Abstract
Objectives
To compare the safety and efficacy of combined laser iridoplasty followed by surgical iridectomy (LI-SI) versus trabeculectomy in the management of medically unresponsive acute primary angle closure (APAC) with minimal cataract.
Patients and methods
This was a randomized controlled trial conducted among patients with medically unresponsive APAC without significant cataract. Study participants were randomized into: LI-SI or unaugmented trabeculectomy. Primary outcome of the study was the rate of post-operative surgical complications in the first 3 months after surgery. Secondary outcome assessed at 1 year was whether treatment was completely successful (IOP < 21 mmHg without IOP lowering drops), or partially successful (IOP < 21 mmHg with IOP lowering drops). Failure was defined as IOP ≥ 21 mmHg with IOP lowering drops.
Results
The study included 67 eyes of 67 patients (59 females/8 males = 7.4/1) who were randomized into 2 groups: LI-SI (Group 1, 37 eyes), and trabeculectomy (Group 2, 30 eyes). There was no statistical difference between the two groups at baseline. Overall, there were more post-operative complications in Group 1 versus Group 2 (45.9% versus 33.3% - p = 0.23), although all responded well to medical treatment and resolved without sequelae. Complete success was found in 97.1% (34/35 eyes) in Group 1 and 92.6% in group 2 (p = 0.19, Fisher’s exact test).
Conclusions
There was a higher rate of post-operative complications after LI-SI compared to trabeculectomy performed for medically unresponsive APAC with minimal cataract. Both procedures had similar surgical outcomes at 1 year.
Similar content being viewed by others
Introduction
Acute primary angle closure (APAC) is a relatively common ophthalmic emergency in Vietnam and can potentially be blinding due to the risk of severe damage to the optic nerve from prolonged high intraocular pressure (IOP).
In many parts of the world, trabeculectomy is performed for medically unresponsive APAC with severe cornea oedema and high IOP. However, trabeculectomy can potentially result in serious post-operative complications such as flat anterior chamber (AC), malignant glaucoma, bleb leak, choroidal detachment, hypotony or infection and has limited success rate after APAC [1]. As the main mechanism underlying APAC is acute pupillary block, it is possible that surgical iridectomy alone may be sufficient for medically unresponsive APAC as it minimizes the risks associated with trabeculectomy [2]. Surgical iridectomy could be combined with pre-operative laser peripheral iridoplasty to widen the angle as well as to treat any plateau iris component. This option may be particularly feasible in APAC eyes without significant cataract.
We thus conducted this randomized controlled trial to compare the safety and efficacy of laser peripheral iridoplasty followed by surgical iridectomy (LI-SI) compared with trabeculectomy for medically unresponsive APAC without significant cataract.
Patients and methods
From June 2017 to June 2019, patients with medically unresponsive APAC without significant cataract were recruited from three centers in Hanoi, Vietnam: Ha Dong Eye Hospital, the Ophthalmology Department of Military Hospital 103, and the Vietnam National Eye Hospital. All participants were followed-up for one year post-surgery. Written informed consent was obtained from all participants. Study protocol was approved by the hospitals’ respective ethics committees and by the Hanoi Department of Science and Technology Ethics Committee. The study was registered with the local Vietnam trial registry (822/QĐ-UBND-2017) and was performed according to the tenets of the Declaration of Helsinki.
Diagnostic criteria used for APAC were based on those suggested by Tan et al. [3] and was defined as uncontrolled IOP ≥ 22 mm Hg with maximal medical treatment for at least 3 days, with an angle opening less than Shaffer grade II for at least 180° on indentation gonioscopy. Angle opening was graded based on gonioscopy, according to the Shaffer grading system. Gonioscopy was performed when IOP was maximally reduced and the cornea was relatively clear following the application of a hyperosmotic solution. A non-significant cataract was defined as less than grade II using the lens opacities classification system (LOCS) III. Patients with inflammatory disease of the anterior segment, such as corneal ulcer, uveitis, or secondary closed angle glaucoma, and patients with a history of trauma, intraocular surgery, or vitreoretinal diseases were excluded from this study.
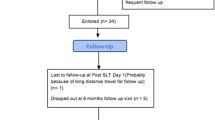
Randomization was performed by the Eye Department, 103 Military Hospital. Patients were randomized (1:1) using a random number generator, and the treatment assigned was conveyed to the treating surgeon via a phone call prior to surgery. Eligible eyes were randomized into two groups: LI-SI (Group 1) and Trabeculectomy (Group 2) (Fig. 1). If patient was randomized to Group 1, the surgeon would perform laser iridoplasty first, then surgical iridectomy the day after. All 3 centers had laser machines located close by to the operating theatre for logistic purposes. Subsequent follow-up examinations were performed by other investigators, technicians and optometrists, and were masked to the trial randomization.
Surgical procedure
Laser peripheral iridoplasty
Pilocarpine 2% eyedrop was applied twice (5 min apart) before the procedure to constrict the pupil. One drop of Brimonidine Tartrate 0.15% was used 30 min before the procedure to minimize IOP spike after laser. Argon (532 nm) laser iridoplasty was performed using a 1 mirror or 3 mirror Goldmann lens. Laser spots were placed as close as possible to the iris root without touching the trabeculae with the following settings: spot size 200–500 µm, duration 200–500 millisecond, power 200–400 mW. The power was adjusted according to the tissue reaction: with power increased until iris contraction was seen and decreased when pigment or air bubble were released. Approximately 6–10 applications were performed in each quadrant and all 4 quadrants were treated in one session. Laser peripheral iridoplasty was performed the day prior to surgical iridectomy.
Surgical iridectomy
Topical Alcaine 0.5% anaesthetic drops was applied 15 min before the surgery. Subconjunctival injection of lidocaine 2% was given near the limbus prior to making a 1.5 mm corneal incision. The posterior lip of the incision was gently pressed to extrude the iris. Toothed micro forceps were used to grasp the iris, and the iridectomy was performed with Vannas scissors. The iris epithelium pigment was checked to ensure that a full thickness iridectomy was performed. A flat spatula was then used to reposition the iris back into the anterior chamber (AC), and AC was reformed with Balanced Salt Solution. The incision was subsequently sealed by hydration or with one 10–0 nylon suture. Topical antibiotics (either tobramycin or moxifloxacin) and steroid eye drops (usually dexamethasone) were used routinely for 6 weeks after surgery. For the first 3 weeks after surgery, the drops were administered 6 times per day and subsequently tapered over the next 3 weeks.
Trabeculectomy
The surgical procedure and postoperative management were similar across all three centers. In all cases, modified Cairns-type trabeculectomy was performed with a fornix-based conjunctival flap. A ½ to ¾ thickness scleral flap measuring 3 mm by 4 mm was dissected to clear cornea. After resection of an anterior trabecular block, a basal surgical iridectomy was performed. The sclera flap and conjunctiva were sutured with 10–0 nylon. At the conclusion of the surgery, a subconjunctival injection of dexamethasone and gentamicin was administered. Due to the unavailability of mitomycin-C, there was no intraoperative use of antimetabolites in any patient, but post-operative 5-Fluororacil injections could be administered based on the judgment of surgeons. Topical antibiotics (either tobramycin or moxifloxacin) and steroid eye drops (usually dexamethasone) were used routinely for 6–8 weeks after surgery. For the first 3 weeks after surgery, the drops were administered 6 times per day and then tapered over the next 3–5 weeks.
All surgeries were performed by senior ophthalmic surgeons (T.D., N.N.D., H.N.T.). For Group 1, if IOP was found to be >21 mm Hg on two consecutive post-operative check-ups (at least 1 week apart), IOP-lowering drop(s) were added. If IOP is still uncontrolled, patient would be counseled for trabeculectomy. In Group 2, if IOP was >21 mm Hg within the first month, bleb manipulation, including bleb massage, suture lysis, or needling with 5-FU injection, were attempted by managing surgeons, before which IOP-lowering drops were added.
Study outcome measure
All eyes were examined at 1 day, 1 week, 1 month, 3 months, 6 months and 12 months following surgery. The primary outcome of the study was the rate of post-operative surgical complications within the first 3 months following surgery. The secondary outcome measure was the efficacy of IOP reduction. This was assessed at 1-year post-surgery and was defined as follows: Complete success was defined as IOP < 21 mmHg without IOP-lowering drops, and partial success was defined as IOP < 21 mmHg with IOP-lowering drops. Failure was defined as IOP ≥ 21 mmHg with IOP-lowering drops. A similar analysis using 18 mmHg as the cut-off was also performed. Other secondary outcome criteria were the mean IOP and best corrected visual acuity (BCVA) after 1 year. The mean change in angle width was also compared (evaluated prior to surgery, at 1 month and 12 months post-surgery).
All eyes were also evaluated using ultrasound biomicroscopy (UBM) (prior to surgery and 6 months post-surgery), and the following anterior segment parameters were measured: AC depth (ACD), the trabecular–iris angle area (TIA), the angle recess area (ARA), the angle opening distance at 500 µm from the scleral spur (AOD500), the iris curvature (IC), the trabecular–ciliary process distance (TCPD), and the iris–ciliary process distance (ICPD).
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (SPSS Version 10.0 for Windows). The Student’s t-test was used to compare means and the Fischer’s exact test was used to compare categorical data.
Sample size and power calculation
sample size was estimated based on previously published studies [3, 4]. We used the following formula for the sample size estimation, based on the estimated rate of post-operative complications in the first 3 months after surgery.
α: type 1 error, set to 0.05.
β: type 2 error, set to 0.2.
p1: rate of post-operative complications in Group 1, estimated to be 15%
p2: rate of post-operative complications in Group 2, estimated to be 31.3% [1]
q1 = 1 – p1
q2 = 1 – p2
Based on the above estimates, the sample size required was 30 eyes per group.
Results
Out of 70 patients who were screened, 67 patients (59 females/8 males) were recruited and randomized into 2 groups: Group 1: LI-SI (37 eyes), Group 2: Trabeculectomy (30 eyes). Of these, 35/37 patients in Group 1 and 27 eyes/30 eyes in Group 2 completed the 12 months follow-up.
The mean age of study subjects was 57.67 ± 7.67 years old (range, 32 to 75 years old). The mean age in Group 1 was 57.95 ± 7.56 years, while the mean age in Group 2 was 57.33 ± 7.93 years (p = 0.43, Fisher’s Exact test). Prior to surgeries, overall best corrected visual acuity (BCVA) was severely affected with 34.3% between 20/80 – Count fingers (CF) at 3 meters and 29.9% with VA less than CF at 3 meters. Mean LogMAR BCVA was 0.96 ± 0.61 LogMAR in group 1 and 1.16 ± 0.72 LogMAR (p = 0.22, test T student) in group 2. The mean IOP of the 2 groups was 31.81 ± 7.43 mmHg and 31.60 ± 8.22 mmHg, respectively (p = 0.91, test T student), with an overall mean IOP of 31.72 ± 7.73 mmHg (15–47 mmHg) (Table 1)
Complications
We observed more post-operative complications in Group 1 than in Group 2, especially postoperative hyphaema. There were 2/37 cases of mild hyphaema within the first week in Group 1 (5.4% versus 0% in Group 2), which fortunately, responded well to medical treatment and resolved without sequelae. Corneal oedema was observed in Group 1 (7/37 = 18.9%) as well as in Group 2 (6/30 = 20%) within the first month after surgery. In all cases, corneal oedema did not last longer than 1 month. The endothelial cell count was not different between 2 groups after 1 year of follow-up (group 1: 2202 ± 543 versus group 2: 2300 ± 560, p = 0.85). In general, the rate of complications in Group 1 was higher than Group 2 (45.9% vs 33.3%). However, the difference was not significant (p = 0.23). (Table 2)
Over the year, there was improvement of BCVA in almost all studied eyes. Comparing the 2 groups, the difference in VA was not significant at any time point. At 12 months after surgery, mean VA in group 1 was 0.51 ± 0.45, and in group 2 was 0.49 ± 0.34 (p = 0.86, test ANOVA) (Fig. 2a).
IOP was controlled in both groups on the immediate post operative day (p < 0.0001, paired T test), and remained stable thereafter throughout the 12 months of follow-up. Mean IOP in both groups was similar in all time points except at 1 month; mean IOP in group 1 was 16.82 mmHg versus 14.36 mmHg in group 2 (p = 0.012, ANOVA test). There was no difference in mean IOP at 12 months in both groups: Group 1–16.60 mmHg and Groups 2–16.07 mmHg (p = 0.51, ANOVA) (Fig. 2b). The rate of complete success was also equivalent in 2 groups. At 12 months, complete success was 97.1% (34/35 eyes) in Group 1 and 92.6% (25/27 eyes) in Group 2 (p = 0.19 Fisher’s exact test). In Group 1, one eye failed IOP reduction despite maximal medical therapy and required trabeculectomy In group 2, two eyes needed one medication to control IOP, and was defined a partial success. In Group 2, there were 2 cases that needed additional procedures (suture removal, bleb massage and 5-FU injections) to control IOP at month 1.
Preoperatively, there were no significant differences in any UBM parameters between both groups (p > 0.05). After 12 months, there were 3 parameters that changed significantly including TIA, ARA and AOD500. As expected, those 3 parameters were greatly improved in Group 1 (p = 0.003, p = 0.002, p = 0.001, respectively, pared samples t test) showing the angle was significantly widened in Group 1 as compared to Group 2. Between the two groups, the mean ACD deepened more in Group 1 than in Group 2 (p = 0.02) (Table 3).
Discussion
Refractory APAC is considered as a serious therapeutic challenge in ophthalmology. In such a situation, Aung et al. found that the success rate (in terms of IOP control) with trabeculectomy was low, at only 56.2% over 2 years [1]. However, if the IOP was under control before the surgery while the angle remained closed (Shaffer grade II or less over 180[0]), the success rate of trabeculectomy could improve up to 87.5% [1]. In this study, the results of trabeculectomy in Group 2 was surprisingly good in comparison with previous reports. This may be due to 2 possible reasons: (1) we attempted maximal medical treatment (IOP lowering and steroids) over a longer period of time before surgical intervention, that prevented us from operating on a “hot eye” condition even when IOP was sometimes not entirely normalized; (2) the scleral flap was closed tightly with releasable suture(s) that helped to prevent early complications such as choroidal detachment or malignant glaucoma [5].
Overall, IOP control was excellent in both groups with absolute success rate 97.3% in Group 1 (only 1 case of failure) and 93.3% in Group 2 (the 2 remaining cases needed only one additional IOP lowering medication). The comparable IOP results in both groups suggest the redundancy in creating a filtration bleb in the management of APAC. Surgical iridectomy as an initial procedure for acute PAC was first proposed by Von Graefe in 1856 and later further certified by several studies [4, 6, 7]. The success rate varied from 70% to 76% after at least 1 year of follow-up. More recently, this procedure was largely replaced by laser iridotomy which was believed to be much less invasive. However, the size of the iridotomy opening is usually much smaller that the surgical iridectomy opening. A larger iridectomy would guarantee the flow of aqueous from the posterior chamber even while the pupil is dilated. The anterior chamber is usually left unmanipulated during laser iridotomy. In contrast, the AC is reformed after surgical iridectomy, which helps to reopen the angle and break any newly formed peripheral anterior synechiae (PAS).
After either laser iridotomy or surgical iridectomy, only one mechanism of angle closure is resolved – pupillary block. Other component(s)/mechanism(s) such as plateau iris is untouched. Recent imaging studies have proved that the latter mechanism could contribute to about 44.6% of all PACG cases in Vietnamese patients [8]. This observation may explain why an APAC attack could recur in eyes with a patent iridotomy [9]. In our study, we added Peripheral Iridoplasty as an additional treatment to surgical iridectomy for 2 reasons: (1) Iridoplasty helps to further break the acute attack [10, 11] and also relieve the second mechanism of angle closure – plateau iris; (2) Iridoplasty may help to further open the angle by flattening the iris root and breaking newly formed PAS. We believe that the excellent 1-year IOP results of Group 1 in this study may be due to the additional effect of iridoplasty to surgical iridectomy. In the LI-SI group, we also found that the ACD was significantly deeper and the TIA tended to be wider compared to the trabculectomy group. On the other hand, performing a surgical iridectomy preserved the limbal conjunctiva for future trabeculectomy if needed. One of big disadvantages of performing trabeculectomy in APAC was that this results in bypassing of the natural pathway of aqueous humor outflow – the trabecular meshwork. We believe that this explains why, the AC became shallower, and the angle narrower in comparison to before surgery [12].
Our study had some limitations. Masking of the surgical treatment performed was not possible. While we tried to recruit patients without significant cataract, it was difficult to accurately assess the degree of cataract at presentation due to cornea oedema in many cases. The study participants were Vietnamese, and the findings may not be applicable to other populations. There could have been a learning curve with surgical iridectomy, which may explain the higher than expected rates of hyphaema. While we did power the study to detect differences in complication rates between the two surgical procedures, it is possible that we may not have detected a difference in terms of IOP between both groups due to inadequate sample size. While the occurrences of complications were accounted for, it was difficult to account for the severity of each complication measure. In a paper published during the recruitment phase of this study [13], glaucoma randomised trials were seen to report frequency of complications, but commonly left out severity of the complications. Hence, it is possible that the complication rates between both groups be significant after adjusting for severity. Finally, we did not use adjunctive mitomycin-C for the trabeculectomy group as this is not available in Vietnam.
In conclusion, medically unresponsive APAC without significant cataract can be treated effectively with either LI-SI or trabeculectomy without severe complications. However, there seemed to be a higher rate of complications associated with LI-SI.
Summary
What was known before
-
Medically unresponsive APAC is usually a nightmare to treat, and treatment with trabeculectomy is undertaken in many parts of the world. This however leads to potentially serious post-operative complications. Pupil block from APAC can largely be resolved with laser peripheral Iridotomy, but in some cases, plateau iris may cause the Iridotomy to fail. If successful, this can potentially mitigate the serious complications associated with Trabeculectomy.
What this study adds
-
We explored options to the treatment of refractory APAC by comparing laser peripheral Iridotomy with surgical iridectomy against trabeculectomy surgery, with outcomes and complications compared. The non superiority of trabeculectomy shown shows that these potentially sight threatening complications can be mitigated with less invasive measures. With this paper, refractory APAC can be treated effectively without incurring the complications associated with Trabeculectomy.
References
Aung T, Tow SL, Yap EY, Chan SP, Seah SK. Trabeculectomy for acute primary angle closure. Ophthalmology. 2000;107:1298–302. https://doi.org/10.1016/s0161-6420(00)00137-8.
Angle-closure glaucoma update: Treatments. American Academy of Opthamology. https://www.aao.org/focalpointssnippetdetail.aspx?id=c882a35a-180a-4cfd-807f-9388a537395d.
Tan AM, Loon SC, Chew PT. Outcomes following acute primary angle closure in an Asian population. Clin Exp Ophthalmol. 2009;37:467–72. https://doi.org/10.1111/j.14429071.2009.02060.x.
Playfair TJ, Watson PG. Management of acute primary angle closure glaucoma: a long-term follow-up of the results of peripheral iridectomy used as an initial procedure. Br J Ophthalmol. 1979;63:17–22. https://doi.org/10.1136/bjo.63.1.17.
Spaeth GL. Trabeculectomy for acute primary angle closure. Ophthalmology. 2001;108:1008. https://doi.org/10.1016/s0161-6420(00)00610-2.
Krupin T, Mitchell KB, Johnson MF, Bernard B. The long-term effects of iridectomy for primary acute angle closure glaucoma. Am J Ophthalmol. 1978;86:506–9.
Fleck BW, Wright E, Fairley EA. A randomised prospective comparison of operative peripheral iridectomy and Nd: YAG laser iridotomy treatment of acute angle closure glaucoma: 3 year visual acuity and intraocular pressure control outcome. Br J Ophthalmol. 1997;81:884–8. https://doi.org/10.1136/bjo.81.10.884.
Do T, Nguyen Xuan H, Dao Lam H, Tran DT, Giang Nguyễn TT, Hiện Nguyên DN. et al. Ultrasound biomicroscopic diagnosis of angle-closure mechanisms in vietnamese subjects with unilateral angle-closure glaucoma. J Glaucoma. 2018;27:115–20. https://doi.org/10.1097/IJG.0000000000000856.
Laser peripheral iridotomy for pupillary-block glaucoma. American Academy of Ophthalmology. Ophthalmology. 1994;101:1749–58. https://doi.org/10.1016/s0161-6420(13)31434-1.
Lam DS, Lai JS, Tham CC, Chua JK, Poon SYA. Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: a prospective, randomized, controlled trial. Ophthalmology. 2002;109:1591–6. https://doi.org/10.1016/s0161-6420(02)01158-2.
Chan PP, Pang JC, Tham CC. Acute primary angle closure-treatment strategies, evidences and economical considerations. Eye (Lond). 2019;33:110–9. https://doi.org/10.1038/s41433-018-0278-x.
Man X, Chan NCY, Baig N, Kwong YYY, Leung YLD, Li CHF, et al. Anatomical effects of clear lens extraction by phacoemulsification versus trabeculectomy on anterior chamber drainage angle in primary angle-closure glaucoma (PACG) patients. Graefes Arch Clin Exp Ophthalmol. 2015;253:773–8. https://doi.org/10.1007/s00417-015-2936-z.
Sii S, Barton K, Pasquale LR, Yamamoto T, King AJ, Azuara-Blanco A, et al. Reporting harm in glaucoma surgical trials: Systemic review and a consensus-derived new classification system. Am J Ophthalmol. 2018;194:153–62. https://doi.org/10.1016/j.ajo.2018.07.014.
Funding
This study was funded by the Budget for Science and Research of The People committee of Hanoi. The data that support the findings of this study are available on request from the corresponding author, [DT]. The data are not publicly available due to individual data privacy.
Author information
Authors and Affiliations
Contributions
TD Concept and design Data acquisition, analysis and interpretation Drafting and revision of manuscript Statistical analysis Funding approval NDN Concept and design Data acquisition, analysis and interpretation Drafting and revision of manuscript Funding approval HXN Concept and design Data acquisition, analysis and interpretation Drafting and revision of manuscript Funding approval HTN Data acquisition, analysis and interpretation Funding approval KR-PF Drafting and revision of manuscript HTV Data acquisition, analysis and interpretation Drafting and revision of manuscript Statistical analysis CVN Data acquisition, analysis and interpretation Drafting and revision of manuscript Statistical analysis HTTP Drafting and revision of manuscript TA Drafting and revision of manuscript HDTNN Drafting and revision of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, H.X., Nguyen, N.D., Nguyen, H.T. et al. Comparing combined laser iridoplasty and surgical iridectomy with trabeculectomy in treatment of refractory acute primary angle closure without significant cataract: a randomized controlled trial. Eye 37, 2139–2144 (2023). https://doi.org/10.1038/s41433-022-02311-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02311-z
- Springer Nature Limited