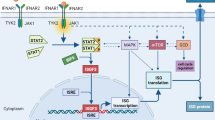
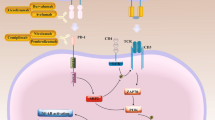
Abstract
The existing conventional treatments for breast cancer, including immune checkpoint blockade, exhibit limited effects in some cancers, particularly triple-negative breast cancer. Epigenetic alterations, specifically DNMT and HDAC alterations, are implicated in breast cancer pathogenesis. We demonstrated that DNMTs and HDACs are overexpressed and positively correlated in breast cancer. The combination of DNMT and HDAC inhibitors has shown synergistic antitumour effects, and our previously designed dual DNMT and HDAC inhibitor (termed DNMT/HDACi) 15a potently inhibits breast cancer cell proliferation, migration, and invasion and induces apoptosis in vitro and in vivo. Mechanistically, 15a induces a viral mimicry response by promoting the expression of endogenous retroviral elements in breast cancer cells, thus increasing the intracellular level of double-stranded RNA to activate the RIG-I–MAVS pathway. This in turn promotes the production of interferons and chemokines and augments the expression of interferon-stimulated genes and PD-L1. The combination of 15a and an anti-PD-L1 antibody had an additive effect in vivo. These findings indicate that this DNMT/HDACi has immunomodulatory functions and enhances the effectiveness of immune checkpoint blockade therapy.

A novel dual DNMT and HDAC inhibitor induces viral mimicry, which induces the accumulation of dsRNA to activate tumoral IFN signalling and cytokine production to enhance the immune response in breast cancer.
Similar content being viewed by others
Introduction
Although there has been notable progress in the early detection and treatment of breast cancer, the considerable heterogeneity and aggressiveness of the disease make detection and treatment challenging [1,2,3]. As a result, breast cancer remains the leading cause of cancer-related death in women, second only to lung cancer [4,5,6]. The adoption of immune checkpoint blockade (ICB) therapy, which has demonstrated early success in treating melanoma and lung cancer, has been relatively slow. Therefore, it is crucial to develop innovative strategies and therapies to improve patient survival rates [7, 8]. Among the recently identified tumour characteristics in 2022, nonmutation epigenetic reprogramming has emerged as a novel feature [9]. Notably, cancer is a complex disease involving both genetic and epigenetic alterations. Epigenetic mechanisms exert crucial influences on multiple aspects of cancer biology, including the promotion of primary tumour growth and invasion, as well as the regulation of immune responses within the tumour microenvironment [10,11,12]. These mechanisms involve intricate interactions between DNA methylation and histone modification. Among the various classes of enzymes involved in epigenetic regulation, DNA methyltransferase (DNMT) and histone deacetylase (HDAC) have emerged as particularly significant. In fact, targeted drugs, such as the DNMT inhibitor decitabine [13, 14] and the HDAC inhibitor vorinostat (SAHA), have been developed to specifically target these enzymes [15].
Within mammals, there are four distinct members of the DNMT family, namely, DNMT1, DNMT3A, DNMT3B, and DNMT3L [16]. The overexpression of these DNMTs facilitates the methylation of cytosine within CpG islands, thereby leading to the suppression of tumour suppressor genes (TSGs) in human cancer [17]. This phenomenon contributes to the initiation and progression of cancer. Conversely, DNMT inhibitors (DNMTis) reactivate TSGs [18], augment tumour immunogenicity, and induce the secretion of cytotoxic cytokines by various immune cells, including macrophages, natural killer (NK) cells, and CD8+ T cells. Ultimately, these actions promote the death of tumour cells. The upregulation of the MHC I class molecules has been observed. Presently, the primary mechanism through which DNMTis exert their immune function is by targeting cancer cells through the induction of viral mimicry. This is achieved by reducing the methylation level of the endogenous retrovirus (ERV) gene promoter and activating the expression of endogenous retroviral elements [19,20,21]. Consequently, the intracellular levels of double-stranded RNA (dsRNA) are increased, leading to an inflammatory immune response. This response further promotes the production of type I and type III interferons and induces the production of a variety of interferon-stimulated genes (ISGs) and cytokines [19, 22, 23], thereby inhibiting tumour cell immune escape.
HDACs, a group of epigenetic enzymes, are intricately associated with tumorigenesis. In conjunction with histone acetyltransferases (HATs), HDACs govern the equilibrium of histone and protein acetylation through the processes of lysine acetylation and deacetylation [24]. HDACs exert significant regulatory effects on numerous key cellular processes, such as cell proliferation, cellular differentiation, and programmed cell death [25]. Furthermore, the expression and activity of HDACs are frequently disrupted in cancer, whereby HDACs counteract lysine acetylation on histones, thereby inducing chromatin remodelling and altering the expression of TSGs [26, 27], ultimately contributing to increased susceptibility to carcinogenesis. Inhibiting HDACs can result in the release of oncogenic transcription suppressors, leading to cell cycle arrest and apoptosis. Moreover, HDAC inhibitors (HDACis) upregulate the expression of T-cell chemokines, namely, CCL5 and CXCL10, thereby establishing a direct correlation with increased T-cell infiltration within tumours [S1.
Flow cytometry analysis
MDA-MB-231 and HCC1937 were treated with either vehicle or 15a for 24 or 48 h. Using Cell Cycle and Apoptosis Kit (Yesen) and Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime), the samples were made according to the instructions. CytoFLEX flow cytometry was used to detect the number of cells in different cycles and the level of apoptosis.
RNA extraction and quantitative real-time PCR assay (qRT‒PCR)
According to the instructions, total RNA was extracted from the cells using the Eastep Super total RNA extraction kit, and the extracted total RNA was quantifiable by Nanodrop spectrophotometer with acceptable purity. For qRT‒PCR, the total RNA was reverse-transcribed by Bio-Rad PCR after different volume samples and 4 μL Hifair® III 1st Strand cDNA Synthesis SuperMix reagent were added according to the calculation (reversing 1 μg of RNA). Using 2 μL cDNA, 10 μL Hieff® qPCR SYBR Green Master Mix (Low Rox Plus), and 0.5 μL forward and reverse primers, using 7500 Fast system under 20 μL volume reaction condition, qPCR was performed using 20 μg cDNA at 95 °C, 5 min, 95 °C, 10 s 60 °C 30 s 40 cycles. The primers used in this study are listed in the Supplementary Table S2.
Transwell evaluation
The 8 μm pore membrane chamber (Corning) was placed in a 24-well plate, and the cells with a density of 4 × 105 were placed in the chamber. The 24-well plate was added with 1 mL medium containing either vehicle or different concentrations of 15a, which was treated for 48 h. After the chamber was washed three times with 1× PBS, adding 1 mL Bourn’s Tissue fixative into the chamber and fixing for 1 h. Then washing three times with 1× PBS after fixing, add 1 mL crystal violet solution dyeing solution for 1 h, and finally wash three times with 1× PBS, wipe the chamber gently with a cotton swab, and then take images with an inverted fluorescence microscope after drying. Three fields of view were taken from each sample.
Colony-forming assay
MDA-MB-231 and HCC1937 cells were seeded in 6-well plates at a density of 300 cells/mL and treated with either vehicle or 15a for 14–21 d, replacing the medium with fresh medium every 72 h. Subsequently, the formed colonies were fixed with 1 mL Bourn’s Tissue fixative for 1 h, washed three times with 1× PBS after fixation, added 1 mL crystal violet solution staining solution for 1 h, and finally washed three times with 1× PBS, dried and then photographed with an inverted fluorescence microscope. Three fields of view were taken for each sample.
Wound healing assay
MDA-MB-231 and HCC1937 cells were cultured in 6-well plates at a density of 2 × 105 cells/mL. Upon reaching confluence, the cells were subjected to serum starvation using FBS-free medium for a duration of 12 h. Prior to cell seeding, three parallel lines were marked on the bottom of the 6-well plates. Subsequently, wounds were induced by vertically scratching the cell monolayer with 200 µL sterile pipette tips along the aforementioned lines. Following a thorough washing and medium replacement, the cells were exposed to either a control solution or varying concentrations of 15a. The wounds adjacent to the lines were captured through photography at the initial time point and 48 h following the act of scratching, employing an inverted fluorescence microscope equipped with a 10× objective lens. For each sample, three distinct fields of view were documented.
Thermal shift assay
The cells resuspended in 1× protease inhibitor was subjected to freezing using liquid nitrogen and subsequent thawing at 37 °C in a water bath. Once approximately 60% of the cells were thawed, they were transferred to ice to continue thawing, repeating this process three times. The soluble proteins in the supernatant were separated from the cell precipitation by centrifugation at 20,000× g for 20 min at 4 °C. The resulting supernatants were then treated with both a vehicle and 15a. Following a 5 min incubation at room temperature with either a vehicle or drugs, the lysates were partitioned into multiple 50 µL aliquots within new 200 µL PCR tubes. These aliquots were then individually heated at varying temperatures for 3 min using a thermal cycler, followed by a 3 min cooling period at room temperature. Subsequently, the heated lysates underwent centrifugation at 20,000× g for 20 min at 4 °C to separate soluble proteins from precipitated proteins. The resulting soluble proteins were utilized for western blotting, with their protein concentration determined through the employment of a BCA assay.
Transient transfection cell assay
Cells were initially plated at a confluency range of 50% to 60% in 60 mm plates and incubated for a duration of 24 h. Subsequently, the cells were transiently transfected using Lipofectamine RNAiMAX Transfection Reagent, employing two sets of pooled siRNAs per gene, specifically DNMT1, HDAC1, MDA5, RIG-I and MAVS, which were procured from GenePharma. To serve as a control, nonspecific scrambled siRNA was utilized. Following a 24-hour period of transfection, the cells were lysed for the purpose of immunoblotting analysis. The gene sequence can be found in Supplementary Table S3.
Construction of stable cells
Extraction of plasmid: take a conical bottle and pour 100 mL LB medium, seal with tissue culture sealing film and rubber band, and then sterilize with high-pressure steam. After cooling to room temperature, ampicillin was added (final concentration was 100 μg/mL), 500 μL of sequenced bacterial solution was added, and the bed was shaken at 37 °C at 200× g overnight. After the bacterial solution was cloudy, the plasmid was extracted in large quantities (Beyotime), and the gene sequence was shown in Supplementary Table S4. (2) Packaged virus: HEK-293T cells were incubated in DMEM medium at 37 °C. The mixture (4 μg target plasmid, 1 μg pMD2.G envelope plasmid, 3 μg psPAX2 package plasmid and 10 μL (1 mg/mL) PEI (Beyotime, C0537) was added when the density of cells was 80%–90%. The mixture was prepared with FBS-free medium to 400 μL and then left for 10 min. Drips were added to 293 T cells in 10 cm petri dish. After 6–8 h, the medium was discarded and was added into 5 mL DMEM medium. After 24 h, the venom was collected and was centrifuged for 1000× g for 5 min, and the supernatant was divided into 1 mL tubes and stored at −80 °C. (3) Infected cells: the target cells were spread in a 6 cm dish and incubated overnight at 37 °C. When the cell density was 80–90%, the old medium was discarded and 1 mL of disease venom and 1 μL polybrene (8 mg/mL, Beyotime, C0351) were added. After 4 h, the disease venom was sucked away and added to 3 mL of complete medium, or directly added to 2 mL of complete medium. In order to screen out the successfully infected cells, complete medium containing puromycin was added to the next passage and subsequent culture for culture, and after the state of the target cells was stable, western blot assay and qRT‒PCR assay were used to verify whether the knockdown was carried out.
Immunofluorescence (IF)
Breast cancer cells with a density of 50–60% were cultured in confocal petri dish (Nest, KH-TB-A1111) for 48 h, then fixed with −20 °C pre-cooled ice methanol at room temperature for 10 min, and washed three times with 1× PBS after fixation. Goat serum blocking buffer (1× PBS/5% normal serum / 0.3% Triton X-100) was added, enclosed at room temperature for 1 h, washed three times with 1× PBS, STAT1 (1:200) and J2 (1:200) antibody, incubated overnight at 4 °C, recovered primary antibody on the second day, washed three times with 1× PBS, and added rabbit secondary antibody (1:200) or mouse secondary antibody (1:50), after incubation at room temperature for 1 h, wash three times with 1× PBS, add DAPI anti-fluorescence quencher (Yesen, 36308ES20), and incubate in dark light for 5 min before taking photos. The primary and secondary antibodies were formulated with a dilute release buffer (1× PBS / 1% BSA / 0.3% Triton X-100).
Methylation-specific PCR (MSP) analysis
Cells were subjected to treatment with a vehicle and different concentrations of 15a. The Blood/Cell/Tissue Genomic DNA Extraction Kit (Tangen) was used to extract DNA from these cells. Subsequently, the DNA was processed using the D5005/EZDNAMethylation-GoldKit kit (Zymo Research) and the methylation-specific PCR kit (Tangen). During this process, unmethylated cytosine was converted to uracil, while methylated cytosine remained unaltered. Based on this alteration in the DNA bases, primers targeting methylated and unmethylated sequences were designed and amplified using PCR.
In vivo functional assays
A total of 2 × 105 4T1 cells were subcutaneously inoculated into female BALB/c wild-type mice aged 6–8 weeks, which were obtained from Guangdong Pharmaceutical Company. Once the tumour volume reached 80–100 mm3, the mice were randomly assigned to two groups, with five mice in each group. The groups were administered either a vehicle (10% DMSO, 45% PEG300, and 45% 1× PBS) or 15a (dissolved in vehicle) intravenously once daily. Tumour size was measured using vernier calipers every three days. The mean tumour volume for each group was calculated using the formula (length × width × width)/2 and expressed in cubic millimeters. The animals were killed on day 21, and the removed tumour tissue was weighed and photographed. For in vivo combination therapy, mice were treated with vehicle or 15a alone injected on once every three days or in combination with anti-PD-L1 antibody (BioXcell, 100 µg injected on days 7, 10, 13). The experiments were conducted in accordance with the regulations of the Agency’s Animal Management and Use Committee and were approved by the Committee.
Hematoxylin and eosin (HE) staining
The organ specimens, comprising the heart, liver, spleen, lung, and kidney, were subjected to a series of procedural steps, followed by staining with the HE staining kit (Biosharp).
Immunohistochemistry (IHC)
The tumour specimen underwent a series of procedures, including dewaxing and washing with 1× PBS, treatment with 3% H2O2 for 20 min to prevent endogenous peroxidase activity, heat-induced epitope retrieval at EDTA (pH 9.0) for 2 min, and incubation in a blocking buffer containing 10% goat serum for 30 min at room temperature. Subsequently, the sections were exposed to primary antibodies against PD-L1 (CST, 64988, 1:200) and CD8α (CST, 98941, 1:200) overnight at 4 °C, followed by incubation with secondary antibodies (MaxVision) for 1 h at room temperature. Finally, the sections were developed using diaminobenzidine (DAB). The IHC profiler plugin in Image J software was utilized to automatically analyze the percentage and staining intensity of positive cells in each image [55, 56].
Database analysis
Gene correlation analysis was retrieved from the TCGA database (https://portal.gdc.cancer.gov/) RNA expression profiles and matched clinicopathological information of breast cancer patients. Spearman correlation analysis was conducted on DNMT1 and HDAC1 in tumor and non-tumor groups using R4.2.1 to explore the relationship between DNMT1 and HDAC1. A statistically significant difference was defined as a difference with a p-value < 0.05. Kaplan–Meier overall survival curves of human breast tumors generated using Kaplan–Meier Plotter according to DNMT1 and HDAC1 gene expression levels. The R package ggplot2 was used to plot gene difference analysis, correlation analysis and Kaplan–Meier survival curves.
Statistical analysis
All experiments requiring statistical analysis were independently conducted with a minimum of three replications. The experimental data are presented as the mean value ± standard deviation (SD) or standard error of the mean (SEM). Statistical analysis was carried out using GraphPad Prism 8.0. Differences were assessed an unpaired Student’s t test for two-sample comparisons and one-way analysis of variance (ANOVA) for multiple-sample comparisons. A statistically significant difference was defined as a p-value < 0.05. Spearman correlation analysis was employed for assessing correlations between DNMT1 and HDAC1 expression levels. Kaplan–Meier survival curves were analyzed using the Mantel–Cox log-rank test. Inclusion/exclusion criteria were all pre-established and no samples or animals were excluded from the analysis.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Cancer Netw 2020;18:479–89.
Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res 2018;24:511–20.
Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009;15:539–50.
Verma M. Cancer epigenetics: risk assessment, diagnosis, treatment, and prognosis. Preface. Methods Mol Biol 2015;1238:v–vi.
Garmpis N, Damaskos C, Garmpi A, Kalampokas E, Kalampokas T, Spartalis E, et al. Histone deacetylases as new therapeutic targets in triple-negative breast cancer: progress and promises. Cancer Genom Proteom 2017;14:299–313.
Shen M, Pan H, Chen Y, Xu YH, Yang W, Wu Z. A review of current progress in triple-negative breast cancer therapy. Open Med 2020;15:1143–9.
Onkar SS, Carleton NM, Lucas PC, Bruno TC, Lee AV, Vignali DAA, et al. The great immune escape: understanding the divergent immune response in breast cancer subtypes. Cancer Discov 2023;13:23–40.
So JY, Ohm J, Lipkowitz S, Yang L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther 2022;237:108253.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022;12:31–46.
Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen RW, Vatapalli R, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget 2014;5:587–98.
Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol 2020;17:75–90.
Emran AA, Chatterjee A, Rodger EJ, Tiffen JC, Gallagher SJ, Eccles MR, et al. Targeting DNA methylation and EZH2 activity to overcome melanoma resistance to immunotherapy. Trends Immunol 2019;40:328–44.
Yu J, Qin B, Moyer AM, Nowsheen S, Liu T, Qin S, et al. DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine. J Clin Investig 2018;128:2376–88.
Dhillon S. Decitabine/Cedazuridine: First approval. Drugs 2020;80:1373–8.
Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 2007;110:313–22.
Wang L, Zhao Y, Bao X, Zhu X, Kwok YK, Sun K, et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res 2015;25:335–50.
Kuang SQ, Bai H, Fang ZH, Lopez G, Yang H, Tong W, et al. Aberrant DNA methylation and epigenetic inactivation of Eph receptor tyrosine kinases and ephrin ligands in acute lymphoblastic leukemia. Blood 2010;115:2412–9.
Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang S, et al. Caloric restriction delays age-related methylation drift. Nat Commun 2017;8:539.
Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, et al. DNA-Demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 2015;162:961–73.
Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 2017;169:361.
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471–5.
Chen R, Ishak CA, De Carvalho DD. Endogenous retroelements and the viral mimicry response in cancer therapy and cellular homeostasis. Cancer Discov 2021;11:2707–25.
Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249–53.
Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer 2010;10:669–82.
Winter M, Moser MA, Meunier D, Fischer C, Machat G, Mattes K, et al. Divergent roles of HDAC1 and HDAC2 in the regulation of epidermal development and tumorigenesis. EMBO J 2013;32:3176–91.
Siu LL, Pili R, Duran I, Messersmith WA, Chen EX, Sullivan R, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol 2008;26:1940–7.
Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez-Chavez A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol 2011;29:2052–9.
Zheng H, Zhao W, Yan C, Watson CC, Massengill M, **e M, et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res 2016;22:4119–32.
Li L, Hao S, Gao M, Liu J, Xu X, Huang J, et al. HDAC3 inhibition promotes antitumor immunity by enhancing CXCL10-mediated chemotaxis and recruiting of immune cells. Cancer Immunol Res 2023;11:657–73.
Woods DM, Sodré AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res 2015;3:1375–85.
Li X, Su X, Liu R, Pan Y, Fang J, Cao L, et al. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene 2021;40:1836–50.
Yang W, Feng Y, Zhou J, Cheung OK, Cao J, Wang J, et al. A selective HDAC8 inhibitor potentiates antitumor immunity and efficacy of immune checkpoint blockade in hepatocellular carcinoma. Sci Transl Med 2021;13:eaaz6804.
Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE. Inhibition of histone deacetylases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. Mol Cancer Res 2008;6:873–83.
Harada T, Ohguchi H, Grondin Y, Kikuchi S, Sagawa M, Tai YT, et al. HDAC3 regulates DNMT1 expression in multiple myeloma: therapeutic implications. Leukemia 2017;31:2670–7.
Sheikh TN, Chen X, Xu X, McGuire JT, Ingham M, Lu C, et al. Growth inhibition and induction of innate immune signaling of chondrosarcomas with epigenetic inhibitors. Mol Cancer Ther 2021;20:2362–71.
Bruyer A, Maes K, Herviou L, Kassambara A, Seckinger A, Cartron G, et al. DNMTi/HDACi combined epigenetic targeted treatment induces reprogramming of myeloma cells in the direction of normal plasma cells. Br J Cancer 2018;118:1062–73.
Yuan Z, Sun Q, Li D, Miao S, Chen S, Song L, et al. Design, synthesis and anticancer potential of NSC-319745 hydroxamic acid derivatives as DNMT and HDAC inhibitors. Eur J Med Chem 2017;134:281–92.
Yang J, Xu J, Wang W, Zhang B, Yu X, Shi S. Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther 2023;8:210.
Qiu L, Xu W, Lu X, Chen F, Chen Y, Tian Y, et al. The HDAC6-RNF168 axis regulates H2A/H2A.X ubiquitination to enable double-strand break repair. Nucleic acids Res 2023;51:9166–82.
Bárcena-Varela M, Caruso S, Llerena S, Álvarez-Sola G, Uriarte I, Latasa MU, et al. Dual targeting of histone Methyltransferase G9a and DNA-Methyltransferase 1 for the treatment of experimental hepatocellular carcinoma. Hepatology 2019;69:587–603.
Ozyerli-Goknar E, Bagci-Onder T. Epigenetic deregulation of apoptosis in cancers. Cancers 2021;13:3210.
Daskalakis M, Brocks D, Sheng YH, Islam MS, Ressnerova A, Assenov Y, et al. Reactivation of endogenous retroviral elements via treatment with DNMT- and HDAC-inhibitors. Cell Cycle 2018;17:811–22.
Fresquet V, Garcia-Barchino MJ, Larrayoz M, Celay J, Vicente C, Fernandez-Galilea M, et al. Endogenous retroelement activation by epigenetic therapy reverses the Warburg effect and elicits mitochondrial-mediated cancer cell death. Cancer Discov 2021;11:1268–85.
Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol 2019;4:914–24.
Luszczek W, Cheriyath V, Mekhail TM, Borden EC. Combinations of DNA methyltransferase and histone deacetylase inhibitors induce DNA damage in small cell lung cancer cells: correlation of resistance with IFN-stimulated gene expression. Mol Cancer Ther 2010;9:2309–21.
Ren Y, Li S, Zhu R, Wan C, Song D, Zhu J, et al. Discovery of STAT3 and Histone Deacetylase (HDAC) dual-pathway inhibitors for the treatment of solid cancer. J medicinal Chem 2021;64:7468–82.
Wu SY, **ao Y, Wei JL, Xu XE, ** X, Hu X, et al. MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer 2021;9:e002528.
Yu H, Liu J, Bu X, Ma Z, Yao Y, Li J, et al. Targeting METTL3 reprograms the tumor microenvironment to improve cancer immunotherapy. Cell Chem Biol 2023;S2451-9456:00291–X.
Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RC, et al. Epigenetic therapy ties MYC depletion to reversing immune evasion and treating lung cancer. Cell 2017;171:1284–1300.e1221.
Pfister SX, Ashworth A. Marked for death: targeting epigenetic changes in cancer. Nat Rev Drug Discov 2017;16:241–63.
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012;150:12–27.
Guo E, **ao R, Wu Y, Lu F, Liu C, Yang B, et al. WEE1 inhibition induces anti-tumor immunity by activating ERV and the dsRNA pathway. J Exp Med 2022;219:e20210789.
Stone ML, Chiappinelli KB, Li H, Murphy LM, Travers ME, Topper MJ, et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc Natl Acad Sci USA 2017;114:E10981–e10990.
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014;28:1280–8.
Ding J, Wang C, Sun Y, Guo J, Liu S, Cheng Z. Identification of an autophagy-related signature for prognosis and immunotherapy response prediction in ovarian cancer. Biomolecules 2023;13:339.
Pavliuk-Karachevtseva A, Mihalik J, Biel R, Rybárová S, Hodorová I. Chosen antioxidant enzymes GPx4 and GPx8 in human colorectal carcinoma: Study of the Slovak population. Medicine 2022;58:298.
Acknowledgements
This work was supported by grants from the Stable Support Project of Shenzhen (Project No. 20231120105309001), State Key Laboratory of Chemical Oncogenomics Open Funding (SKLCO202204). We also thank the Instrumental Analysis Center of Shenzhen University for its assistance with confocal microscopy analysis.
Author information
Authors and Affiliations
Contributions
BC and YJ designed and supervised the experiments and revised the manuscript. WZ provided critical reading and revision of the manuscript. BC and WH wrote the manuscript and analyzed the data. ZS and ZY designed and synthesized the compounds. LL, XZ and YH contributed new reagents. WH performed cell line studies and western blot assay. WH performed quantitative real-time PCR assay and immunofluorescence assay. WH, QZ and YT performed animal studies. WH and QL performed database analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Shenzhen University (permission no. AEWC-202300007).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, W., Zhu, Q., Shi, Z. et al. Dual inhibitors of DNMT and HDAC induce viral mimicry to induce antitumour immunity in breast cancer. Cell Death Discov. 10, 143 (2024). https://doi.org/10.1038/s41420-024-01895-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-01895-7
- Springer Nature Limited