF? Chem. Commun. 52, 2932–2935 (2016).
Zhang, L. et al. Enhanced cuprophilic interactions in crystalline catalysts facilitate the highly selective electroreduction of CO2 to CH4. J. Am. Chem. Soc. 143, 3808–3816 (2021).
Schmidbaur, H. Proof of concept for hydrogen bonding to gold, Au···H-X. Angew. Chem. Int. Ed. 58, 5806–5809 (2019).
Lei, Z., Pei, X.-L., Ube, H. & Shionoya, M. Reconstituting C-centered hexagold(I) clusters with N-heterocyclic carbene ligands. Bull. Chem. Soc. Jpn. 94, 1324–1330 (2021).
Han, X. S. et al. Structure determination of alkynyl‐protected gold nanocluster Au22(tBuC≡C)18 and its thermochromic luminescence. Angew. Chem. Int. Ed. 59, 2309–2312 (2020).
Yonemoto, D. T., Papa, C. M., Mongin, C. F. & Castellano, N. Thermally activated delayed photoluminescence: deterministic control of excited-state decay. J. Am. Chem. Soc. 142, 10883–10893 (2020).
Ford, P. C., Cariati, E. & Bourassa, J. Photoluminescence properties of multinuclear copper(I) compounds. Chem. Rev. 99, 3625–3648 (1999).
Huang, R.-W. et al. Tandem silver cluster isomerism and mixed linkers to modulate the photoluminescence of cluster‐assembled materials. Angew. Chem. Int. Ed. 57, 8560–8566 (2018).
Huang, R.-W. et al. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 9, 689–697 (2017).
Gao, X. et al. Evaluation of spin-orbit couplings with linear-response time dependent density functional methods. J. Chem. Theory Comput. 13, 515–524 (2017).
Zhou, D. et al. Thermally stable donor–acceptor type (alkynyl)gold(III) TADF emitters achieved eqes and luminance of up to 23.4% and 70300 cd m−2 in vacuum‐deposited oleds. Adv. Sci. 6, 1802297 (2019).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Liu, Z., Lu, T. & Chen, Q. An Sp-hybridized all-carboatomic ring, cyclo[18]carbon: electronic structure, electronic spectrum, and optical nonlinearity. Carbon 165, 461–467 (2020).
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986).
Neese, F. Software update: the ORCA program system-version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 12, e1606 (2022).
Lu, T. sobMECP program. http://sobereva.com/286 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 92061201, S.-Q.Z.; U21A20277, X.-Y.D.; 21825106, S.-Q.Z.; 21975065, X.-Y.D.; and 62175189, G.X.) and Zhengzhou University. This work was also supported by the Zhongyuan Thousand Talents (Zhongyuan Scholars) Program of Henan Province (234000510007, S.-Q.Z.) and The Excellent Youth Foundation of Henan Scientific Committee (No.232300421022, X.-Y.D.). The authors also appreciate the assistance of Professor Yong Pei from **angtan University in the calculation.
Author information
Authors and Affiliations
Contributions
S.-Q.Z. conceived and designed the experiments. X.-H.M. conducted the synthesis and characterization. J.L. and P.L. performed the calculations. J.-H.H. and Z.H. drew figures in the main text. G.X. is in charge of the fabrication of OLED devices. X.-Y.D. and S.-Q.Z. analyzed the experimental results. X.-H.M., X.-Y.D., and S.-Q.Z. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, XH., Li, J., Luo, P. et al. Carbene-stabilized enantiopure heterometallic clusters featuring EQE of 20.8% in circularly-polarized OLED. Nat Commun 14, 4121 (2023). https://doi.org/10.1038/s41467-023-39802-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39802-w
- Springer Nature Limited