Abstract
With the expansion of the aging population, age-associated sarcopenia (AAS) has become a severe clinical disease of the elderly and a key challenge for healthy aging. Regrettably, no approved therapies currently exist for treating AAS. In this study, clinical-grade human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) were administrated to two classic mouse models (SAMP8 mice and D-galactose-induced aging mice), and their effects on skeletal muscle mass and function were investigated by behavioral tests, immunostaining, and western blotting. Core data results showed that hUC-MSCs significantly restored skeletal muscle strength and performance in both mouse models via mechanisms including raising the expression of crucial extracellular matrix proteins, activating satellite cells, enhancing autophagy, and impeding cellular aging. For the first time, the study comprehensively evaluates and demonstrates the preclinical efficacy of clinical-grade hUC-MSCs for AAS in two mouse models, which not only provides a novel model for AAS, but also highlights a promising strategy to improve and treat AAS and other age-associated muscle diseases.

This study comprehensively evaluates the preclinical efficacy of clinical-grade hUC-MSCs in treating age-associated sarcopenia (AAS), and demonstrates that hUC-MSCs restore skeletal muscle strength and performance in two AAS mouse models via raising the expression of extracellular matrix proteins, activating satellite cells, enhancing autophagy, and impeding cellular aging, which highlights a promising strategy for AAS and other age-associated muscle diseases.
Similar content being viewed by others
Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder characterized by the degenerative loss of skeletal muscle strength and mass, involving the accelerated loss of muscle mass and function [1,2,3]. Studies have reported that aging appears to result in the disturbance of homeostasis in skeletal muscle and an imbalance of tissue regeneration, leading to an overall loss of skeletal muscle. Cellular changes in sarcopenic muscle include a reduction in the size and number of myofibers. This is due to the decreased number of fast myosin fibers with age, together with intramuscular and intermuscular fat infiltration, and a decreased number of satellite cells [4]. Sarcopenia could be divided into primary sarcopenia (or age-associated sarcopenia, AAS), caused by aging, and secondary sarcopenia (or disease-associated sarcopenia, DAS), caused by diabetes mellitus, cancer, chronic obstructive pulmonary disease, or heart failure [4,5,6], which also requires appropriate treatment of the underlying disease.
With the expansion of the aging population, the problem of AAS becomes increasingly severe, which seriously impacts the lifestyle of the elderly [7, 8]. Despite physical exercise is proven to be the most effective preventative measure for AAS in mouse models, it is often impractical or inefficient for elderly individuals with reduced functional capacities. Several clinical pharmaceuticals, such as testosterone, growth factors and dehydroepiandrosterone, have been reported to be helpful in treating AAS, but with controversial effects [3, 9]. Unfortunately, there are currently no approved therapies for AAS, making it imperative to develop new treatments.
Mesenchymal stem cells (MSCs) have gained widespread use in cell therapy due to their anti-fibrosis, immunomodulatory properties, as well as their ability to release various biologically active molecules [10,11,12]. It has been reported that MSCs could enhance muscular regeneration in animal and cellular models [13,14,15,16,17,18]. Particularly, human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) are advantageous due to their higher yield without the invasive procedures or ethical issues, as well as their ability to secrete a wide range of multifunctional factors [19, 20]. Our group has previously developed a complete system for obtaining clinical-grade hUC-MSCs according to the current Good Manufacturing Practice (cGMP) guidelines. The hUC-MSCs obtained through this system have been shown to meet the quality criteria of the National Institute of Food and Drug Control (NIFDC) and possess good preclinical efficacy in the intervention or treatment of neurodegeneration associated with aging [21, 22]. Furthermore, therapeutic effects of hUC-MSCs have been demonstrated on muscular atrophy experimental models [15, 17, 23]. Although these studies lack a more comprehensive evaluation of preclinical efficacy, some positive effects bring new hope for the treatment of AAS. Therefore, we speculated that hUC-MSCs might be a superior source for reversing muscle dysfunction in AAS.
Here, for the first time, we comprehensively evaluated the preclinical efficacy of clinical-grade hUC-MSCs on two AAS mouse models, including the SAMP8 mice (a senescence-accelerated mouse commonly used as the AAS model) and D-galactose (D-gal)-induced aging model (a systemic and homogeneous aging model with the acceleration of senescence). Both models were proven to have the typical phenotype of AAS in our results. Based on the behavioral test, hematoxylin-eosin (H&E) staining, immunostaining and western blotting, we found that administration of hUC-MSCs effectively improved muscle strength, restored muscle morphology and performance of aging skeletal muscle in AAS mouse models. The mechanisms involved included raising the expression of crucial extracellular matrix proteins, activating skeletal satellite cells, enhancing autophagy, and impeding the cellular senescence by down-regulating p16/p53-p21 axis.
Collectively, our results demonstrated that hUC-MSCs transplantation could improve skeletal muscle dysfunction from multiple aspects, including cellular components, cell structure and cell function, and ultimately restore muscle strength in AAS mice. Additionally, our study clarified the underlying mechanism of systematically targeting AAS therapy, at least partially through reconstructing myocyte autophagy to provide self-energy supply and down-regulating the classic p16/p53-p21 axis to delay myocyte aging. More importantly, our study provided a promising strategy for the prevention and treatment of AAS and other age-associated muscle diseases.
Results
Clinical-grade hUC-MSCs improved muscle strength and restored skeletal muscle morphology both in SAMP8 mice and D-gal-induced aging mice
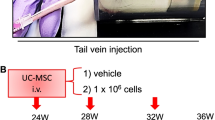
Based on the detection of the cellular viability, morphology, differentiation potential and surface markers, the clinical-grade hUC-MSCs conformed to the quality standards of MSCs [21, 22] (Fig. S1). After treatment with hUC-MSCs, the behavior features of AAS mouse models were evaluated by grip test and anti-fatigue test, which are considered core metrics of sarcopenia [21, 22]. The results indicated that compared with the P8-PBS group, the mice treated with hUC-MSCs exhibited enhanced grip strength and anti-fatigue abilities (Fig. 1B, C), similar to the R1 group.
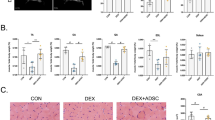
A Illustrated in schematic form is the chronological sequence used for hUC-MSCs or PBS treatment, behavioral tests, immunostaining, western blotting and others. B, C Evaluation of muscle strength was performed by measuring grip** (PR1&PBS = 0.0018; PMSC & PBS = 0.0012) and anti-fatigue (PR1 & PBS = 0.0001; PMSC & PBS = 0.0001) capacities. The time of Latency to fall in the Rota Rod system was used to reflect the muscle endurance, and grip** test was used to show the grip strength of SAMP8 mice (n = 6–7 per group). D The representative cross-sections of gastrocnemius muscle were stained with H&E to observe better the morphology of muscle cells in R1, P8-PBS, and P8-MSC groups (scale bar = 100 μm). E, F Quantitative analysis of muscle fiber cross-sectional area (PR1 & PBS < 0.0001; PMSC & PBS < 0.0001) (μm2) and muscle fiber diameter (PR1 & PBS = 0.0029; PMSC & PBS < 0.0001) (μm2) in SAMP8 mice. (n = 8 or 10 views per group from 5–6 male mice; all data shown as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001).
The mice were euthanasia subsequently (Figs. 1A, S2A), and the gastrocnemius muscles were collected and subjected to histopathological tests by cross section and vertical section [24]. To better characterise the muscle size on different treatment, we conducted H&E staining on the cross section of gastrocnemius muscle and measured the area and diameter of muscle cells. Initially, we observed a significant reduction in the cross-sectional area and diameter in the P8-PBS group, which were restored in the P8-MSC group. A similar treatment effect was noted in the D-gal-induced aging model (Fig. S2). These results demonstrated the treatment of hUC-MSCs could restore muscle functions and morphology in AAS mouse models.
hUC-MSCs restored the ratio of slow and fast motor units of skeletal muscle in two mouse models
Skeletal muscle fibers can be classified into slow myosin (type I) fibers and fast myosin (type II) fibers [25], which play a critical role in accurately assessing the extent of muscle fiber impairment in aging muscle [26]. The primary cause of age-related loss of muscle mass is a decrease in the total number of both slow and fast myosin fibers, with the preferential atrophy of fast myosin fibers being secondary [27,28,29]. In order to estimate the potential of hUC-MSCs to prevent the slight decrease in fast fibers abundance, we examined the proportion of slow and fast myosin fibers in extensor digitorum longus (EDL). The EDL of skeletal muscle was then consecutively sectioned and stained for fast and slow muscle respectively. We observed that the unstained portion of the slow muscle section coincided with the deeply stained portion of the fast muscle section. Statistical results indicated that compared to the P8-PBS group, the ratio of slow and fast muscles in both the R1 group and P8-MSC group was lower (Fig. 2). Likewise, the same phenomenon of muscle contraction could also be found in the D-gal-induced aging model (Fig. S3). These results demonstrated that hUC-MSCs treatment restored the ratio of slow and fast motor units, thereby enhancing skeletal muscle performance.
A The representative immunohistochemical images of extensor digitorum longus (EDL) muscle cells illustrated the localization of fast myosin and slow myosin in R1, P8-PBS and P8-MSC mice. The square denoted the same muscle cell (scale bar = 100 μm). B The percentage of fast myosin and slow myosin in EDL muscle cells of SAMP8 mice (PR1 & PBS = 0.0173; PMSC & PBS = 0.0050) (n = 8 or 10 views per group from 5–6 male mice; all data shown as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001).
hUC-MSCs regulated the extracellular matrix of muscle cells in AAS mouse models
The extracellular matrix (ECM) plays a crucial role in the growth of muscle cells by creating the cellular niche and mediating the signal transduction [30]. With aging, the ECM surrounding muscle cells could undergo change, such as decreased stability of the muscle fiber sarcolemma (MFS) and weakened cell adhesion function [31]. In order to describe the changes in AAS models, we detected the expression of dystrophin and laminin, which played critical roles in stabilizing the sarcolemma of muscle fiber and participate in cell communication [31,32,33,34]. The immunofluorescent images showed that the muscle cells in SAMP8 mice exhibited lower levels of dystrophin and laminin proteins expression than those in the R1 mice. Following treatment with hUC-MSCs, the expression levels of dystrophin and laminin were noticeably increased, implying restoration of the muscle cells ECM (Fig. 3). Similarly, the elevated expressions of dystrophin and laminin in the D-gal-induced aging model were also observed after hUC-MSCs treatment (Fig. S4). These findings indicated that hUC-MSCs preserved muscle cell adhesion, improved their microenvironment and advanced muscle toughness and tensile strength in AAS by restoring the muscle ECM.
A The representative immunohistochemical images of ECM depicted dystrophin protein expression in the SAMP8 mouse model (scale bar = 100 μm), with individual cells expressing positive protein shown under high magnification within the square (scale bar = 25 μm). The percentage of dystrophin among different groups in the visual field area was quantified (PR1 & PBS = 0.0001; PMSC & PBS = 0.0001) (C) (n = 8 or 10 views per group from 5–6 male mice). B Representative immunohistochemistry images indicated the expression of Laminin protein in SAMP8 mice (scale bar = 50 μm). The average fluorescence value of Laminin protein expression was quantified according to the random visual field in different SAMP8 mice groups (PR1 & PBS = 0.0032; PMSC & PBS = 0.0001) (D) (n = 8 or 10 views per group from 5–6 male mice; all data shown as mean ± SEM, *P < 0.05, **P < 0.01, *** P < 0.001).
hUC-MSCs restrained the decline in the number of muscle satellite cells in two mouse models
The decline in regenerative capacity of skeletal muscle with aging is attributed to the depletion and exhaustion of muscle stem cells (MuSCs), also known as satellite cells. To decipher whether hUC-MSCs could prevent this decline by reducing the depletion of MuSCs, we analyzed the expression of Pax7, the specific marker of MuSCs in skeletal muscle cells [35, 36]. Fluorescence image and Western blot revealed that the quantity of Pax-7+ cells in SAMP8 mice was lower than in R1 mice. Nevertheless, the number of MuSCs was significantly recovered after hUC-MSC treatment (Fig. 4). Moreover, in the D-gal-induced aging model, intraperitoneal injection of hUC-MSCs restored the number of MuSCs (Fig. S5). These results suggested that hUC-MSCs treatment can significantly recover the number of MuSCs in two AAS models, which maintained the stability of the stem cell pool, promoted proliferation and differentiation, and remodeled muscle fibers.
A, B Representative immunohistochemical images of satellite cells in skeletal muscle showed the expression of Pax-7, and the number of Pax-7+ cells in different visual fields in SAMP8 mice was calculated (PR1 & PBS < 0.0001; PMSC & PBS < 0.0001; PR1 & MSC = 0.0006) (scale bar = 25 μm; n = 8 or 10 views per group from 5–6 male mice). C, D The expression of Pax-7 in gastrocnemius muscle of R1, P8-PBS and P8-MSC groups was detected by western blot and statistically analyzed (PR1 & PBS = 0.0691; PMSC & PBS = 0.0001; PR1 & MSC = 0.0008) (n = 3 per group; all data shown as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001).
hUC-MSCs increased autophagy and delayed muscle cells senescence via p16-Rb/p53-p21 axis
It is widely acknowledged that cellular autophagy is closely intertwined with senescence. Decreased autophagy may hasten the aging process, whereas increased autophagy holds the potential for anti-aging effects [37]. To investigate how hUC-MSCs could ameliorate the muscle dysfunctions in AAS mouse models by regulating muscle autophagy, we evaluated the expression levels of Lamp2 [38] and LC3- II/I [39], which are autophagy-related biomarkers. The western blot images showed the expression levels of Lamp2 and LC3- II/I in the P8-MSC group (Fig. 5A, B) and the D-gal-MSC group (Fig. S6A, B) were remarkably elevated as compared to the aging group, indicating that autophagy was stimulated in the presence of hUC-MSCs.
A, B, C, D The expressions of Lamp2(PR1 & PBS = 0.0151; PMSC & PBS = 0.0243), LC3B II/I (PR1 & PBS = 0.0193; PMSC & PBS = 0.0016), p16(PR1 & PBS = 0.0011; PMSC & PBS = 0.0020) and p53(PR1 & PBS = 0.0001; PMSC & PBS = 0.0001) in muscle cells of R1, P8-PBS and P8-MSC mice were detected by western blot (n = 3 per group; all data shown as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001). E, F Representative immunofluorescence images showed the expression of p16(PR1 & PBS < 0.0001; PMSC & PBS < 0.0001; PR1 & MSC = 0.0221), p53(PR1 & PBS = 0.0005; PMSC & PBS = 0.0003) and p21(PR1 & PBS = 0.0001; PMSC & PBS = 0.0001) in skeletal muscle in R1, P8-PBS and P8-MSC mice (scale bar = 50 μm) and quantified its expression based on the mean fluorescence value in various perspectives of view (n = 8 or 10 views per group from 5–6 male mice; all data shown as mean ± SEM, ***P < 0.001).
Several pathways, such as inhibition of p53, p16, and p21 activation, are implicated in the acceleration of senescence [40] and the inhibition of autophagy [41]. Western blot and immunofluorescence images demonstrated that the expression levels of p16, p53 and p21 were decreased in the P8-MSC group (Fig. 5C–F) and the D-gal-MSC group (Fig. S6C–F) as compared to the P8-PBS and the D-gal-PBS groups, respectively, suggesting that hUC-MSCs treatment could inhibit the activation of senescence-related pathways.
Collectively, hUC-MSCs played a pivotal role in revitalizing myocyte autophagy to furnish self-energy supply, and it could downregulate the classic p16 / p53-p21 axis to decelerate myocyte aging.
Discussion
AAS, the primary clinical malady affecting the elderly, poses a significant challenge for achieving healthy aging [Immunohistochemical experiment The mice were perfused transcardially with ice-cold saline, followed by 50 mL of 4 % paraformaldehyde (PFA), took out their muscle tissue, and fixed in 4 % PFA overnight. Each tissue was embedded in paraffin and sectioned coronally into 5-μm-thick slices. A standard histological immunohistochemical protocol was performed, which involved dewaxing and rehydration of the sections, incubating them with 3% H2O2 in methanol, and retrieving the antigen by placing the slides in target retrieval solution at 95 °C for 20 min. The sections were then incubated overnight with Monoclonal Anti-Myosin (Skeletal, Fast) antibody (Sigma, Germany, M4276), Monoclonal Anti-Myosin (Skeletal, Slow) antibody (Sigma, Germany, M8421), Rabbit polyclonal to Anti-Dystrophin (Abcam, USA, ab15277), or Anti-Myosin Heavy Chain Antibody (Sigma, Germany, 05-716) at 4 °C. After incubation with secondary antibody at room temperature for 30 min, the slides were incubated with HRP-peroxidase complex at room temperature for 30 another minutes. Reaction products were visualized using 3,3-diaminobenzidine (DAB) for counterstaining. The sections of the gastrocnemius muscle were subjected to dewaxing and rehydration, followed by fixation in pre-cooled acetone at 4 °C for 25 min, and permeabilization in PBS containing 0.1% Triton X-100 for 15 min. The sections were then blocked for 1 h with 10 % normal Donkey Serum, before incubation with Rabbit polyclonal to Anti-Laminin (Abcam, USA, ab11575) or Anti-Pax7 Rabbit pAb antibody (Servicebio, China, GB113190) and Anti-p21 Rabbit pAb (Servicebio, China, GB11153). Incubation was carried out overnight at 4 °C followed by washing with PBS three times and incubation with the secondary antibody (Invitrogen, USA) for one hour at room temperature, followed by another round of washing. The nucleus was observed using 2 μM DAPI (Servicebio, China, G1012). The sections of the gastrocnemius muscle underwent dewaxing and rehydration procedures, followed by hematoxylin staining for a duration of 10 min. The sections were then washed with running water, differentiated with 0.7% hydrochloric acid and ethanol for a few seconds, and rinsed with tap water. After 15 min, the sections were stained with 95% ethanol, alcohol-based eosin, 95% ethanol (I, II), 100% ethanol (I, II), xylene and xylene (I, II) for 30 seconds each, and finally covered a film. H&E staining was used for distinguishing skeletal muscle (red). Western blots were performed and analyzed as previously described [22]. Briefly, gastrocnemius muscle samples were homogenized in RIPA buffer, and then centrifuged at 12000 × g for 30 min at 4 °C. The quantification of protein in supernatant was accomplished using a BCA kit (Beyotime Biotechnology, Wuhan, China). The protein samples were boiled in the presence of sample buffer at 95 °C for 3 min. The proteins were subjected to the separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto a nitrocellulose membrane. The target protein was probed by a corresponding antibody and then visualized by enhanced chemiluminescence (ECL) reagent and imaged by the chemiluminescence imaging system Amersham Imager 680 (General Electric Company, USA). Antibodies used for Western blots were: Anti-Pax7 (Abcam, USA, ab199010), Anti-alpha smooth muscle Actin (Abcam, USA, ab5694), Anti-LAMP2-Lysosome Marker (Abcam, USA, ab25631), Anti-Beta actin (Abcam, USA, ab8226), Anti-Cleaved LC3B (Sigma, Germany, L7S43), Anti-ARPC5/p16 ARC (Abcam, USA, ab51243), p53 (1C12) Mouse mAb (Cell Signaling Technology, USA, 1C12). Origin images of all western blot have been uploaded as a single ‘Supplemental Material’ file. The cell density, nuclear area, cell area, and antibody expression in Immunohistochemical experiment, Immunofluorescent staining and Hematoxylin-Eosin staining were acquired via images by Tissue FAXS (Tissue Gnostics GmbH, Vienna Austria) with a Zeiss Axio Imager Z2 Microscope System at ×200 magnification. The cross-sectional area of 500 selected skeletal muscle fibers in the stained sections was measured and calculated using ImageJ software (version 1.8.0, National Institutes of Health) and Image-Pro Plus (version 6.0.0, media cybernetics). Statistical analyses were executed with GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA) and presented as the mean value accompanied by the plus or minus standard error of the mean. The statistical significance of the differences among the three groups was determined using one-way analysis of variance (ANOVA), followed by Tukey post hoc tests, as depicted in the bar graph. A value of P <0.05 was considered to be statistically significant.Immunofluorescent staining
Hematoxylin-Eosin staining
Western blot analysis
Quantitative and statistical analysis
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Jt Bone Spine. 2019;86:309–14.
Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–4.
Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradere JP, Le Gonidec S, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24:1360–71.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–46.
Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle. 2019;10:956–61.
Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7.
Pasco JA, Williams LJ, Jacka FN, Stupka N, Brennan-Olsen SL, Holloway KL, et al. Sarcopenia and the Common Mental Disorders: a Potential Regulatory Role of Skeletal Muscle on Brain Function? Curr Osteoporos Rep. 2015;13:351–7.
Martinez BP, Batista AK, Gomes IB, Olivieri FM, Camelier FW, Camelier AA. Frequency of sarcopenia and associated factors among hospitalized elderly patients. BMC Musculoskelet Disord. 2015;16:108.
Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev. 2012;33:314–77.
Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20.
Magatti D, Molteni M, Cardinali B, Rocco M, Ferri F. Modeling of Fibrin Gels Based on Confocal Microscopy and Light-Scattering Data. Biophys J. 2017;112:410.
Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev. 2014;34:1100–26.
Sandona M, Di Pietro L, Esposito F, Ventura A, Silini AR, Parolini O, et al. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front Bioeng Biotechnol. 2021;9:652970.
Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, et al. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev. 2016;2016:9047821.
Kim MJ, Kim ZH, Kim SM, Choi YS. Conditioned medium derived from umbilical cord mesenchymal stem cells regenerates atrophied muscles. Tissue Cell. 2016;48:533–43.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7.
Park CM, Kim MJ, Kim SM, Park JH, Kim ZH, Choi YS. Umbilical cord mesenchymal stem cell-conditioned media prevent muscle atrophy by suppressing muscle atrophy-related proteins and ROS generation. Vitr Cell Dev Biol Anim. 2016;52:68–76.
Li TS, Shi H, Wang L, Yan C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med Sci Monit. 2016;22:4651–60.
Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell 2012;10:709–16.
Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–73.
Cao N, Liao T, Liu J, Fan Z, Zeng Q, Zhou J, et al. Clinical-grade human umbilical cord-derived mesenchymal stem cells reverse cognitive aging via improving synaptic plasticity and endogenous neurogenesis. Cell Death Dis. 2017;8:e2996.
Jia Y, Cao N, Zhai J, Zeng Q, Zheng P, Su R, et al. HGF Mediates Clinical‐Grade Human Umbilical Cord‐Derived Mesenchymal Stem Cells Improved Functional Recovery in a Senescence‐Accelerated Mouse Model of Alzheimer’s Disease. Adv Sci 2020;7:1903809.
Piao L, Huang Z, Inoue A, Kuzuya M, Cheng XW. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem cell Res Ther. 2022;13:226.
Manini TM, Clark BC. Letter to the editor: results from a Web-based survey to identify dynapenia screening tools and risk factors. J Cachexia Sarcopenia Muscle. 2016;7:499–500.
Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–6.
Murach KA, Dungan CM, Kosmac K, Voigt TB, Tourville TW, Miller MS, et al. Fiber ty** human skeletal muscle with fluorescent immunohistochemistry. J Appl Physiol. 2019;127:1632–9.
Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–90.
Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–94.
Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–7.
Carmignac V, Durbeej M. Cell-matrix interactions in muscle disease. J Pathol. 2012;226:200–18.
Labat-Robert J. Cell-matrix interactions in aging: role of receptors and matricryptins. Ageing Res Rev. 2004;3:233–47.
Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–28.
Haenggi T, Fritschy JM. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cell Mol Life Sci. 2006;63:1614–31.
Hunter DD, Porter BE, Bulock JW, Adams SP, Merlie JP, Sanes JR. Primary sequence of a motor neuron-selective adhesive site in the synaptic basal lamina protein S-laminin. Cell 1989;59:905–13.
Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015;21:854–62.
Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–42.
Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–6.
Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104.
Sun Y, Berleth N, Wu W, Schlutermann D, Deitersen J, Stuhldreier F, et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 2021;12:1028.
Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–96.
Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011;146:682–95.
**e WQ, He M, Yu DJ, Wu YX, Wang XH, Lv S, et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle. 2021;12:538–54.
Sepulveda-Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:1164–76.
Rolland Y, Czerwinski S, Abellan VKG, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–50.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23.
Papachristou E, Ramsay SE, Lennon LT, Papacosta O, Iliffe S, Whincup PH, et al. The relationships between body composition characteristics and cognitive functioning in a population-based sample of older British men. BMC Geriatr. 2015;15:172.
Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Disco. 2015;14:58–74.
Reyhani S, Abbaspanah B, Mousavi SH. Umbilical cord-derived mesenchymal stem cells in neurodegenerative disorders: from literature to clinical practice. Regen Med. 2020;15:1561–78.
Wang QQ, **g XM, Bi YZ, Cao XF, Wang YZ, Li YX, et al. Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stromal Cells May Attenuate Sarcopenia in Aged Mice Induced by Hindlimb Suspension. Med Sci Monit. 2018;24:9272–81.
Derave W, Eijnde BO, Ramaekers M, Hespel P. Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp Gerontol. 2005;40:562–72.
Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM). Exp Gerontol. 1997;32:117–27.
Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol. 2010;48:626–32.
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res. 2006;84:647–54.
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC, Liu J. The relation between D-galactose injection and mitochondrial DNA 4834 bp deletion mutation. Exp Gerontol. 2006;41:628–34.
Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, et al. D-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology 2007;8:373–81.
Azman KF, Zakaria R. D-Galactose-induced accelerated aging model: an overview. Biogerontology 2019;20:763–82.
Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol 1985;45:397–458.
Sullivan VK, Powers SK, Criswell DS, Tumer N, Larochelle JS, Lowenthal D. Myosin heavy chain composition in young and old rat skeletal muscle: effects of endurance exercise. J Appl Physiol. 1995;78:2115–20.
Messa G, Piasecki M, Hill C, McPhee JS, Tallis J, Degens H. Morphological alterations of mouse skeletal muscles during early ageing are muscle specific. Exp Gerontol. 2019;125:110684.
Scott RW, Arostegui M, Schweitzer R, Rossi F, Underhill TM. Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell. 2019;25:797–813.
Uezumi A, Ikemoto-Uezumi M, Zhou H, Kurosawa T, Yoshimoto Y, Nakatani M, et al. Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia. J Clin Invest. 2021;131:e139617.
Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–32.
Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75.
Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–77.
Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science 2008;319:1352–5.
Sousa-Victor P, Garcia-Prat L, Munoz-Canoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2022;23:204–26.
Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, et al. Autophagy maintains stemness by preventing senescence. Nature 2016;529:37–42.
Hunsberger JG, Rao M, Kurtzberg J, Bulte J, Atala A, LaFerla FM, et al. Accelerating stem cell trials for Alzheimer’s disease. Lancet Neurol. 2016;15:219–30.
Byrne AB, Garcia AG, Brahamian JM, Mauri A, Ferretti A, Polack FP, et al. A murine model of dengue virus infection in suckling C57BL/6 and BALB/c mice. Anim Model Exp Med. 2021;4:16–26.
Acknowledgements
This work was supported by grants from National Key Research and Development Program of China (2017YFA0103100 and 2017YFA0103103 to XTP), Guangzhou Health Care and Cooperative Innovation Major Project (201604020009 to YLJ, 201803040005 to WY) and The National Nature Science Foundation of China (81671385 and 82271467 to YLJ).
Funding
This work was supported by grants from National Key Research and Development Program of China (2017YFA0103100 and 2017YFA0103103 to XTP), Guangzhou Health Care and Cooperative Innovation Major Project (201604020009 to YLJ, 201803040005 to WY) and The National Nature Science Foundation of China (81671385 and 82271467 to YLJ).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YLJ, CW, BCZ, JLZ, ALW, NC, TLL, RYS, LJH and YHL. The first draft of the manuscript was written by YLJ, CW, BCZ, ALW, WY and XTP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Financial support and final approval of the manuscript were XTP, WY and YLJ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
For human specimens, the Ethics Committee approved all procedures involving human subjects in this study at the Sun Yat-Sen Memorial Hospital (Approval number: 2021-01-01). For animal experiments, The Committee approved the Ethics of Animal Experiments of the Academy of Military Medical Sciences.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Anastasis Stephanou
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Zhao, B., Zhai, J. et al. Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice. Cell Death Dis 14, 321 (2023). https://doi.org/10.1038/s41419-023-05843-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05843-8
- Springer Nature Limited