Abstract
The fine equilibrium of bone homeostasis is maintained by bone-forming osteoblasts and bone-resorbing osteoclasts. Here, we show that TAM receptors MERTK and TYRO3 exert reciprocal effects in osteoblast biology: Osteoblast-targeted deletion of MERTK promotes increased bone mass in healthy mice and mice with cancer-induced bone loss, whereas knockout of TYRO3 in osteoblasts shows the opposite phenotype. Functionally, the interaction of MERTK with its ligand PROS1 negatively regulates osteoblast differentiation via inducing the VAV2-RHOA-ROCK axis leading to increased cell contractility and motility while TYRO3 antagonizes this effect. Consequently, pharmacologic MERTK blockade by the small molecule inhibitor R992 increases osteoblast numbers and bone formation in mice. Furthermore, R992 counteracts cancer-induced bone loss, reduces bone metastasis and prolongs survival in preclinical models of multiple myeloma, breast- and lung cancer. In summary, MERTK and TYRO3 represent potent regulators of bone homeostasis with cell-type specific functions and MERTK blockade represents an osteoanabolic therapy with implications in cancer and beyond.
Similar content being viewed by others
Introduction
Bone represents a dynamic tissue constantly renewed and reshaped by osteoclastic bone resorption and osteoblastic bone formation. The process of bone formation is dependent on osteoblasts1. Their function is tightly regulated by a signaling pathway network controlling differentiation from mesenchymal stem cell-derived osteoprogenitor cells via osteoblasts towards osteocytes2. For example, the WNT signaling pathway induces osteoblast differentiation via the binding of WNT ligands to LRP5/63. The discovery of the WNT antagonist Sclerostin led to the development of anti-sclerostin antibodies (Romosozumab), which were recently approved in the indication of postmenopausal osteoporosis in the United States and Europe. Despite its success, the bone anabolic effect of Romosozumab is short-lived, and therapy needs to be followed by established antiresorptive agents to reach long-term effects in osteoporosis4,5,6. Furthermore, it is associated with potential adverse cardiac events5. The investigation of additional pathways controlling osteoblast differentiation and function is therefore essential to improve osteoanabolic treatments.
The TAM family of receptor tyrosine kinases, consisting of TYRO3 (BRT, DTK, RSE, SKY, and TIF), AXL (ARK, TYRO7, and UFO), and MERTK (EYK, NYM, and TYRO12) and their cognate ligands growth-arrest-specific gene-6 (GAS6) and protein S (PROS1) represent cell surface transmembrane receptors. They trigger phosphorylation and activation of multiple downstream signaling proteins influencing tissue homeostasis in several organ systems by modulating key processes, including tissue repair, inflammation, cell survival, proliferation, and migration7,8,9,10,11,12,13. The GAS6-TYRO3 axis induces osteoclast differentiation and it was consistently shown that germline deletion of TYRO3 leads to increased bone mass14. However, the tissue-specific roles of TAM receptors in the bone, especially in osteoblasts, are unknown.
TAM receptors and their ligands PROS1 and GAS6 are frequently overexpressed in cancer and mediate tumor-stroma interaction to limit anti-tumor immunity and fuel cancer growth15,16. Bone is a major site of cancer metastases, often presenting as osteolytic bone metastases17. Osteolytic bone metastases lead to skeletal-related events (SREs), including pathological fractures and pain, that require palliative interventions18. When established in the bone, cancer cells secrete factors, which educate osteoblasts to promote tumor growth and suppress their bone-forming capacity19. Several studies hypothesize that restoring osteoblast function could mitigate metastatic osteolytic bone disease20,21,22.
In this work, we demonstrate that osteoblasts are controlled by the TAM receptors MERTK and TYRO3, which regulate key cellular functions, including differentiation, migration, and bone formation. Furthermore, we show that cancer cells exploit this regulatory mechanism and aggravate osteolytic bone disease via MERTK. Treatment of bone metastasis-bearing mice with a MERTK-targeting small molecule inhibitor alleviated bone destruction and prolonged life span in mice, suggesting MERTK blockade as an osteoanabolic therapy in osteopenic bone diseases.
Results
Expression of TAM receptors in osteoblasts
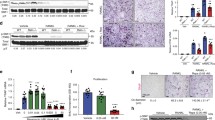
To gain insight into the role of TAM receptors in osteoblast biology, we performed gene expression analysis of Mertk, Tyro3, Axl, Gas6, and Pros1 mRNA in primary murine calvarial cell osteoblast cultures. Upon osteogenic induction, we observed an upregulation of Mertk, Gas6, and Pros1 during the culture period of 3 weeks, whereas Axl was downregulated. Tyro3 showed a short peak of twofold enhanced expression in the early differentiation phase with constant expression levels thereafter (Fig. 1a).
a mRNA expression analysis of TAM receptor family Mertk, Tyro3, Axl, Gas6, and Pros1 in primary murine osteoblast cultures after 0, 7, 14, and 21 days (n = 3 biological replicates) (Mertk expression d0 vs. d7: p = 0.0002; d0 vs. d14: p = 0.0042; d0 vs. d21: p = 0.0172), (Tyro3 expression d0 vs. d7: p = 0.009; d0 vs. d14: p = 0.9604; d0 vs. d21: p = 0.2928), (Axl expression d0 vs. d7: p = 0.0161; d0 vs. d14: p = 0.0152; d0 vs. d21: p = 0.0331), (Gas6 expression d0 vs. d7: p = 0.0011; d0 vs. d14: p = 0.0002; d0 vs. d21: p = 0.001), (Pros1 expression d0 vs. d7: p = 0.0157; d0 vs. d14: p = 0.0181 d0 vs. d21: p = 0.054). b, c Microcomputed tomography (μCT) of the metaphyseal proximal region of tibias from 8-week-old Mertkflox/flox and Col1a1-cre+;Mertkflox/flox female mice (top, longitudinal view of cortical and cancellous bone; bottom, longitudinal view of cancellous bone) (b). Quantification of bone volume (BV/TV) of cancellous bone determined by μCT analysis (c) (Mertkflox/flox, n = 9; Col1a1-cre+;Mertkflox/flox, n = 14). d, e Representative pictures of Calcein Demeclocycline labeling of Mertkflox/flox and Col1a1-cre+;Mertkflox/flox female mice (d). Bone formation rate of Col1a1-cre+;Mertkflox/flox mice after 8 weeks (e) (Mertkflox/flox, n = 5; Col1a1-cre+;Mertkflox/flox, n = 8). f–h Representative pictures (f) and histomorphometric analysis of osteoblast (g), and osteoclast number (h) by TRAP/Hematoxylin staining in femur from Col1a1-cre+;Mertkflox/flox mice. Green arrows pointing to osteoblasts visible as cuboidal or polygonal mononuclear cells on the endosteal bone surface (Mertkflox/flox, n = 5; Col1a1-cre+;Mertkflox/flox, n = 8). i Alizarin Red staining of ex vivo calvarial cell osteoblast culture from Col1a1-cre+;Mertkflox/flox mice. j Analysis of MERTK protein in osteoblast cultures from Mertkflox/flox mice treated with recombinant CRE recombinase. ß-ACTIN run on a separate gel. k Alizarin Red staining of MERTK KO calvarial cell cultures treated with PROS1 (100 nM). l, m RT-qPCR analysis of Alpl (l) and Bglap (m) mRNA expression in MERTK KO osteoblasts (n = 3 biological replicates). n Alizarin Red staining of wild-type calvarial cell cultures treated with PROS1 (100 nM) and MERTK-inhibitor R992 (200 nM). o, p RT-qPCR analysis of Alpl (o) and Bglap (p) mRNA expression (n = 3 biological replicates). Data were means ± SEMs. Statistical significance was determined by a two-tailed unpaired t-test.
Conditional deletion of Mertk and Tyro3 in osteoblasts
The increased expression levels of Mertk and Tyro3 led us to investigate their function in osteoblasts. Therefore, we created an osteoblast-targeted knockout of Mertk and Tyro3 in mice. We used the 2.3-kb mouse collagen type I, alpha 1 (Col1a1) promotor for cre recombinase expression (Col1a1-cre+), which is well described for being exclusively expressed in osteoblast lineage and odontoblasts without significant leakage to other tissues23. We deleted Mertk and Tyro3 by crossing Col1a1-cre+ mice with Mertkflox/flox mice1 and Tyro3flox/flox mice (SFig. 1a, b), generating Col1a1-cre+;Mertkflox/flox and Col1a1-cre+;Tyro3flox/flox conditional knockout mice (referred to as Mertk−/−OB and Tyro3−/−OB). Col1a1-cre−;Mertkflox/flox and Col1a1-cre−;Tyro3flox/flox littermates were used as controls throughout the experiments. Mertk−/−OB and Tyro3−/−OB mice exhibited no obvious skeletal defects and normal tooth eruption. However, whereas body weight in Mertk−/−OB (SFig. 2a) was not altered, Tyro3−/−OB mice showed slightly decreased body weight in comparison to littermate controls (SFig. 2b). Quantitative RT-PCR analysis of mRNA extracted from ex vivo osteoblast cultures confirmed decreased expression of Mertk (SFig. 3a) and Tyro3 (SFig. 3b), showing efficient deletion comparable to in vitro expression of other genes e.g. including Smad1 under the Col1a1 promoter24.
Role of MERTK in osteoblasts
Microcomputed tomography (μCT) analysis of the metaphyseal tibia of 8-week-old Mertk−/−OB mice in comparison to controls showed an increase in bone volume (+28.9%) (Fig. 1b, c), trabecular number (+21.8%) (SFig. 4a) and trabecular thickness (+5.8%) (SFig. 4b), whereas trabecular separation was decreased (−22.2%) (SFig. 4c). Midshaft evaluation of femurs of Mertk−/−OB mice did not show significant changes in cortical bone thickness (SFig. 4d), indicating that Mertk might play a more significant role on trabecular bone than on cortical bone. Dynamic histomorphometry showed an increased bone formation rate in Mertk−/−OB mice (Fig. 1d, e). Osteoblast numbers in Mertk−/−OB mice were increased, whereas osteoclast numbers were not affected (Fig. 1f–h). Ex vivo calvarial cell cultures of Mertk−/−OB mice showed increased osteoblast matrix mineralization (Fig. 1i). These data demonstrate that TAM receptor MERTK negatively regulates physiological bone remodeling by inhibition of osteoblastic bone formation.
To further study the role of MERTK in osteoblast differentiation in vitro, we induced stable knockout of Mertk using the Cre-Lox recombination technology and delivered recombinant CRE to calvarial cell cultures from Mertkflox/flox mice. Efficient MERTK knockout was evaluated by SDS-PAGE (Fig. 1j). Treatment of calvarial cells with MERTK-ligand PROS1 inhibited osteoblast matrix mineralization in control conditions while inducing it in the absence of MERTK receptor (Fig. 1k). Consistent findings were obtained when measuring alkaline phosphatase (Alpl) mRNA expression on day 7 and Osteocalcin (Bglap) on day 21: PROS1 decreased expression of these osteoblast differentiation markers, whereas MERTK KO osteoblasts showed increased expression. Notably, PROS1 could not decrease the osteoblast differentiation marker in the absence of MERTK (Fig. 1l, m). Furthermore, the MERTK-specific small molecule inhibitor R992 effectively suppressed the inhibitory effect of PROS1 on matrix mineralization and differentiation marker expression in wild-type cells (Fig. 1n–p). Altogether, these results show that the PROS1-MERTK axis inhibits osteoblast differentiation and matrix mineralization.
MERTK regulates cell morphology and migration by modulation of actin cytoskeletal rearrangement, primarily via small GTPase RHOA25, and it was shown that this pathway is detrimental to terminal osteoblast differentiation and bone formation26.
Therefore, we characterized in a first step osteoblast cell morphology which is mainly regulated by the F-actin cytoskeleton, whose arrangement is constantly renewed to enable different mechanical forces needed for diverse cellular functions such as differentiation, adhesion, or migration27. Osteoblast differentiation is associated with low F-actin content and reduced stress fiber formation28. Stress fibers are thick crosslinked F-actin bundles promoting cellular retraction via interaction with myosin motor proteins29,30. Confocal microscopy of F-actin immunofluorescence staining revealed that PROS1 promoted high F-actin staining intensity and could prominently induce stress fiber formation, whereas MERTK KO osteoblast cultures exhibited low F-actin content and a reduced number of stress fiber-containing cells. PROS1 could not induce stress fiber formation in the absence of MERTK (Fig. 2a–c). As the assembly and function of actomyosin stress fibers depend on myosin regulatory light chain (RLC) phosphorylation30, we stained for RLC phosphorylation of Ser19, which was reduced in MERTK KO osteoblasts (Fig. 2a, d). These results indicate that MERTK has profound effects on osteoblast cytoskeletal arrangement, with MERTK inducing cellular retraction and stress fiber formation altogether impeding osteoblast differentiation.
a–d Confocal imaging of pRLC and F-actin staining of MERTK KO osteoblasts on glass coverslips treated with TAM receptor ligand PROS1 (100 nM) (a). F-actin intensity (n = 3, mean of 50 measurements in three fields) (b), stress fiber-containing cells (n = 3, mean of 100 measurements in three fields) (c), and pRLC intensity (n = 3, mean of 50 measurements in three fields) (d) was quantified. e Immunoblot of wild-type osteoblasts treated with different concentrations of PROS1 (0, 50, 100, 200 nM) showing activated GTPγs-bound RHOA, total-RHOA, and ß-ACTIN. ß-ACTIN run on a separate gel. f Immunoblot of wild-type osteoblasts treated with PROS1 (100 nM) and MERTK-inhibitor R992 (200 nM) showing activated GTPγs-bound RHOA, total-RHOA, phosphorylated VAV2, an association of MERTK and VAV2 and ß-ACTIN. ß-ACTIN run on a separate gel. g Alizarin Red staining of wild-type calvarial cells treated with PROS1 (100 nM) and ROCK-inhibitor Y27632 (10 μM). h RT-qPCR analysis of Alpl mRNA expression (n = 3 biological replicates). i Calvarial cells were cultured in an osteogenic medium with exogenous addition of PROS1 and Y27632. Cultures were stained by F-actin staining. The top view shows representative pictures of the cell morphology of calvarial cell cultures after 5 days. The bottom view shows representative pictures of single-cell analysis on fibronectin substrate (representative image section). j The percentage of stress fiber-containing cells was quantified (n = 3, mean of 100 cells in three fields). Data were means ± SEMs. Statistical significance was determined by a two-tailed unpaired t-test.
Evaluation of RHOA signaling revealed that PROS1 dose-dependently induced activated GTPyS-RHOA in osteoblasts (Fig. 2e). It is known that MERTK can bind SH2 domain proteins, particularly the vav-proto oncogene (vav) family of guanine nucleotide exchange factors (GEFs) for Rho-family GTPases31,32. Previous data show that tyrosine phosphorylation of MERTK and VAV leads to VAV dissociation from MERTK and downstream GDP to GTP exchange of RHOA31. VAV3 and VAV1 are expressed only in mature osteoblasts, whereas VAV2 is present throughout osteoblast differentiation33, suggesting that these RHO-GEFS could be involved in the activation of RHOA by MERTK because it binds VAV proteins constitutively. As we observed the biological effects of MERTK in all stages of calvarial cell culture, we hypothesized that VAV2 is the main substrate of MERTK, leading to RHOA/ROCK activation. We could demonstrate that blocking of MERTK phosphorylation by R992 promotes high MERTK-VAV2 protein interaction leading to low VAV2 phosphorylation levels and inhibition of RHOA activation (Fig. 2f). PROS1 could neither decrease osteoblast matrix mineralization and expression of Alpl mRNA nor induce stress fiber formation in the presence of ROCK-inhibitor Y27632 (Fig. 2g–j). These data demonstrate that the PROS1-MERTK axis negatively regulates osteoblastic differentiation and bone formation via the VAV2-RHOA-ROCK pathway.
As RHOA regulates osteoblast motility, our results suggest a role of MERTK in this process. We performed wound healing assays upon the addition of PROS1 with MERTK KO osteoblasts. Here, we observed increased osteoblast migration induced by PROS1 in control conditions, which was abrogated in MERTK KO osteoblasts (SFig. 5a, b). Migrating mesenchymal cells, including osteoblasts, are dependent on a polarized cytoskeletal arrangement and application of cytoskeletal forces to the extracellular matrix via Integrin receptors with focal adhesion formation34,35. Analysis of focal adhesion protein vinculin revealed that focal adhesion formation was increased by PROS1. MERTK KO osteoblasts exhibited decreased vinculin staining intensity and PROS1 could not induce focal adhesions in the absence of MERTK (SFig. 5c, d). Furthermore, PROS1 increased and loss of MERTK decreased the percentage of cells with a leading and a trailing edge, suggesting that MERTK controls polarization of migrating osteoblasts (SFig. 5e). These results indicate that MERTK induces osteoblast migration and focal adhesion formation.
Altogether, our data suggest that MERTK activates the VAV2-RHOA-ROCK pathway in osteoblasts inducing cellular retraction and stress fiber formation, resulting in increased motility, which counteracts osteoblastic bone formation.
Role of TYRO3 in osteoblasts
Evaluation of Tyro3−/−OB mice revealed decreased bone volume with a decreased trabecular number. Trabecular separation was increased, whereas trabecular thickness was not significantly affected (Fig. 3a, b and SFig. 6a–c). Midshaft evaluation of the femur revealed decreased cortical thickness (SFig. 6d). Bone formation rate and osteoblast numbers were decreased, whereas osteoclast numbers were not affected (Fig. 3c–g). These results suggest that TAM receptor TYRO3 promotes osteoblastic bone formation and exerts opposing biological functions in contrast to MERTK. Correspondingly ex vivo calvarial cell cultures of Tyro3−/−OB mice showed decreased osteoblast matrix mineralization, indicating that TYRO3 directly enhances osteoblast function (Fig. 3h).
a, b Microcomputed tomography (μCT) of the metaphyseal proximal region of tibias from 8-week-old Tyro3flox/flox and Col1a1-cre+;Tyro3flox/flox female mice (top, longitudinal view of cortical and cancellous bone; bottom, longitudinal view of cancellous bone) (a). Quantification of bone volume (b) of cancellous bone determined by μCT analysis (Tyro3flox/flox, n = 8; Col1a1-cre+;Tyro3flox/flox, n = 10). c, d Representative pictures of Calcein Demeclocycline labeling of Tyro3flox/flox and Col1a1-cre+;Tyro3flox/flox female mice (c). Bone formation rate of Col1a1-cre+;Tyro3flox/flox mice after 8 weeks (Tyro3flox/flox, n = 5; Col1a1-cre+;Tyro3flox/flox, n = 8) (d). e–g Representative pictures (e) of histomorphometric analysis of osteoblast (f) and osteoclast number (g) by TRAP/Hematoxylin staining in femur from Col1a1-cre+;Tyro3flox/flox mice. Green arrows pointing to osteoblasts visible as cuboidal or polygonal mononuclear cells on the endosteal bone surface (Tyro3flox/flox, n = 8; Col1a1-cre+;Tyro3flox/flox, n = 10). h Alizarin Red staining of ex vivo calvarial cell osteoblast culture from Col1a1-cre+;Tyro3flox/flox mice. i Analysis of TYRO3 protein in osteoblast cultures from Tyro3flox/flox mice treated with recombinant CRE recombinase. j Alizarin Red staining of Tyro3 KO calvarial cell cultures treated with PROS1 (100 nM). k, l RT-qPCR analysis of Alpl (k) and Bglap (l) mRNA expression in Tyro3 KO osteoblasts (n = 3 biological replicates). m–p Confocal imaging of pRLC and F-actin staining of TYRO3 KO osteoblasts on glass coverslips treated with TAM receptor ligand PROS1 (100 nM) (m). F-actin intensity (n = 3, mean of 50 measurements in three fields) (n), stress fiber-containing cells (n = 3, mean of 100 measurements in three fields) (o), and pRLC intensity (n = 3, mean of 50 measurements in three fields) (p) was quantified. Data were means ± SEMs. Statistical significance was determined by a two-tailed unpaired t-test.
We investigated the effects of PROS1 on osteoblast differentiation in the presence and absence of TYRO3. First, we found almost complete knockout of TYRO3 upon the addition of CRE recombinase to Tyro3flox/flox calvarial cells (Fig. 3i). Addition of PROS1 decreased matrix mineralization in TYRO3 KO osteoblast cultures (Fig. 3j). TYRO3 KO osteoblasts showed decreased expression of Alpl and Bglap which was further reduced by treatment with PROS1, suggesting that PROS1-TYRO3 promotes osteoblast differentiation (Fig. 3k, l).
As we observed cytoskeletal regulation of osteoblasts by TAM receptor MERTK, we hypothesized that TYRO3 coming from the same receptor tyrosine kinase family may exert similar functions. Interestingly, we observed opposing effects of TYRO3 vs. MERTK in osteoblast cytoskeletal rearrangement: TYRO3 KO osteoblasts showed enhanced F-actin content with increased stress fiber formation and elevated pRLC activity, which could be further increased by PROS1 (Fig. 3m–p). Concomitantly, migration and focal adhesion formation was increased and PROS1 could further enhance these processes in TYRO3 KO osteoblasts (SFig. 7a–d). In TYRO3 KO osteoblasts, cells containing a leading and a trailing edge were increased and PROS1 could further increase this phenotype (SFig. 7e). These results suggest that the PROS1-TYRO3 axis promotes low F-actin content in osteoblasts with decelerated migration leading to increased osteoblast function.
Cytoskeletal regulation of osteoblasts by MERTK vs. TYRO3
To validate our findings investigating the biological effects of MERTK and TYRO3 in osteoblasts in the same experiment, we performed siRNA-mediated knockdown of Mertk and Tyro3. Mertk and Tyro3 expression was silenced by 86.2% (SFig. 8a) and 74.4% (SFig. 8b), respectively 3 days after transfection. Confocal microscopy of F-actin immunofluorescence staining revealed that PROS1 increases F-actin intensity, induces stress fiber formation and consistently pRLC intensity in osteoblasts in control conditions. These effects were inhibited by silencing of Mertk (SFig. 9a–e). In contrast, treatment of Tyro3-silenced osteoblasts with PROS1 increased F-actin intensity, induces high-stress fiber formation and increased pRLC intensity, indicating that PROS1-MERTK axis promotes actin polymerization and stress fiber formation, whereas the PROS1-TYRO3 axis inhibits these effects (SFig. 9a–e).
We performed additional wound healing assays upon the addition of PROS1 with Mertk and Tyro3 siRNA-silenced osteoblasts. We observed increased osteoblast migration induced by PROS1 in control conditions, which was abrogated in Mertk-silenced osteoblasts. In contrast, PROS1 treatment in Tyro3-silenced osteoblasts increased osteoblast migration (SFig. 10a, b). These results show that the PROS1-MERTK axis induces osteoblast migration, while the PROS1-TYRO3 axis inhibits migration. Consistently, analysis of focal adhesion protein vinculin revealed that focal adhesion formation was increased by PROS1 in NTsiRNA controls. In Mertk-silenced osteoblasts, PROS1 could not increase vinculin staining intensity, indicating that the PROS1-MERTK axis promotes osteoblast focal adhesion formation. Tyro3-silenced osteoblasts showed increased vinculin staining intensity, which could be further increased by PROS1, indicating that the PROS1-TYRO3 axis inhibits focal adhesion formation (SFig. 10c, d).
Consistently, PROS1 increased the percentage of cells with a leading and a trailing edge in control osteoblasts, whereas in Mertk-silenced osteoblasts, PROS1 treatment could not increase osteoblast polarization. In Tyro3-silenced osteoblasts, cells containing a leading and a trailing edge were increased and PROS1 could further increase this phenotype, indicating that the PROS1-MERTK axis induces and PROS1-TYRO3 axis inhibits osteoblast polarization (SFig. 11a, b). Cell spreading experiments demonstrated that loss of Mertk accelerates while loss of Tyro3 decelerates osteoblast spreading. PROS1 did not significantly affect spreading capability in our experimental setting (SFig. 11c).
Stimulation with PROS1 led to increased levels of GTP-bound RHOA in control osteoblasts, which was abrogated in Mertk-silenced osteoblasts. In contrast, Tyro3-silenced osteoblasts showed higher PROS1 mediated RHOA activation levels compared to control (SFig. 12).
Altogether our results suggest that MERTK activates the RHOA-ROCK pathway in osteoblasts inducing cellular retraction and stress fiber formation, resulting in increased motility, which counteracts matrix mineralization. In contrast, TYRO3 promotes osteoblast differentiation by antagonizing these effects. Therefore, we hypothesized that MERTK could represent a novel therapeutic target for osteoanabolic pharmacotherapy.
Effect of pharmacologic MERTK blockade on bone formation in healthy mice
Consequently, we examined the effect of the small molecule R992 on bone formation in healthy mice. R992 is a novel MERTK-selective inhibitor discovered through rational drug design. Figure 4a shows the structure and chemical formula of R992. The drug forms H-bonds with three amino acid residues in the ATP pocket of MERTK (Fig. 4b). R992 is an orally bioavailable, potent, and selective inhibitor displaying low nanomolar cell-based activity against MERTK. In biochemical assays, R992 exhibits seven to eight-fold selectivity over AXL and TYRO3 (Fig. 4c). Furthermore, R992 blocks MERTK phosphorylation in human and murine cells (Fig. 4d, e).
a Structure and chemical formula of R992: 3-(Butylamino)−5-((1r,4r)−4-hydroxycyclohexyl)−8-((4-methylpiperazin-1-yl)methyl)pyrimido[4,5-c]isoquinolin-6(5H)-one (C27H38N6O2). b Illustration of R992 docked in MERTK and the H-bonds R992 forms with three residues in the ATP pocket (MET-647, PRO-672, and ASP-741). c R992 on-target and off-target activity. d, e Immunoblots of MERTK phosphorylation and AKT signaling in human (d) and murine (e) cells. f μCT of the metaphyseal proximal region of tibias of 10-week-old healthy C57BL/6J mice treated after 2 weeks of treatment with vehicle or R992. g Quantification of trabecular bone volume (BV/TV) of the proximal tibia determined by μCT analysis (Vehicle n = 7 and R992 n = 7). h, i Representative pictures (h) and analysis (i) of bone formation rate by Calcein and Demeclocycline double labeling (Vehicle n = 7 and R992 n = 7). j, k Representative pictures of Alizarin red staining of calvarial cells cultures treated with different doses of R992 on day 21 (j). The number of mineralized nodules was quantified (k) (n = 3 biological replicates). l Analysis of osteoblast differentiation marker Alpl, Runx2, and Osx on day 7 in R992-treated calvarial cell cultures in comparison to control treated cultures (n = 3 biological replicates). Data were means ± SEM. Statistical significance was determined by a two-tailed unpaired t-test.
Eight-week-old C57BL/6J mice were treated orally 2x/day with 60 mg/kg R992 continually for 2 weeks. μCT analysis of cancellous bone in R992-treated mice revealed increased bone volume (+59.5%) (Fig.4f, g). Analysis of bone formation revealed an increased bone formation rate (+43.5%) in R992-treated mice (Fig. 4h, i). Consistently, osteoblast cultures showed that R992 could dose-dependently increase bone nodule formation and increase osteoblast differentiation markers Alpl, SP7, and Runx2 (Fig. 4j–l).
Effect of MERTK blockade on tumor–osteoblast interaction
Skeletal-related events induced by bone-seeking tumors, including multiple myeloma, breast- and lung cancer result in severe pain and fractures, causing substantial morbidity in patients17,36,37,38. The osteolytic disease is caused by an imbalance in bone remodeling, favoring osteoclast-mediated bone resorption over osteoblast-mediated bone formation36. As many cancer types express MERTK and TYRO3 as well as their ligand PROS1, we hypothesized that tumor cells may exploit the TAM-receptor-dependent regulation of osteoblasts and inhibit their function via MERTK39,40,41,Immunoprecipitation/SDS-PAGE For the harvest, cells were washed in ice-cold PBS and lysed in RIPA Buffer (Thermo Fisher), containing PhosSTOP phosphatase inhibitor cocktail (Roche) and protease inhibitor cocktail (Roche). Equivalent amounts of protein were incubated with respective primary antibodies for 2 h, followed by incubation with protein G-Sepharose (Thermo Fisher) for 3 h. The beads were washed three times in the RIPA buffer, resuspended in the appropriate volume of RIPA containing Laemmli gel loading buffer, and subjected to SDS-PAGE. The proteins were electrotransferred to nitrocellulose membranes and blocked in 5% bovine serum albumin for 1 h. Blocked membranes were probed with primary antibodies overnight at 4 °C in the same buffer, followed by secondary antibody conjugated to HRP in blocking solution for 1 h shaking at room temperature. Bands were visualized using enhanced chemiluminescence (ECL) detection. GTP-bound RHOA was analyzed by performing a pull-down assay with Cell Biolabs, Inc. Rho Activation Assay kit. Calvarial cells were transfected and cultured as described. Three days after osteogenic induction, cells were incubated in serum-free α-MEM medium for 5 h and then stimulated with human-purified Protein S (Enzyme Research Laboratories). Cells were collected at the indicated time points and processed following the manufacturer’s instructions. Mouse P1NP (Novus biologicals) and Trap5b (immunodiagnostic systems) levels were determined in peripheral blood using ELISA kits according to the manufacturer’s instructions. Human Igλ concentration was assessed in blood plasma from myeloma-bearing mice according to the manufacturerʼs instructions (Human Lambda ELISA KIT; Bethyl Laboratories Inc, Montgomery, TX, USA). The bone marrow of myeloma-bearing mice was flushed and MM cells were stained for CD138 (BioLegend). Events were captured using BD FACS Fortessa. The mice were fixed with 4% paraformaldehyde for 48 h and the tibia was decalcified with EDTA. Paraffin blocks were cut into 5-μm-thick sections. Two non-serial sections of each bone were assessed. TRAP staining was performed for 30 min followed by nuclear counterstaining with hematoxylin. The number of osteoclasts and osteoblasts at the bone surface was measured using the osteomeasure system (Osteometrics). Osteoclast precursor cells per bone marrow area or tumor area was quantified using Osteomeasure system (Osteometrics). About 2 mg/ml Calcein (Sigma) and 2 mg/ml Demeclocycline (Sigma) were injected i.p. 7 and 2 days before sacrificing the animals. Tibia were collected and fixed in 4% paraformaldehyde (PFA) for 48 h. For analysis, tibiae were embedded in methyl methacrylate. Samples were cut using 5-μm sagittal sections. Quantitative bone histomorphometric measurements were performed according to standard protocols using an OsteoMeasure system (Osteometrics). μCT was used for 3D analyses of long bones. Long bones of mice were analyzed using high-resolution μCT with a fixed isotropic voxel size of 10 μm (70 peak kV at X μA 400 ms integration time; Viva80 microCT; Scanco Medical). All analyses were performed on digitally extracted bone tissue using 3D distance techniques (Scanco Medical), as reported previously56. Region of interest (ROI) was defined manually by drawing contours in slices. Data were means ± SEMs. Statistical significance was determined by a two-tailed unpaired t-test, unless otherwise stated. Survival analysis was carried out using the Kaplan–Meier function (Mantel–Cox-test/log-rank test). Significant outliers were calculated and excluded from the analysis. All statistical analyses were performed using GraphPad Prism 5 software. All cell culture experiments were performed at least three times with similar results. For data presentation, a representative experiment was chosen and included in the manuscript. Further information on research design is available in the Nature Research Reporting Summary linked to this article.Detection of RHOA activation
ELISA
Flow cytometry
Histology
Analysis of bone formation rate
μCT
Statistics and reproducibility
Reporting summary
Data availability
All data supporting the findings described in this manuscript are available in the article and in the Supplementary Information and from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Sims, N. A. & Martin, T. J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3, 481 (2014).
Rutkovskiy, A., Stenslokken, K. O. & Vaage, I. J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 22, 95–106 (2016).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
McClung, M. R. et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 (2014).
Solling, A. S. K., Harslof, T. & Langdahl, B. The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther. Adv. Musculoskelet. Dis. 10, 105–115 (2018).
Saag, K. G. et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 377, 1417–1427 (2017).
Fourgeaud, L. et al. TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244 (2016).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017).
Chan, P. Y. et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science 352, 99–103 (2016).
Prasad, D. et al. TAM receptor function in the retinal pigment epithelium. Mol. Cell Neurosci. 33, 96–108 (2006).
Zagorska, A. et al. Differential regulation of hepatic physiology and injury by the TAM receptors Axl and Mer. Life Sci Alliance 3, e202000694 (2020).
Rothlin, C. V., Carrera-Silva, E. A., Bosurgi, L. & Ghosh, S. TAM receptor signaling in immune homeostasis. Annu. Rev. Immunol. 33, 355–391 (2015).
Tondo, G., Perani, D. & Comi, C. TAM receptor pathways at the crossroads of neuroinflammation and neurodegeneration. Dis. Markers 2019, 2387614 (2019).
Ruiz-Heiland, G. et al. Deletion of the receptor tyrosine kinase Tyro3 inhibits synovial hyperplasia and bone damage in arthritis. Ann. Rheum. Dis. 73, 771–779 (2014).
Graham, D. K., DeRyckere, D., Davies, K. D. & Earp, H. S. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 14, 769–785 (2014).
Gadiyar, V., Patel, G. & Davra, V. Immunological role of TAM receptors in the cancer microenvironment. Int. Rev. Cell Mol. Biol. 357, 57–79 (2020).
David Roodman, G. & Silbermann, R. Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep. 4, 753 (2015).
Coleman, R. E. et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat. Rev. 36, 615–620 (2010).
Kolb, A. D., Shupp, A. B., Mukhopadhyay, D., Marini, F. C. & Bussard, K. M. Osteoblasts are “educated” by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res. 21, 31 (2019).
Hesse, E. et al. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight 5, e125543 (2019).
Paton-Hough, J. et al. Preventing and repairing myeloma bone disease by combining conventional antiresorptive treatment with a bone anabolic agent in murine models. J. Bone Min. Res. 34, 783–796 (2019).
Chen, Y. C., Sosnoski, D. M. & Mastro, A. M. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 12, 215 (2010).
Dacquin, R., Starbuck, M., Schinke, T. & Karsenty, G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev. Dyn. 224, 245–251 (2002).
Wang, M. et al. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthr. Cartil. 19, 751–762 (2011).
Lee, Y. J., Park, H. J., Woo, S. Y., Park, E. M. & Kang, J. L. RhoA/phosphatidylinositol 3-kinase/protein kinase B/mitogen-activated protein kinase signaling after growth arrest-specific protein 6/mer receptor tyrosine kinase engagement promotes epithelial cell growth and wound repair via upregulation of hepatocyte growth factor in macrophages. J. Pharm. Exp. Ther. 350, 563–577 (2014).
Negishi-Koga, T. et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 17, 1473–1480 (2011).
Svitkina, T. The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 10, a018267 (2018).
Zouani, O. F., Rami, L., Lei, Y. & Durrieu, M. C. Insights into the osteoblast precursor differentiation towards mature osteoblasts induced by continuous BMP-2 signaling. Biol. Open 2, 872–881 (2013).
Tojkander, S., Gateva, G. & Lappalainen, P. Actin stress fibers–assembly, dynamics and biological roles. J. Cell Sci. 125, 1855–1864 (2012).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009).
Mahajan, N. P. & Earp, H. S. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J. Biol. Chem. 278, 42596–42603 (2003).
Shelby, S. J., Colwill, K., Dhe-Paganon, S., Pawson, T. & Thompson, D. A. MERTK interactions with SH2-domain proteins in the retinal pigment epithelium. PLoS ONE 8, e53964 (2013).
Faccio, R. et al. Vav3 regulates osteoclast function and bone mass. Nat. Med. 11, 284–290 (2005).
Jannie, K. M. et al. Vinculin-dependent actin bundling regulates cell migration and traction forces. Biochem. J. 465, 383–393 (2015).
Vallenius, T. Actin stress fibre subtypes in mesenchymal-migrating cells. Open Biol. 3, 130001 (2013).
O’Donnell, E. K. & Raje, N. S. Myeloma bone disease: pathogenesis and treatment. Clin. Adv. Hematol. Oncol. 15, 285–295 (2017).
Brook, N., Brook, E., Dharmarajan, A., Dass, C. R. & Chan, A. Breast cancer bone metastases: pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 96, 63–78 (2018).
Rossi, A., Gridelli, C., Ricciardi, S. & de Marinis, F. Bone metastases and non-small cell lung cancer: from bisphosphonates to targeted therapy. Curr. Med. Chem. 19, 5524–5535 (2012).
Ubil, E. et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Investig. 128, 2356–2369 (2018).
Che Mat, M. F. et al. Silencing of PROS1 induces apoptosis and inhibits migration and invasion of glioblastoma multiforme cells. Int. J. Oncol. 49, 2359–2366 (2016).
Saraon, P. et al. Proteomic profiling of androgen-independent prostate cancer cell lines reveals a role for protein S during the development of high grade and castration-resistant prostate cancer. J. Biol. Chem. 287, 34019–34031 (2012).
**e, S. et al. Mer receptor tyrosine kinase is frequently overexpressed in human non-small cell lung cancer, confirming resistance to erlotinib. Oncotarget 6, 9206–9219 (2015).
Waizenegger, J. S. et al. Role of growth arrest-specific gene 6-Mer axis in multiple myeloma. Leukemia 29, 696–704 (2015).
Linger, R. M., Keating, A. K., Earp, H. S. & Graham, D. K. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin. Ther. Targets 14, 1073–1090 (2010).
Smart, S. K., Vasileiadi, E., Wang, X., DeRyckere, D. & Graham, D. K. The emerging role of TYRO3 as a therapeutic target in cancer. Cancers 10, 474.(2018).
Krishnan, V., Dhurjati, R., Vogler, E. A. & Mastro, A. M. Osteogenesis in vitro: from pre-osteoblasts to osteocytes: a contribution from the Osteobiology Research Group, The Pennsylvania State University. Vitr. Cell Dev. Biol. Anim. 46, 28–35 (2010).
Tang, Y. et al. Mertk deficiency affects macrophage directional migration via disruption of cytoskeletal organization. PLoS ONE 10, e0117787 (2015).
Lemke, G. & Rothlin, C. V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336 (2008).
Burstyn-Cohen, T., Heeb, M. J. & Lemke, G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J. Clin. Invest. 119, 2942–2953 (2009).
Seeman, E. & Martin, T. J. Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat. Rev. Rheumatol. 15, 225–236 (2019).
Holland, S. J. et. al. Small molecule inhibitors of the anti-inflammatory TAM receptor MerTK. [abstract]. Cancer Res. 76, 4869 (2016).
Kristinsson, S. Y., Minter, A. R., Korde, N., Tan, E. & Landgren, O. Bone disease in multiple myeloma and precursor disease: novel diagnostic approaches and implications on clinical management. Expert Rev. Mol. Diagn. 11, 593–603 (2011).
Weber, K., Mock, U., Petrowitz, B., Bartsch, U. & Fehse, B. Lentiviral gene ontology (LeGO) vectors equipped with novel drug-selectable fluorescent proteins: new building blocks for cell marking and multi-gene analysis. Gene Ther. 17, 511–520 (2010).
Weber, K., Bartsch, U., Stocking, C. & Fehse, B. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 16, 698–706 (2008).
Lineham, E., Tizzard, G. J., Coles, S. J., Spencer, J. & Morley, S. J. Synergistic effects of inhibiting the MNK-eIF4E and PI3K/AKT/ mTOR pathways on cell migration in MDA-MB-231 cells. Oncotarget 9, 14148–14159 (2018).
Bouxsein, M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 25, 1468–1486 (2010).
Acknowledgements
The position of J.E. was supported by the priority program μbone from the DFG (LO1863/5-1) and he received the E.W. Kuhlmann scholarship from the University Cancer Center Hamburg (UCCH). S.L. was supported by a Heisenberg professorship (DFG), by the priority program μbone from the DFG (LO1863/5-1), and is currently supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 758713), by the priority program μbone from the DFG (LO1863/5-1) and by the Hector Stiftung II. I.B.-B. was supported by (BE6658/1-1) and is currently supported by the priority program μbone from the DFG (BE6658/2-1). E.H. and H.T. received funding from the German Research Foundation (SPP 2084 to E.H. (HE 5208/5-1) and H.T (TA 1154/2-1) and Emmy Noether Program to H.T. (TA 1154/1-1 and TA 1154/1-2)). R992 was obtained from Rigel Inc. (South San Francisco, CA, USA). The authors would like to thank Michael Horn and the UCCH in vivo optical imaging core facility for help with the intracardial injections and imaging of our mouse models. Confocal microscopy was conducted at the UKE Microscopy Imaging Facility (DFG Research Infrastructure Portal: RI_00489). FACS analysis with BD Fortessa was performed at the FACS Sorting Core Unit at University Hospital Hamburg-Eppendorf. We thank Bao-Uyen Huynh and Sabrina Noster on behalf of the Animal Facility (FTH) at University Hospital Hamburg-Eppendorf for taking care of the mice housing and breeding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.E., J.Z., V.G., N.B., T.V.L., M.E.V.D., A.B.-H., S.B., I.S.D., and E.H. performed experiments. K.R., E.M., T.B.-C, E.J.A., S.G., C.R., E.H., and H.T. provided animals, techniques, and intellectual feedback H.T., J.E., K.P., C.B., I.B.-B., and S.L were involved in data interpretation, in the writing, review, and/or revision of the manuscript. J.E., I.B.-B., and S.L. conceived the project, supervised the research, and wrote the original manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.B., I.S.D., and E.M. are employees of Rigel Inc. S.L. has received speaker honoraria from Rigel Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Raymond Birge and Matthew Greenblatt for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Engelmann, J., Zarrer, J., Gensch, V. et al. Regulation of bone homeostasis by MERTK and TYRO3. Nat Commun 13, 7689 (2022). https://doi.org/10.1038/s41467-022-33938-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33938-x
- Springer Nature Limited
This article is cited by
-
Efferocytosis and Bone Dynamics
Current Osteoporosis Reports (2024)