Abstract
While the majority of cells contain a single nucleus, cell types such as trophoblasts, osteoclasts, and skeletal myofibers require multinucleation. One advantage of multinucleation can be the assignment of distinct functions to different nuclei, but comprehensive interrogation of transcriptional heterogeneity within multinucleated tissues has been challenging due to the presence of a shared cytoplasm. Here, we utilized single-nucleus RNA-sequencing (snRNA-seq) to determine the extent of transcriptional diversity within multinucleated skeletal myofibers. Nuclei from mouse skeletal muscle were profiled across the lifespan, which revealed the presence of distinct myonuclear populations emerging in postnatal development as well as aging muscle. Our datasets also provided a platform for discovery of genes associated with rare specialized regions of the muscle cell, including markers of the myotendinous junction and functionally validated factors expressed at the neuromuscular junction. These findings reveal that myonuclei within syncytial muscle fibers possess distinct transcriptional profiles that regulate muscle biology.
Similar content being viewed by others

Introduction
Skeletal muscle forms through the fusion of mononucleated muscle progenitor cells during development1,2. Mature, multinucleated myofibers are composed of different contractile and metabolic machinery that result in independent muscle fiber types (IIb, IIx, IIa, and I)3. In addition to possessing fiber type diversity, skeletal muscle also contains a stem cell population (satellite cells) that can be activated upon a stimulus and ultimately fuse with myofibers or each other, thereby allowing regeneration4. The requirement of multinucleation for skeletal muscle function lacks experimental validation, but explanations include that each nucleus is capable of controlling a finite volume of cytoplasm and that regions of the myofiber perform specialized functions necessitating localized transcripts.
Compelling recent studies have enhanced our understanding of the transcriptional diversity of skeletal muscle. Single-cell RNA-sequencing and mass cytometry studies have outlined the major mononuclear cell types present in skeletal muscle, ranging from 10 to 15 cell types depending on cluster assignments and the granularity of subty**5,6,7. The major cell types always include the following broad categories: fibroadipogenic progenitors (FAPs), tenocytes, endothelial cells, smooth muscle cells, immune cells (B cells, T cells, macrophages, neutrophils), neural/glial cells, and satellite cells. Notably, mature myofibers are a small minority of captures in single-cell studies despite their dominance within the tissue, owing to the fact that multinucleated cells are not easily isolated in single-cell dissociation approaches5,6,7.
Myofibers are known to exhibit functional specialization at postsynaptic endplates (neuromuscular junction: NMJ), where a small cluster of myonuclei are responsible for formation and maintenance of the synaptic apparatus8,9,10, a Unbiased clustering of nuclei from postnatal (P) day 21 presented in a UMAP revealed all major populations in addition to unique myonuclei outside the canonical Type IIb and Type IIx myonuclei. These nuclei populations were marked by Nos1 and Enah, respectively. b Subclustering of myonuclear populations from P21 muscle revealed sarcomere assembly myonuclear states. c Feature plots assessing expression of mature muscle markers (Ttn, Myh1, Myh4, and Ckm) and differentiation markers (Myod1, Myog, Mymk) from the myonuclear populations in b. d Violin plots showing the sarcomere assembly myonuclei are enriched for a transcriptional profile associated with early myofibrillogenesis (expression of Nrap, Enah, Flnc, Myh9, Myh10, Fhod3).
One interpretation of P21 sarcomere assembly myonuclei is that they are the most recently fused nuclei and have not yet established their fiber type. In this scenario, these sarcomere assembly myonuclei could be in a more immature state and suggest that they progress through a postfusion maturation program to establish their adult identity. Another possibility is that these myonuclear states are entrained after the majority of myonuclear accretion has occurred (around P21). To distinguish these possibilities, we generated 8611 nuclear transcriptomes from the tibialis anterior at P10 when fusion is ongoing and recently fused nuclei would be present. Here we were unable to detect robust independent clustering of Enah+ myonuclear populations and instead detected myonuclear populations similar to adult muscle (Fig. 3a, Supplementary Fig. 12a). Interestingly, we did detect a cluster of cells at P10, which were close to satellite cells, but were not present at P21 or 5 months of age. We hypothesized that this population represented myocytes (activated satellite cells) since fusion is ongoing at P10. We subclustered the satellite cell and myocyte populations and detected the presence of differentiation and fusion genes including Myog and Mymk in the myocyte population, while Pax7 was enriched in the satellite cell population (Supplementary Fig. 12b). The presence of myocytes demonstrated ongoing fusion at P10 and the absence of this population at P21 confirmed that the majority of fusion was terminated in these samples. The lack of sarcomere assembly populations at P10 indicates that they do not represent a maturational phase that myonuclei transition through proximal to fusion, but instead more nuclei adopt this state in response to a physiological stimulus during the postfusion growth of skeletal muscle. We validated the transient expansion of Enah+ myonuclei at P21 by performing smRNA-FISH on tibialis anterior muscles from P10, P21, and 5-month-old mice (Fig. 3b). Flnc and Enah expression was present at all ages, but the percentage of Flnc+/Enah+ nuclei was significantly increased in P21 compared to P10 and 5 months (Fig. 3b). This result validated the P21 snRNA-seq data while also confirming our initial observation of low-level heterogeneity and Enah+ myonuclei in adult muscle (Supplementary Fig. 5c).
a UMAP representing snRNA-seq data from a postnatal (P) day 10 tibialis anterior shows the presence of an activated muscle progenitor population (myocytes) but the absence of defined sarcomere myonuclear states. b smRNA-FISH for Flnc and Enah shows an expansion of sarcomere assembly state myonuclei in P21 tibialis anterior compared to P10 and adult muscle. Flnc+ Enah+ nuclei were quantified at each timepoint (n = 3). Data are presented as mean ± standard deviation. A one-way ANOVA with Tukey post-hoc comparison was used to determine statistical significance, **P < 0.01. Scale bar: 20 μm. Source data are provided as a Source Data file.
In addition to functional diversity found in normal development, transcriptional heterogeneity can arise from pathological or compensatory processes associated with aging34,35,36. Skeletal muscle undergoes dramatic age-related changes with respect to functional deterioration and regenerative decline37,38,39,40, and so we next asked if the aging process impacted myonuclear transcription. We profiled 18,087 nuclei from 24- and 30-month-old tibialis anterior muscles (Fig. 4a, b, Supplementary Figs. 13 and 14). In 30-month muscle only, we observed emerging myonuclear populations that were not present in 5 or 24 months, including clusters expressing Nr4a3, Ampd3, and Enah, respectively (Fig. 4b). We integrated myonuclei from 5, 24, and 30 months and found a consistent aging-related gene-expression signature including upregulation of Nr4a3 and Smad3 at both aged timepoints (Fig. 4c, Supplementary Data 3), indicating that this may be a progressive feature of aging muscle. Smad3 has been implicated during the muscle aging process41,42, while Nr4a3 has a role for metabolic adaptations to exercise but has not been studied during aging43,44. smRNA-FISH for Nr4a3 in the gastrocnemius muscle confirmed elevated expression in myonuclei in aged muscle, following a heterogeneous pattern (Fig. 4d). The Ampd3+ population were identified as myonuclei based on the presence of the myogenic regulatory factor Myf6, the pro-atrophy gene Fbxo32, and neuromuscular markers Musk, Chrnb1, and Hdac4 (Supplementary Fig. 15). The cluster was also enriched for genes associated with the immune response, apoptosis, and proteasomal degradation (Tnfrsf23, Traf3, and Psma5) (Supplementary Fig. 15). Taken together, these data suggest that the Ampd3+ population could represent a denervated state that is dysfunctional (Supplementary Fig. 15). Surprisingly, the Enah+ myonuclear cluster was found to be highly similar to the Enah+ sarcomere assembly state from P21 muscle, with numerous marker genes in common including Atf3, Flnc, and Nrap (Fig. 4e). The presence of an Enah+ cluster at 30 months presents further evidence for the appearance of this sarcomere assembly population throughout the lifespan, appearing most prominently in development and aging.
a UMAP of snRNA-seq data from the tibialis anterior of a 24-month-old mouse displaying similar clusters as at 5 months of age. b UMAP of snRNA-seq data from 30-month-old muscle displaying the presence of unique clusters of myonuclei marked by Ampd3, Enah, and Nr4a3. c Heatmap of integrated myonuclei from 5, 24, and 30-month tibialis anterior muscle showing a consistent transcriptional signature of aging muscle. Columns are individual nuclei belonging to each to timepoint. d Representative image of smRNA-FISH for Nr4a3 validated its upregulation in myonuclei in 30-month skeletal muscle (n = 3 for each group). Scale bar: 10 μm. e Feature plots of key marker genes of the Enah+ population within 30-month myonuclei.
In order to more systematically investigate the presence of sarcomere assembly myonuclei over time, as well as to determine patterns of nuclear composition across the lifespan, we integrated our snRNA-seq data from the tibialis anterior at P10, P21, 5 months, 24 months, and 30 months (Fig. 5a). Notably, integration confirmed the independent clustering of Enah+ and Ampd3+ populations (Fig. 5a). Enah+ myonuclei were present in all timepoints but increased in P21 muscle (Fig. 5b), consistent with our smRNA-FISH findings and analysis of individual datasets. Intriguingly, we also identified a population of nuclei (Ckm+ Col1a1+) expressing a number of skeletal muscle-specific genes (e.g., Tnni2, Tnnt3, Ckm) as well as marker genes involved in extracellular matrix remodeling (e.g., Col1a1, Col3a1, Col5a3, Col6a1, and Dcn) (Fig. 5a, Supplementary Data 1). This population was overrepresented in development, comprising 4.7% of nuclei at P10 compared to 0.9% of nuclei at 5 months (Fig. 5b). This cluster could represent an intermediate population that shares characteristics of myonuclei as well as cells of fibroblastic or tendon origin. We confirmed that our combined datasets are likely to represent a faithful trajectory of development and aging, as an independent approach for combined analysis without batch correction found overlap** cells in all clusters, indicative of temporally related cell states (Supplementary Fig. 16).
a Integrated UMAP of snRNA-seq data from postnatal day (P) 10, P21, 5-month, 24-month, and 30-month tibialis anterior muscle. b Proportional composition of nuclear types across the lifespan. Major categories (FAPs, endothelial cells, smooth muscle) include closely-related subpopulations. Myonuclei are combined (top graph) and subsetted (bottom graph) to display myonuclear composition dynamics.
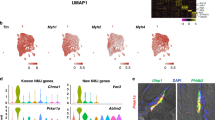
In addition to revealing the emergence of shared transcriptional responses at distinct stages of the life cycle, our data also establish an atlas of myonuclear transcriptomics and reveal transcripts associated with rare myonuclear populations such as the NMJ. Previous studies have elegantly uncovered unique gene expression in NMJ-associated nuclei, which allowed for identification of fundamental molecular components of postsynaptic function45,46,47. However, our data reveal numerous previously unreported genes associated with the NMJ, likely because our approach was able to resolve global transcription at the nuclear level. This represents a unique opportunity to further reveal mechanisms of postsynaptic development and function. To test the validity of genes not previously associated with the NMJ, we confirmed localization of 4 of the genes not previously associated with the NMJ (Ufsp1, Lrfn5, Ano4, and Vav3) by smRNA-FISH and co-labeling of the NMJ marker acetylcholine receptor (AchR) (Fig. 6a). Moreover, we found that multiple top NMJ genes identified here showed significant changes in mRNA levels following hindlimb denervation, an expression pattern associated with postsynaptic regulation (Fig. 6b)48. While the expression of the majority of those genes increased after denervation, similar to the canonical NMJ factor Musk49, Ufsp1 and D430041D05Rik exhibited a decrease in expression, while Pdzrn4 did not change (Fig. 6b). To assess the functional relevance of these factors for NMJ formation and maintenance, we used an in vitro culture system in which C2C12 myoblasts, when plated on laminin, form aneural NMJ-like clusters that are marked by AChR (Fig. 6c)50,51,52. We treated C2C12 cells with a control siRNA or siRNAs targeting Musk, Ufsp1, Vav3, B4galnt3, or Gramd1b, then assayed for AChR clustering. Reduced expression of the target genes was observed after treatment with the appropriate siRNA (Fig. 6d). Musk reduction results in a blockade of AChR clustering (Fig. 6e), showing fidelity of the system50. Obvious AChR clustering abnormalities were not observed for cultures where Vav3 or B4galnt3 was reduced, but Ufsp1 and Gramd1b were identified as regulators of the NMJ (Fig. 6e). Loss of Gramd1b resulted in reduced AChR clusters, whereas reduction of Ufsp1 elicited an increase (Fig. 6f). These data indicate that Ufsp1 is a negative regulator of AChR clustering and NMJ formation, consistent with its downregulation following denervation. Reduction of Ufsp1 or Gramd1b did not impact overall myogenesis or transcript levels of canonical NMJ genes (Fig. 6g), suggesting they specifically regulate the NMJ in a transcriptionally independent manner. These data highlight the utility of nuclear-level resolution for the discovery of factors that regulate the biology of skeletal muscle.
a Representative images from smRNA-FISH for Ufsp1, Lfrn5, Vav3, and Ano4 on skeletal muscle sections showing co-localization with the canonical NMJ protein, acetylcholine receptor (AChR) (n = 3). AChR was visualized through α-bungarotoxin labeling. b Quantitative real-time PCR (qPCR) analysis for the indicated genes not previously associated with the NMJ from normal (n = 4) and denervated (n = 5) muscle. c Schematic for a siRNA screen in C2C12 myoblasts designed to test the function of candidate NMJ genes. siRNA was transfected two days after differentiation and three days later α-bungarotoxin was used to analyze AChR clustering as a surrogate for NMJ formation. d qPCR analysis for the genes targeted with siRNA. A scrambled siRNA was used as a control. e Representative images of C2C12 myotube cultures after treatment with various siRNAs and staining with α-bungarotoxin. Cells were also stained with phalloidin and DAPI. f Quantification of AChR clusters per field of view from e. g qPCR analysis for genes associated with myogenesis and NMJ formation. Scale bars: a 50 μm (top left panel), 10 μm (top right and bottom panels), e 50 μm. Data in d, f, and g are from three independent experiments. All data are represented as mean ± standard deviation. An unpaired two-sided t-test was used to determine statistical significance, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are provided as a Source Data file.
Discussion
Altogether, our single-nucleus profiling of skeletal muscle reveals intricate transcriptional dynamics of myonuclear states across the lifespan of the mouse. To this end, an interactive data portal is publicly available at https://research.cchmc.org/myoatlas. An unexpected finding was the presence of a unique sarcomere assembly transcriptional signature in a subset of myonuclei, most prominent in develo** muscle after postnatal fusion and myonuclear accrual was terminated. We speculate that expansion of the sarcomere assembly state is determined by stage-specific growth signals following the tapering of developmental fusion, or is reflective of the magnitude of growth occuring during this phase. Further experiments will be required to identify the key regulatory factors that drive the sarcomere assembly myonuclear state and to elucidate its contribution to postnatal development and muscle maintenance.
Our analyses also provide a comprehensive resource for further study of rare but essential myonuclear populations. We identify numerous genes for the specialized NMJ and MTJ compartments and provide evidence that previously uncharacterized NMJ factors are functional regulators of postsynaptic function. Little is known regarding the regulatory mechanisms that drive NMJ-related transcription in postsynaptic myonuclei, while restricting this program in other nuclei within the syncytium. Interrogation of factors with demonstrated functional roles in NMJ formation, particularly those that may act in a negative regulatory capacity, will be critical to further understanding in this area. In addition, independent approaches now indicate the existence of a Col22a1+ population of myonuclei located within myofibers at the mammalian MTJ and provide robust transcriptomes for these nuclei53. These results should provide a powerful resource for addressing unresolved questions with respect to the cellular composition and transcriptional regulation of the MTJ, such as mechanisms of crosstalk between myofibers and tendon cells12 and the implication of MTJ-related factors in inherited myopathies13,14,15.
Additionally, integration of tibialis anterior snRNA-seq data from all timepoints revealed a population of nuclei with muscle-specific markers but also a signature enriched for extracellular matrix-related genes such as Col1a1, Col3a1, Col5a3, Col6a1, and Dcn. This population bears some similarity to the MTJ-B population reported in the accompanying manuscript53, a second MTJ population in addition to the Col22a1+ myonuclei identified in both studies. It is possible that the inclusion of transcriptionally similar non-muscle mononuclear cells in our data, for example FAPs and tenocytes, precludes the emergence of the second MTJ population in our individual datasets, which instead appears only upon integration of all timepoints. Further experimentation would be necessary to confirm the correlation of these populations.
Our snRNA-seq successfully captured known nuclear diversity while also uncovering transcriptional states across all ages interrogated. While we analyzed all nuclei from muscle, approaches to enrich specifically for myonuclei53 or to improve transcript detection levels may reveal further insights into myonuclear heterogeneity. One caveat of our work is the need for functional validation of previously uninidentified myonuclear populations, which will be the focus of future investigations. Nonetheless, snRNA-seq can be leveraged towards greater understanding of myonuclear dynamics in pathological states such as acute or chronic injury or in human myopathies. Altogether, our snRNA-seq data have revealed rare myonuclear populations and provided a powerful resource for understanding of myonuclear transcriptional heterogeneity across the lifespan. Further studies will help to clarify the mechanisms that lead to assignment of certain nuclei to specialized compartments within a shared myofiber cytoplasm.
Methods
Mice and associated experimental procedures
All of the mice used in this study were C57BL/6 wild-type males. Mice were housed at 22.2 °C with humidity of 30–70% and a 14 h light/10 h dark cycle. Mice were fed ad libitum and all snRNA-seq experiments were performed in the morning. For isolation of nuclei for snRNA-seq, tibialis anterior muscles were taken from a single mouse (5-month, 24-month, 30-month) or pooled from 2 mice (P21), or 4 mice (P10) to collect sufficient nuclei for sequencing. Soleus muscles were pooled from 4 mice. Unilateral hindlimb denervation was performed on C57BL/6 wild-type male mice by cutting the left sciatic nerve in the mid-thigh region after the mouse was anesthetized with isoflurane. The gastrocnemius muscle was harvested 3 days after denervation and the contralateral muscles were used as control. For smRNA-FISH experiments, muscles were dissected, embedded in 10% tragacanth/PBS (Sigma) for cross-sections or OCT compound (Fisher) for longitudinal sections, and frozen in 2-methylbutane cooled in liquid nitrogen. 10-μm sections were prepared for all experiments. For RNA isolation, muscles were flash-frozen in liquid nitrogen and stored at −80 °C until use. All mouse procedures were approved by Cincinnati Children’s Hospital Medical Center’s Institutional Animal Care and Use Committee (IACUC2017-0053).
Purification of nuclei from mouse skeletal muscle
Tibialis anterior or soleus muscles were isolated from mice immediately following euthanasia, minced, and placed in homogenization buffer (0.25 M sucrose and 1% BSA in Mg2+-free, Ca2+-free, RNase-free PBS). The minced tissue was further homogenized using an Ultra-Turrax T25. The homogenate was then incubated for 5 min with addition of Triton-X100 (2.5% in RNase-free PBS, added at a 1:6 ratio). Samples were then filtered through a 100-μm strainer, centrifuged (3000 × g for 10 min at 4 °C) to form a crude pellet, and resuspended in sorting buffer (2% BSA/RNase-free PBS) and filtered again through a 40 μm strainer. Hoechst dye was added to label nuclei as well as 0.2 U/μl Protector RNase inhibitor (Roche). Labeled nuclei were purified via FACS (BD Aria, 70 μm nozzle) and collected in sorting buffer containing RNase inhibitor. The resulting nuclear suspension was pelleted with a light centrifugation step (250 × g for 5 min at 4 °C) and the supernatant drawn off to concentrate the nuclei. Aliquots were taken for visualization under a fluorescent microscope to assess nuclear appearance and integrity.
Construction of libraries and generation of cDNA on the 10X Genomics platform
Nuclei were counted using a hemocytometer and the concentration adjusted if needed to meet the optimal range for loading on the 10X Chromium chip. The nuclei were then loaded into the 10X Chromium system using the Single Cell 3′ Reagent Kit v3 according to the manufacturer’s protocol. We aimed to load ~13,000 nuclei for each run. Following library construction, libraries were sequenced on the Illumina NovaSeq 6000 system.
Single-nucleus data analysis
Raw sequencing data of all samples were processed using the cellRanger workflow (version 3.1.0), using a combined intron-exon reference produced as described using the vendor-provided “Generating a Cell Ranger compatible “pre-mRNA” Reference Package” guidelines (https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/advanced/references). In brief, the “pre-mRNA” reference was derived using the default exon-level GTF file provided by 10x Genomics (http://cf.10xgenomics.com/supp/cell-exp/refdata-cellranger-mm10-3.0.0.tar.gz). Using the below awk command, this exon-level GTF file into “pre-MRNA” GTF containing intron transcript definitions ($ awk ‘BEGIN{FS = “\t”; OFS = “\t”} $3 = = “transcript”{$3 = “exon”; print}’ genes.gtf > genes.premrna.gtf). Next, the below mkref command was run to produce the final “pre-MRNA” GTF and genome fasta file ($ cellranger mkref–genome=Mmpre–fasta=genome.fa–genes=genes.premrna.gtf). For each dataset, we corrected for ambient background RNA by filtering with the R package SoupX54. We used the inferNonExpressedGenes() function to determine which genes had the highest probability of being ambient mRNA, and the strainCells() function in order to transform count matrices. Genes expressed per nucleus was between 1446 and 2445, depending on the dataset. Quality control metrics for each dataset, including genes expressed per nucleus, are summarized in Supplementary Data 4.
Further data analysis was carried out using the R (version 3.6.1) package Seurat (version 3.1.0)55. For individual datasets, outlier nuclei were excluded based on unique feature counts. Nuclei with less than 200 expressed features were excluded, as well as nuclei with greater than 3200 expressed features (5-month dataset) or 4000 expressed features (all other datasets). No features that were expressed in 3 or fewer cells were included. Data from filtered nuclei were then normalized logarithmically using the NormalizeData() function. Using the FindVariableFeatures() function, 2000 features with high variable expression across the nuclei were identified and used in a subsequent Principal Component Analysis (PCA) using the RunPCA() function. For clustering and Uniform Manifold Approximation and Projection (UMAP) visualization, the number of components used was unique to each dataset, based on the strength of PCs visualized by elbow plot and PC heatmaps after PCA was performed. The UMAPs and clusters were generated using the FindNeighbors(), FindClusters(), and RunUMAP() functions (R implementation). Of note, the default resolution in this pipeline was 0.5. Violin and Feature plots were generated using the VlnPlot() and FeaturePlot() from the Seurat package, respectively. Heatmaps were generated using the DoHeatmap() function, displaying the top marker genes by highest average log fold change for each cluster. For subclustering, the function subset() was used to select populations of interest from the data and create a new Seurat object with just those identities, followed by standard Seurat preprocessing. In order to analyze the 5-month dataset with higher dimensionality, the function SCTransform() was used in place of standard preprocessing methods19.
The FindAllMarkers() function was used on each dataset to generate markers for each cluster. Cell types and nuclear identities were assigned based on a combination of previously reported marker genes along with gene ontology analysis of uniquely expressed genes. Gene enrichment or differential expression was considered significant with an adjusted p value < 0.05. Regarding gene ontology, the top 100 marker genes of each cluster were analyzed using ToppGene56.
In order to integrate multiple snRNA-seq datasets, the preprocessed datasets to be integrated were run through the FindIntegrationAnchors() function. Resulting integration anchors were used in the IntegrateData() function, creating a new integrated Seurat object. Following this, the standard workflow for visualization and clustering was used. To generate UMAPs of cell identities split or grouped based on their original dataset, the parameters split.by and group.by were set to “orig.ident”. Heatmaps were generated from subpopulations using the DoHeatmap() function. For the heatmap comparing gene expression across aging (5 months/24 months/30 months), a custom heatmap was created and the full list of differentially expressed genes was reported in Supplemental Data 3. In order to determine differential expression between populations of interest across datasets, the function FindMarkers() was used. In order to identify genes that were consistently changed in aging myonuclei, pairwise comparisons of each aging dataset with the 5-month dataset were performed, and a common aging gene signature was identified. To assess integration of the data, without batch correction, we performed an additional unsupervised analysis using the software ICGS2 (http://altanalyze.org). ICGS2 was run using the default parameters, with cell cycle removal on all combined timepoint snRNA-Seq samples.
To ensure the absence of artifactual clusters from nuclei doublets, we computationally predicted heterotypic doublets using the software DoubletDecon57. We examined marker genes (MarkerFinder algorithm) within each Seurat3 produced set of clusters and subclusters and combined main clusters. By definition, no large clusters can be doublets (>20% of the size of the largest nuclei population). No small-to-medium sized primary and subclusters were enriched in putative DoubletDecon predictions (>70%) or lacked unique marker genes, suggesting such populations were not doublets.
To identify transcription factor (TF) binding sites that are enriched within the promoter regions of Nos1+ and Enah+ genes, we used the HOMER motif enrichment algorithm58 and a large library of human position weight matrix (PWM) binding site models obtained from the CisBP database, build 2.059. We used a definition of 1000 bp upstream and downstream of the transcription start site (TSS) to generate consensus predictions.
Single-molecule RNA fluorescent in situ hybridization
Single-molecule FISH experiments were performed using RNAscope (ACDBio) following the manufacturer’s protocols. We used fresh-frozen cross-sections of quadriceps or gastrocnemius muscles where indicated, as well as fresh-frozen longitudinal sections of tibialis anterior. Following the completion of the RNAscope fluorescent assay, an immunostaining step was carried out to label myofibers. Sections were blocked for 30 min using 1% BSA, 1% heat-inactivated goat serum, and 0.025% Tween-20/PBS, followed by incubation for 30 min at RT with anti-laminin (1:100; Sigma L9393). Secondary AlexaFluor antibodies (1:200) (Invitrogen) were then applied at room temperature for 1 h. For visualization of neuromuscular junctions, an α-Bungarotoxin AlexaFluor conjugate (1:100; Thermo Fisher) was added with the secondary antibody. For staining of muscle fibers, we used anti-myosin primary antibodies (MF 20, 1:20, Developmental Studies Hybridoma Bank) and incubated for 1 h at RT. Sections were mounted using VectaShield with DAPI (Vector Laboratories). Slides were imaged using a Nikon A1R confocal system.
Cell culture
C2C12 cells were acquired from American Type Culture Collection. Cells were grown in DMEM (Gibco) containing 10% heat-inactivated bovine growth serum (BGS) and supplemented with penicillin-streptomycin (1%), and incubated at 37 °C with 5% CO2. C2C12 cells were differentiated by switching medium to DMEM with 2% heat-inactivated horse serum with antibiotics. In order to induce formation of aneural AChR clusters, 35 mm plates were precoated with 10 μg/ml natural mouse laminin protein (ThermoFisher 23017015) suspended in L-15 medium (Thermo Fisher) with 0.2% NaHCO350. Cells were transfected with siRNAs on day 2 of differentiation using Lipofectamine 2000 (Thermo Fisher) according to the manufacturer’s protocol. All siRNAs were purchased from Santa Cruz.
On day 5 of differentiation, cells were rinsed with PBS and processed for RNA isolation or immunocytochemistry. For the latter, cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Cells were then permeabilized with 0.2% Triton X-100/PBS for 20 min at room temperature, followed by blocking in 3% BSA/PBS for 30 min. We then incubated with phalloidin (1:100, Thermo Fisher) and α-Bungarotoxin (1:200, Thermo Fisher) AlexaFluor conjugates for 1 h at room temperature. Cells were then rinsed with PBS and nuclei were stained with Hoechst 33342 solution (Thermo Fisher). Imaging was performed using an upright Nikon FN 1 microscope on a Nikon A1R confocal.
Quantitative real-time PCR
RNA was isolated from homogenized muscle tissue and plated cells using Trizol reagent (Invitrogen) according to standard protocols. cDNA synthesis was carried out using the MultiScribe™ kit (Applied Biosystems) and qPCR reactions were performed on a Bio-Rad CFX96™ Real-Time System using PowerUp™ SYBR Green Master Mix (Applied Biosystems). Primers used for qPCR are listed in Supplementary Table 1.
Statistics
GraphPad Prism 8 software was used for statistical analysis, excluding single-nucleus RNA-sequencing data. Unpaired two-sided t-tests or one-way ANOVAs with Tukey post-hoc comparison were used to determine statistical significance, which was set at p values < 0.05. For qPCR analysis, ΔCt values were used to assess significance, and ΔΔCt values were graphed to show relative expression compared to control.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.