Abstract
Endometriosis is a gynecological inflammatory disease that is linked with immune cells, specifically macrophages. IL-33 secreted from macrophages is known to accelerate the progression of endometriosis. The periodic and repeated bleeding that occurs in women with endometriosis leads to excess iron in the microenvironment that is conducive to ferroptosis, a process related to intracellular ROS production, lipid peroxidation and mitochondrial damage. It is suggested that eESCs may specifically be able to inhibit ferroptosis. However, it is currently unclear whether IL-33 directly regulates ferroptosis to influence the disease course in endometriosis. In this study, eESCs co-cultured with macrophages or stimulated with IL-33/ST2 were observed to have increased cell viability and migration. Additionally, IL-33/ST2 decreased intracellular iron levels and lipid peroxidation in eESCs exposed to erastin treatment. Furthermore, IL-33/ST2 treatment resulted in a notable upregulation in SLC7A11 expression in eESCs due to the downregulation of negative transcription factor ATF3, thereby suppressing ferroptosis. The P38/JNK pathway activated by IL-33/ST2 was also found to inhibit the transcription factor ATF3. Therefore, we concluded that IL-33/ST2 inhibits the ATF3-mediated reduction in SLC7A11 transcript levels via the P38/JNK pathway. The findings reveal that macrophage-derived IL-33 upregulates SLC7A11 in eESCs through the p38/JNK/ATF3 pathway, ultimately resulting in protection against ferroptosis in eESCs. Moreover, we conducted an experiment using endometriosis model mice that showed that a combination of IL-33-Ab and erastin treatment alleviated the disease, showing the promise of combining immunotherapy and ferroptosis therapy.
Similar content being viewed by others
Introduction
Endometriosis (EMs) is a chronic inflammatory disease that affects nearly 10% of females of reproductive age worldwide [1]. Multiple theories on the pathophysiology of endometriosis, including ectopic implantation, coelomic metaplasia, and immune factors, have been proposed to elucidate the pathophysiology of endometriosis [2]. Notable changes in the local immune microenvironment of endometriosis lesions have been detected, including the infiltration and differentiation of diverse immune cells types and the aggregation of chemokines and cytokines [3]. Macrophages are reported to be one of the predominant cells present in ectopic endometrial lesions, and interleukin-1β (IL-1β)’s release promotes ectopic endometrial stromal cells (eESCs) proliferation [4, 5]. IL-8-Ab reduced the volume of lesions and ameliorated fibrosis and adhesion in monkey endometriosis models [6]. The interplay between infiltrated immune cells and eESCs prompts genetic and epigenetic modifications in the eESCs. Subsequently, these eESCs changes cause molecular changes and dysfunction in immune cells.
Interleukin-33 (IL-33), a member of the IL-1β family, is secreted by macrophages. Previous studies have indicated that IL-33 has the potential to stimulate tumor cell proliferation and neovascularization in ovarian cancer [7]. Knockout of IL-33 in mouse endometriosis models has led to a significant decrease in endometriotic lesion volume [8]. It is also worth mentioning that IL-33 induces macrophage anti-inflammatory polarization and stimulates the formation of splenic red pulp macrophages (RPMs) that regulate erythrocyte homeostasis and support iron recycling [9, 10]. Our team has also discovered a significant promoting effect of macrophages on endometriosis [11]. We thus postulate that macrophage-derived IL-33 may regulate eESCs survival, which ultimately advances the progression of endometriosis. However, the the complete role of IL-33 in the development of endometriosis requires further exploration.
Ferroptosis is a newly discovered programmed cell death process characterized by iron-dependent accumulation of reactive oxygen species (ROS) and lipid peroxidation [12, 13]. The key molecule in ferroptosis, SLC7A11, is a component of the L-cystine/L-glutamic acid reverse transporter system (Xct) that mediates the transmembrane transport of glutamate and cysteine. Cysteine from the extracellular space triggers glutathione (GSH) synthesis, maintaining the GSH/GSSG redox balance. Glutathione peroxidase 4 (GPX4) also plays a critical role in ferroptosis by efficiently reducing lipid hydroperoxides that accumulate in the membrane of cells undergoing ferroptosis [14,15,16]. A study reported that the macrophages of pancreatic ductal adenocarcinoma (PDAC) patients undergo ferroptosis, which suppressed the macrophage defense response against tumor cells [
Materials and methods
Clinical samples
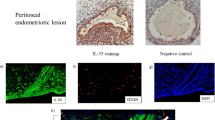
This study was approved by the Ethical Committee of the Second Affiliated Hospital of Harbin Medical University (KY2016-040), and all patients provided informed written consent. The study recruited 26 women with endometriosis who were diagnosed by laparoscopy and histological analysis at the Second Affiliated Hospital of Harbin Medical University from March 2021 to April 2022. The normal endometriosis tissues were collected from 16 patients as the controls. Controls groups patients are whom underwent benign gynecological diseases including uterine prolapsehy, benign cervical lesions and without clinical symptom and sign of endometriosis or adenomyosis.
Cell culture
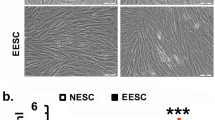
Primary cells were isolated from endometriosis tissues using previously described protocols [11]. Briefly, the tissues were cut into 1 mm3 pieces and then digested using type IV collagenase (0.2% Sigma, USA) for 60 min at 37 °C. The primary cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 15% fetal bovine serum (FBS; Biological Industries, Israel) and 1% penicillin‒streptomycin (Gibco, USA) at 37 °C with 5% CO2. Primary cells were identified using immunofluorescence staining (Supplementary Fig. 1).
The acute monocytic leukemia cell line (THP-1 cells), was purchased from ScienCell, and cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) medium supplemented with 10% FBS and 1% penicillin‒streptomycin (Biological Industries, Israel). THP-1 cells were treated with PMA (200 nM; Sigma) for 48 h to induce cell polarization.
Immunohistochemical staining (IHC)
The sections were incubated overnight and then immersed in xylene and ethanol for deparaffinization. Primary antibodies were incubated for overnight at 4 °C, followed by incubation with secondary antibodies for 20 min at ambient temperature (as listed in Table 1). The sections were then stained using DAB dye (CWBIO, Bei**g, China) and hematoxylin. Finally, the slides were covered with cover slips.
Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from cells or tissues using TRIzol (Invitrogen, USA), isopropyl alcohol, chloroform, and 75% ethanol. Reverse transcription of RNA (500 ng) was accomplished using a cDNA synthesis kit according to the manufacturer’s protocol. Thereafter, cDNA (20 ng) was employed as a template for RT-qPCR using the Top Green qPCR SuperMix kit (TransGen Biotech, China). The primers were obtained from GENEWIZ (GENEWIZ, China) and are listed in Table 2.
Western blotting (WB)
Cells or tissues were lysed using RIPA lysis buffer and 1% PMSF (Beyotime Biotechnology, China). The protein samples were performed using a BCA protein assay kit (Beyotime Biotechnology, China). Samples were loaded into 10% SDS-PAGE gels (Epizyme, China) and then transferred onto PVDF membranes (Millipore, USA). The membrane was blocked using 5% skim milk powder solution for 2 h, after which it was incubated with primary antibodies (as listed in Table 1) overnight at 4 °C and then with secondary antibodies for 2 h at room temperature. The blots were visualized using ECL reagent (Epizyme, China).
ELISA
Cell culture medium from each group was collected and centrifuged at 500 × g, for 5 min. Interleukin-33 (IL-33) concentrations in the culture media supernatant were measured via an ELISA kit (Proteintech, USA) in accordance with the manufacturer’s instructions.
Immunofluorescence (IF)
Cells were fixed and subsequently blocked for 30 min using 5% goat serum albumin (Beyotime Biotechnology, China). The tissue sections were incubated overnight at 4 °C with specific primary antibodies (as listed in Table 1) and with fluorescent secondary antibodies for 1 h at room temperature. Fluorescence images were captured using a fluorescence microscope (Nikon, Tokyo, Japan).
Cell viability assessment
Cell viability was measured by CCK-8 reagent following the manufacturer’s instructions (Beyotime Biotechnology, China). The absorbance readings were taken at 450 nm using a plate reader (Bio-Rad).
Colony formation assay
Cells were subjected to 7 day of culture subsequently, staining was performed for 30 min using crystal violet dye. Imaging was carried out using an iPhone 12, and ImageJ software was then utilized to analyze the obtained data.
Transwell migration assay
Cells were seeded at a density of 1×104/mL in the upper chamber of the 8 μm transwell insert utilizing 100 μL of serum-free DMEM. The lower chamber was filled with 600 μL of DMEM containing 15% FBS. After a 24-h incubation, the cells were fixed and stained.
Wound healing assay
We created a scratch in the cell monolayer with a 200 μL pipette. We recorded images of the scratch area under a microscope immediately after creating the scratch and at 24 and 48 h later.
Cell transfection
We transfected cells with small interfering RNA (siRNA) using the Lipofectamine 3000 transfection kit (Invitrogen, USA) according to the manufacturer’s instructions. Ribobio (Ribobio, China) synthesized all specific siRNAs and siRNA controls. The target sequences of the siRNAs were as follows: siRNA, 5ʹ-TTCTCCGAACGTGTCACGT-3ʹ; siST2-1, 5ʹ-TCTAAUGUCACTAAAUAACUT-3ʹ; siST2-2, 5ʹ-GCGAAUGUCACCAUAUAUATT-3ʹ; siST2-3, 5ʹ-GCCCATGUCATTAAAUAUCAT-3ʹ; siSLC7A11-1, 5ʹ-CCGGCCTGTCACTATTT-3ʹ; siSLC7A11-2, 5ʹ-GGAAGAGATTCAAGTATTA-3ʹ; siSLC7A11-3, 5ʹ-GGAGCTTTCTCGAGAAAG-3ʹ; siATF3-1, 5ʹ-CCGCCTTTCATCTGGATTCTA-3ʹ; and siATF3-2, 5ʹ-GCTGAACTGAAGGCTCAGATT-3ʹ. siATF3-3, 5ʹ-GCTGCAAAGTGCCGAAACA-3ʹ.
Measurement of intracellular iron levels
We used the FerroOrange kit (Do**do, Japan) to detect intracellular Fe2+ levels in eESCs. The cells were incubated with serum-free medium containing 1 μM FerroOrange reagent at 37 °C and 5% CO2 for 30 min. We obtained fluorescence images of the cells using a confocal microscope (Nikon, Tokyo, Japan).
Lipid peroxidation determination
To quantify the levels of lipid peroxidation, we used the LiperFluo and MDA Assay Kit from Do**do (Do**do, Japan) and Beyotime Biotechnology (Beyotime, China), respectively. For the LiperFluo assay, we added LiperFluo reagent (5 μM) diluted in DMEM to the treated cells and incubated them for 30 min at 37 °C and 5% CO2. We evaluated lipid peroxidation levels using fluorescence microscopy (Nikon, Tokyo, Japan) by capturing photographs of the cells. For the MDA assay, cell lysis buffer for Western blotting and IP (Beyotime Biotechnology, China) was used to lyse the cells on ice for 30 min. The absorbance was then measured at 532 nm using a plate reader in line with the manufacturer’s instructions.
Measurement of GSH levels
We measured intracellular GSH levels in the treated cells using a GSH assay kit from Solarbio (Solarbio, Bei**g, China). We lysed the cells entirely by performing four consecutive freeze-thaw cycles. We then mixed the cell lysate with the GSH reagent and measured the OD value of the resulting mixture at 412 nm using a plate reader.
Transmission electron microscopy (TEM)
Samples were fixed with 2.5% glutaraldehyde (Servicebio, China) following a previously established protocol. After dehydration, we cut the samples into thin slices, which were then stained with uranyl acetate and lead citrate for contrast enhancement. Finally, we captured TEM images of the samples using a Hitachi TEM system (Japan).
ChIP
We used the DNA ChIP Assay Kit (Beyotime Biotechnology, China) to immunoprecipitate DNA according to the manufacturer’s instructions. Collected cell sample fragments were diluted in ChIP dilution buffer, followed by incubation with Protein A + G Agarose/Salmon Sperm at 4 °C for 30 min. We later incubated the samples with anti-ATF3 antibody (Affinity, DF3110) or normal rabbit IgG overnight at 4 °C. We purified the samples using a DNA purification kit from Beyotime Biotechnology (China). Finally, we used RT-qPCR to quantify the predicted DNA sequences in the immunoprecipitated samples. The primers used were as follows: SLC7A11, forward 5’-TTGAGCAACAAGCTCCTCCT-3’, reverse 5’-CAAACCAGCTCAGCTTCCTC-3’.
Mouse endometriosis model
Female C57BL/6 mice (7 weeks old) were randomly sorted into four groups (n = 6); the endometriosis group, endometriosis+erastin group, endometriosis+IL-33-Ab group, and endometriosis+erastin+IL-33-Ab group. The endometriosis models were established as described previously [11]. Briefly, the uterus from an estradiol (0.2 ml/mouse)-stimulated donor mouse, was minced into 1 mm3 fragments and immediately injected subcutaneously into the peritoneal cavity of two recipient mice. The abdominal cavity of mice was subcutaneously injected with 300 μL of erastin (20 mg/kg) (MCE, Shanghai, China) alone or combined with 50 μg IL-33-Ab (R&D Systems, USA), or normal saline (Fig. 7A). On Day 10 postsurgery, all mice were sacrificed. The lesion volume was calculated using the Formula V = 1/2Aa2; A: long radius, a: short radius. Ethical approval for all animal research was granted by the Institutional Animal Research Ethics Committee of Harbin Medical University.
Statistical analysis
Each experiment was conducted independently, with a total of three replicates. Statistical was performed using GraphPad Prism 8 (San Diego, USA), and the data are presented as the mean with standard deviation (SD). Student’s t test, one-way ANOVA, and two-way ANOVA were used to compare data in different experimental groups. Statistical significance was determined as p < 0.05. Non-significant differences were designated as “ns” (p ≥ 0.05), whereas ****p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05 represent significant differences.