Abstract
In age-related neurodegenerative diseases, pathology often develops slowly across the lifespan. As one example, in diseases such as Alzheimer’s, vascular decline is believed to onset decades ahead of symptomology. However, challenges inherent in current microscopic methods make longitudinal tracking of such vascular decline difficult. Here, we describe a suite of methods for measuring brain vascular dynamics and anatomy in mice for over seven months in the same field of view. This approach is enabled by advances in optical coherence tomography (OCT) and image processing algorithms including deep learning. These integrated methods enabled us to simultaneously monitor distinct vascular properties spanning morphology, topology, and function of the microvasculature across all scales: large pial vessels, penetrating cortical vessels, and capillaries. We have demonstrated this technical capability in wild-type and 3xTg male mice. The capability will allow comprehensive and longitudinal study of a broad range of progressive vascular diseases, and normal aging, in key model systems.
Similar content being viewed by others
Introduction
There is an increasing need for methods to longitudinally track microvascular alterations in the aging brain. As one example, in Alzheimer’s disease, vascular decline is thought to emerge up to decades before the onset of cognitive symptoms and is widely viewed as one potential contributor to symptomology1. Similarly, changes in vascular structure and dynamics are a signature of several other progressive neurodegenerative diseases, including Parkinson disease2, Huntington disease3, and multiple sclerosis4, suggesting a direct role in disease progression. Deriving effective early treatments for these conditions, and better understanding their etiology, likely requires a better understanding of these evolving vascular changes.
However, studying the role of vascular factors in age-related neurodegenerative diseases has been challenging because of difficulties in longitudinally tracking their development. To date, multi-endpoint terminal approaches have been used for determining such vascular alterations at different ages5,6,7. While effective in some respects, these approaches are analytically less efficient, vulnerable to subject-specific random effects, and require an increasing number of animals when involving more measurement time points (Supplementary Fig. 1). Fluorescence multi-photon microscopy has revolutionized the in vivo visualization of neocortical vascular structure and function (flow)8, but has not met the need for long term, longitudinally repeated imaging. Bleaching and phototoxicity9,10 and the instability of genetically-encoded indicators limit the use of fluorescent methods for stable repeated measurements over several months11,12,13,14.
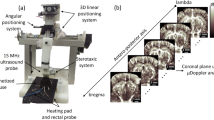
Optical coherence tomography (OCT) is a label-free technology that provides several unique imaging capabilities without the aid of fluorescence15,16,Full size image
Every imaging session included acquisition of Doppler OCT images as well. We used these images to track changes in the diameter and blood flow of individual penetrating vessels (Fig. 2). The diameter of each vessel was measured by fitting its en-face cross-section to a 2D Gaussian function, while the blood flow was measured by the area-integral method19 after noise reduction21. This enabled us to longitudinally track the same set of 151 penetrating vessels of 13 mice for 7 months (gray lines in Fig. 2c, f).
a An example of angiogram MIP with overlaid circles denoting the positions of the penetrating vessels tracked in (b). b An example set of en-face slices of the Doppler velocity maps acquired from the same animal for seven months. Eleven of the same vessels were selected in this animal (circled). WOA, weeks of age. c Time courses of arteriolar diameter of individual vessels (gray; 36 vessels from 7 AD mice, 29 vessels from 6 WT mice, 7 time points) and their LME fits (color). d The LME fits of arteriolar diameter shown together. e LME fits of venular diameter. f Time courses of venular flow of individual vessels (gray; 47 vessels from 7 AD mice, 39 vessels from 6 WT mice, 7 time points) and their LME fits (color). g LME fits of arteriolar flow. h The LME fits of venular flow. The color lines and shades in (c–h) indicate the mean and 95% CI of the LME fits, respectively. and dotted lines indicate AOS. See Supplementary Fig. 2 for individual vessel traces of venular diameter and arteriolar flow.
Arteriolar diameter showed sigmoidal decreases in both AD and WT but faster in AD, with the rates of change defined from the sigmoid fits (Methods) being −13% per month in AD (95% CI, −18 to −9) and −7% per month in WT (95% CI, −11 to −3; p = 0.008; Fig. 2c). In turn, their fractional changes became significantly different between AD and WT at 18 weeks of age (WOA) (p < 0.05, Fig. 2d), which is termed as the age of significance (AOS) hereafter. Second, the venular diameter decreased in AD with the rate of change of −13% per month (95% CI, −18 to −8), significantly different from WT (1.3% per month; 95% CI, 0.9–1.7; p < 0.001), leading to the AOS of 12 WOA (Fig. 2e). Third, the arteriolar flow decreased in both AD and WT but faster in AD (−13% versus −10% per month, p < 0.001; AOS, 21 WOA; Fig. 2g). Fourth, the venular flow decreased in AD only (−16% per month; 95% CI, −25 to −8; AOS, 18 WOA; Fig. 2f, h). Finally, the penetrating vessel density in vessel number per unit area decreased in AD (−6% per month; 95% CI, −11 to −1), but the slopes were not significantly different between AD and WT (p = 0.11). It is interesting to see the AOS be earlier for the structural degenerations than their flow counterparts (see Discussion for detailed interpretation).
Structural and functional properties of capillary vessel networks
To longitudinally track alterations in smaller vessels like capillaries, every imaging session also included acquisition of another OCT angiography dataset with a higher spatial resolution (3 µm) and an optical focus on the cortical capillary bed (“Methods”). To extract as much information as possible from these microangiography images, we first converted grayscale microangiograms into a graph of the capillary vessel network, using our deep learning-based toolbox for enhancement, segmentation and vectorization of vessels22 (Fig. 3a). From these network graphs and the original grayscale images, our methods enabled measurements of numerous angio-architectural properties, including morphological properties like the length, diameter, and tortuosity of each vessel; and topological properties such as the branching order, betweenness, closeness, and shortest cycle of each vessel. Betweenness, closeness, and the shortest cycle are used in graph theory for elucidating how nodes are connected within a network (see Supplementary Fig. 4 for conceptual illustration and their implication when applied to a vascular network). While these properties were measured for each capillary segment (108,964 segments in total across 13 animals and seven ages of measurement), we calculated the mean and heterogeneity of every property per animal per age and then tracked them as a function of age, where the heterogeneity was quantified by the coefficient of variation (COV; the standard deviation divided by the mean). Other related measures include capillary number density, capillary length density, and fractal dimension (see Table 1 for a full list).
a An example of the deep learning-based image processing pipeline results, from the input of microangiogram to its enhancement, vessel segmentation (all shown in MIP), and the vectorized capillary vessels. b Probability density distributions of capillary tortuosity, vessel diameter, capillary length, branching order, and capillary length density. The distributions were gathered from all animals and all measured ages, for each group. c Time courses of the normalized mean capillary length (gray; 7 AD mice and 6 WT mice, 7 time points) and their LME fits (color). d The LME fits of the mean capillary length shown together. e LME fits of the mean shortest cycle. f An example of a 3D betweenness map from an animal at one age, with each vessel colored according to its betweenness value. g LME fits of betweenness. h An example of a 3D closeness map of the same capillary network as (f). i LME fits of closeness COV. The color lines and shades indicate the mean and 95% CI, respectively, and dotted lines indicate AOS. See Supplementary Fig. 3 for all properties whose fractional changes did not become significantly different between AD and WT until the end of measurement (35 WOA). WOA weeks of age.
Prior to analyzing how the properties vary with age, we compared our measures to those reported in the literature when available. The distributions of capillary lengths, diameters, branching orders, and tortuosity obtained from our WT group (Fig. 3b) looked similar in shape and mean value to those previously reported from WT mice23,24,25,26. We also observed agreement with the literature in the capillary length density and the shortest cycle (Supplementary Tables 1 and 2).
LME analysis revealed several differences emerging between AD and WT. Mean capillary length steadily decreased in AD mice only (−1.5% per month; 95% CI, −2.6 to −0.3; AOS, 25 WOA; Fig. 3c, d). Mean capillary tortuosity also significantly increased in AD only (2.2% per month; 95% CI, 1.3–3.1), but its fractional changes did not become significantly different between AD and WT until the end of measurement (35 WOA). Regarding network topology, the shortest cycle increased in AD only (1.9% per month; 95% CI, 0.3–3.4; AOS, 20 WOA; Fig. 3e). The betweenness also increased in AD only (3.5% per month; 95% CI, 1.3–5.8; AOS, 21 WOA; Fig. 3g). In contrast, the closeness did not change significantly with aging in both AD and WT (Supplementary Fig. 3), but heterogeneity (COV) decreased in AD (−3.0% per month; 95% CI, −7.9 to −2.0) whereas it increased in WT (3.6% per month; 95% CI, 0.9–6.4), with an AOS of 18 WOA (Fig. 3i).
To assess the pattern of blood flow through a large microvascular network involving hundreds to thousands capillary vessels, we previously presented a method for high-throughput measurements of red blood cell (RBC) flux based on OCT detection of RBCs passing through a voxel20. However, this method tends to underestimate high flux values27 and requires user-selected thresholds. To overcome these weaknesses and improve accuracy, we developed a deep learning-based method by using two sets of RBC-passage data that were simultaneously obtained with OCT and two-photon microscopy (“Methods”). This new method significantly outperformed the previous method (Fig. 4a, b, Supplementary Text 2).
a Compared to the traditional peak-counting method, the convolutional neural network (CNN)-based method produced the slope closer to 1 and higher R2 (coefficient of determination). The uncertainty of individual estimations of the CNN-based method are color-coded in the right figure, while the traditional peak-counting method does not provide such uncertainty of estimation (Supplementary Text 2). b Distribution of errors of the two methods across different flux ranges (1,888 observations from 119 capillaries across 3 animals). When the CNN-based method filtered out predictions with the lowest 20% confidence, the error became both smaller (narrower distribution) and less biased (the mean closer to 0 RBC/s) even for higher flux values. Each box chart displays the median (red line), the lower and upper quartiles (box), outliers (red dots, computed using the 1.5× interquartile range), and the minimum and maximum values that are not outliers (whiskers). Source data are provided as a Source Data file. c An example of microangiogram constructed from RBC-passage data (top) and the vascular graph vectorized from the microangiogram (bottom). d Distribution of flux values gathered from all animals and all ages. e RBC flux changes as tracked through the same animals (gray; 7 AD mice and 6 WT mice, 7 time points) and their LME fits. f The LME fits of RBC flux shown together. g LME fits of the RBC flux COV. The color lines and shades in (d–g) indicate the mean and 95% CI, respectively, and dotted lines indicate AOS. RBC red blood cell, COV coefficient of variation.
To examine long-term changes in capillary network blood flow, every imaging session included OCT acquisition of RBC-passage data, and the deep learning method was used to produce a 3D map of capillary RBC flux values (Fig. 4c). As a result, WT mice exhibited a gradual increase with aging (2.1% per month; 95% CI, 0.9–3.2; Fig. 4e). This increasing trend agrees with previous findings from WT rats28 although the previous study measured the flux values only at two ages in a non-longitudinal manner. Interestingly, AD mice also showed an increase in flux with aging but at a significantly higher rate (6.0% per month; 95% CI, 3.0–9.0; p < 0.001 against WT), making the AOS to be 22 WOA (Fig. 4f). The capillary flux heterogeneity (COV) decreased in both AD and WT but faster in AD (−6.7% versus −3.0% per month; p < 0.001; AOS, 23 WOA; Fig. 4g).
Temporal relationships between microvascular degenerations and cognitive impairment
To simultaneously obtain the time course of cognitive impairment, the animals also underwent novel object location (NOL) tests every month (Methods; the NOL test assesses spatial cognition and memory29). While WT mice showed no significant changes in the discrimination index of NOL, AD mice showed cognitive decline with the discrimination index becoming significantly lower than WT at 27 WOA (Fig. 5a, b), consistent with previous findings from the identical 3xTg AD model30.
a NOL test scores tracked by individual animals (gray lines; 7 AD mice and 6 WT mice, 9 time points) and their LME fits (color line, mean; color shade, 95% CI). b The LME fits shown together (line, mean; shade, 95% CI). c Chronological graph of all vascular properties whose fractional changes became significantly different between AD and WT mice during the ages of measurement. The graph displays their rates of change in AD at their AOS (structural properties in purple, and flow properties in green). d Significant correlations between the NOL discrimination index and the vascular alterations (p < 0.05, Wald test, no multiple comparison correction was used; see “Methods” for details). Pairs with positive and negative correlations are shown in red and blue respectively. More opaque lines indicate higher maximum correlation, and thinner lines means that the maximum correlation appeared at longer age lags. A correlation with an age lag means that one alteration was correlated to the other one with a certain time delay. Two examples of significant correlations are presented on the right, where each circle represents a measurement from a single animal at a single age point, and the orange line and shade depict the fitted line (mean) and its 95% CI. Source data are provided as a Source Data file.
As listed in Table 1, 10 out of 25 vascular properties showed significant differences in fractional changes between AD and WT during the ages of measurement (11–35 WOA). To illustrate the temporal relationships of these vascular degenerations to the cognitive impairment, we plotted a chronological graph as shown in Fig. 5c. This graph revealed three interesting relationships. First, all the observed vascular degenerations preceded the cognitive decline, up to 15 WOA early (about a seventh of the typical lifespan). Second, degenerations in arterioles and venules appeared earlier than those in capillary vessels. Third, structural changes generally preceded corresponding flow changes (arteriolar/venular diameter versus flow, and capillary betweenness/shortest cycle versus RBC flux; see Discussion for interpretation).
To reveal the correlation between the simultaneously observed vascular alterations and cognitive impairment, we calculated the correlation coefficient with age lags of 0, 4, 8 and 12 weeks in the AD group (Methods). Our analysis found that 19 vascular properties were significantly correlated with the NOL discrimination index (Fig. 5d). Some correlations were expected, such as the positive correlations between the decrease in NOL test score and the decreases in arteriolar and venular diameter and flow. Other correlations were unexpected; for example, the betweenness was strongly negatively correlated with the NOL score. This betweenness result was interesting when considering that it exhibited the youngest AOS among capillary vessel properties (Fig. 5c) and formed the greatest number of inter-correlations with other vascular alterations (Supplementary Fig. 8, see “Discussion” for interpretation).