Abstract
The metabolic pathways through which p53 functions as a potent tumor suppressor are incompletely understood. Here we report that, by associating with the Vitamin D receptor (VDR), p53 induces numerous genes encoding enzymes for peroxisomal fatty acid β-oxidation (FAO). This leads to increased cytosolic acetyl-CoA levels and acetylation of the enzyme 5-Aminoimidazole-4-Carboxamide Ribonucleotide Formyltransferase/IMP Cyclohydrolase (ATIC), which catalyzes the last two steps in the purine biosynthetic pathway. This acetylation step, mediated by lysine acetyltransferase 2B (KAT2B), occurs at ATIC Lys 266, dramatically inhibits ATIC activity, and inversely correlates with colorectal cancer (CRC) tumor growth in vitro and in vivo, and acetylation of ATIC is downregulated in human CRC samples. p53-deficient CRCs with high levels of ATIC is more susceptible to ATIC inhibition. Collectively, these findings link p53 to peroxisomal FAO, purine biosynthesis, and CRC pathogenesis in a manner that is regulated by the levels of ATIC acetylation.
Similar content being viewed by others
Introduction
As a master tumor suppressor, p53 inhibits proliferation and tumorigenesis in a variety of ways that include the induction of cell cycle arrest and the promotion of apoptosis and senescence [1, 2]. More recent studies have uncovered additional roles for p53 in metabolism, including the regulation of glycolysis; the pentose phosphate pathway; mitochondrial oxidative phosphorylation, and amino acid, nucleotide, and lipid biosynthesis [2]. Accumulating evidence suggests that this metabolic regulation is a key aspect of p53’s diverse tumor suppressive activities [3], but many questions remain unresolved.
Dysregulation of fatty acid β-oxidation (FAO) is a frequent feature of cancer metabolic reprogramming [Full size image
To determine whether VDREs regulate peroxisomal FAO genes expression, we cloned WT or mutant VDREs into the promoter region of a firefly luciferase reporter plasmid and observed only the former to be active (Fig. 2e). Luciferase reporters containing peroxisomal FAO gene promoter elements with WT VDREs but not with mutant VDREs were also dramatically up-regulated when treated with Nutlin3, suggesting that the regulation of peroxisomal FAO genes expression by p53 is also VDR dependent (Fig. 2e). Consistent with this, ChIP analysis showed more pronounced binding of VDR to the promoters of peroxisomal FAO pathway genes in p53+/+ cells versus p53−/− cells (Figs. 2f and S2c).
Given the apparent importance of the VDR in promoting the p53 response of peroxisomal FAO genes, we next explored its mechanism of action. Confirming previous findings that VDR is a direct transcriptional target of p53 [23], Nutlin3-treated HCT116 cells, but not p53−/− HCT116 cell, significantly increased VDR mRNA and protein expression (Figs. S2d and S2e). Employing co-immunoprecipitation (Co-IP) experiments, we confirmed previous reports that both WT and mutant p53 interact with the VDR [24] and also found this association to be mediated through the p53 C-terminal regulatory domain (CTD) and the VDR activation function-2 (AF2) domain (Fig. S2f-h). Cell fractionation further demonstrated that p53 reactivation by Nutlin3 caused substantial nuclear translocation of VDR in some cells (Fig. S2i). Collectively, these results strongly suggest that p53 directly interacts with the VDR to enhance peroxisomal FAO-related gene transcription.
Acetyl-CoA from p53-mediated peroxisomal FAO regulates ATIC activity
Peroxisomes play an essential role in lipid metabolism, with each round of FAO producing one mole of a shorter fatty acyl-CoA (-2C), one mole of H2O2, and one mole of acetyl-CoA [5] (Fig. 3a). In this context, we found the total cytosolic acetyl-CoA levels in AOM/DSS CRCs from VP mice tissues and p53−/− cells to be significantly lower than in their WT counterparts (Fig. 3b). Moreover, in a [2H]-docosanoic acid isotopic tracer experiment, the amount of labeled acetyl-CoA (m + 1) derived from peroxisomal FAO was significantly reduced in p53-deficient cells (Fig. 3c) and no detectable differences in H2O2 levels were seen (Fig. S3a). Previous reports indicated that p53 promotes mitochondrial FAO under metabolic stress [2, 25], which may contribute to the increase of cytosolic acetyl-CoA levels. However, [13C]-palmitic acid labeling experiment revealed that the cytosolic acetyl-CoA (M + 2) levels in p53 KD cells to be significantly higher than in p53 WT cells (Fig. 3d). Cytosolic acetyl-CoA is generated primarily from citrate by the lipogenic enzyme ACLY. Secondly, acyl-CoA synthetase short-chain family, member 2 (ACSS2), employs acetate to produce acetyl-CoA in an ATP-dependent manner (Fig. 3a) [9]. Remarkably, the expression of ACLY markedly increased in VP mice, while ACOX1 expression decreased (Fig. 3e). Correspondingly, activation p53 significantly decreased ACLY expression, while ACOX1 increased in CRC cells (Fig. S3b). There was no significant change in ACSS2 expression observed in vitro and in vivo, regardless of treatment (Figs. 3e and S3b). Thus, these results demonstrate the important role of peroxisomal FAO in cytosolic acetyl-CoA production in CRC cells and MEFs with wild-type p53.
a A schematic diagram of the mitochondrial and peroxisomal FAO in mammalian cells and cytosolic acetyl-coA production. ACLY, ATP-citrate lyase; ACSS2, Acyl-CoA synthetase short-chain family members 2; ACOX1, Acyl-CoA oxidase 1. b Cytosolic acetyl-CoA measurement in the tumor tissues from CRC mouse model and HCT116 cells, n = 3 or 5. c Docosanoic-d43 acid isotope profiling analysis revealed acetyl-CoA (m + 1) metabolism is suppressed when p53 deletion in HCT116 and MEFs, n = 3. d Docosanoic-d43 acid and palmitate (13C16) isotope profiling analysis was performed in the HCT116 cells, n = 3. e Western blot analysis of ACLY, ACSS2, and ACOX1 expression in n CRC tumors from Trp53fl/fl and VP mice, n = 3. f Top acetylation of metabolic enzymes that are significantly associated with p53 KO. g A schematic diagram of the purine biosynthesis pathway. h Lysine acetylation of endogenous ATIC was analyzed in CRC tumors from Trp53fl/fl and VP mice by immunoprecipitation using an ATIC antibody, followed by western blot analysis using AcK antibody. i Effect of p53 deletion on ATIC activity in CRC tumors from CRC mouse model, HCT116 and MEFs, n = 3 or 6. j Relative purine nucleotide levels were analyzed by LC-MS/MS in CRC tumors from Trp53fl/fl and VP mice and normalized to per mg tissue, n = 3. Data were presented as mean ± SD. ns, non-significant, P > 0.05.
Beyond its role in energy metabolism and biosynthesis, acetyl-CoA also functions as the acetyl group donor for many proteins [9]. To identify those whose acetylation was regulated by p53, we use MS to analyze all acetylated peptides from the CRCs of Trp53fl/fl and VP mice (Extended Data Table 4). The latter showed a relative paucity of acetylated proteins functioning in glycolysis, the pentose phosphate pathway, fructose and mannose metabolism, the biosynthesis of amino acids, and purine metabolism. The most prominent of these differences were in AKR1C13, PPIB, RACK1, DDX5, and ATIC (Fig. 3f). Because GSEA of our RNA-seq dataset in VP tissues had shown selective enrichment of transcripts involved in the nucleotide biosynthesis and ATIC is the only one of these proteins involved in purine metabolism, we focused on this protein (Fig. S3c).
ATIC is a bifunctional enzyme that catalyzes the last two steps of de novo purine biosynthesis (Fig. 3g) [19]. We, therefore, investigated the role of ATIC in the p53 tumor suppressor network and confirmed that CRCs from VP mice had lower levels of acetylated ATIC (Fig. 3h). Both the activation of p53 by Nutlin3 and the ectopic expression of WT p53 in CRC cells or MEFs dramatically increased the acetylation of both endogenous and exogenous ATIC whereas depleting p53 had the opposite effect (Figs. S3d and S3e). Furthermore, ACOX1 inhibition also led to a decrease in acetyl-CoA levels and ATIC acetylation, which could be reversed by re-expressing ACOX1, while acetylation of histone H3 was unaffected (Fig. S3f).
The acetylation of metabolic enzymes plays important role in regulating their activities [26]. Given that p53 enhanced ATIC acetylation, we next investigated whether this affected ATIC activity. We first found that p53 deletion in WT cells markedly increased ATIC activity whereas p53 activation had the opposite effect (Figs. 3i and S3g). Moreover, p53 knockout also resulted in significantly enhanced purine synthesis in VP mice with CRCs (Fig. 3j). Next, we used MS to identify three acetylated lysine residues (K266, K356, and K524) in ATIC (Extended Data Table 6). We mutated each of these to arginine (R) and found the K266R mutant to be associated with both significantly reduced ATIC acetylation and increased enzymatic activity (Fig. S3h). This indicated that K266 is an acetylation site that negatively regulates ATIC’s activity. This was supported by the finding that K266 is highly conserved across species (Fig. S3i). The K266R mutant also demonstrated higher ATIC activity that was no longer responsive to Nutlin3 and thereby indicating that p53 inhibits ATIC activity by promoting K266 acetylation (Fig. S3j). Collectively, these data show that acetyl-CoA derived from p53-mediated peroxisomal FAO inhibits ATIC activity by promoting ATIC acetylation.
p53 increases ATIC acetylation and promotes ATIC degradation by transcriptionally activating KAT2B
To identify the acetyltransferase(s) responsible for ATIC K266 acetylation, we examined the expression of 13 KAT family member acetyltransferases following Nutlin3 treatment and found only KAT2B expression to be significantly increased in both HCT116 and HIEC cells (Figs. 4a, S4a, and S4b). We also overexpressed several classical acetyltransferases and found that only KAT2B significantly increased ATIC acetylation (Figs. 4b and S4c). Furthermore, KAT2B proved capable of acetylating WT-ATIC and all its mutants except K266R (Fig. S4d). Conversely, depleting KAT2B significantly decreased ATIC acetylation in a manner that could be rescued by KAT2B re-expression (Fig. S4e). An endogenous interaction between KAT2B and ATIC was also documented in CRC cells by Co-IP experiments (Fig. S4f). We also found that inhibiting KAT2B significantly reduced ATIC acetylation and increased its activity in a manner that could not be reversed by Nutlin3 or ACOX1 overexpression (Figs. 4c and 4d). Taken together, these findings show ATIC K266 to be a specific site of KAT2B-mediated acetylation that is mandatory to suppress ATIC activity and that the KAT2B gene is itself a direct transcriptional target of p53.
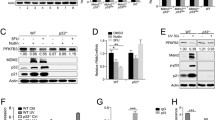
a HCT116 p53+/+ and p53−/− cells were treated with either DMSO or Nutlin3 for 48 h, and the expression of 13 KAT family acetyltransferases genes were assessed by qRT-PCR, n = 3. b Western blot analysis of ATIC acetylation in HCT116 cells with overexpression of KATs. Signal intensity of Ac-ATIC protein was quantified by Image J, the acetylation level of ATIC as indicated was normalized against immunoprecipitated ATIC. c HCT116 cells expressing shCtrl or KAT2B shRNA further infected with control or Flag-ACOX1 vector, followed by western blot analysis of ATIC acetylation (left) and enzyme activity (right), n = 3. d HCT116 cells expressing shCtrl or KAT2B shRNA further treated with or without Nutlin3 for 24 h, followed by western blot analysis of ATIC acetylation (left) and enzyme activity (right), n = 3. e Protein expression of ATIC in CRC cells treated with or without Nutlin3 for 48 h. f ATIC immunoblots of lysates from HCT116 cells treated with CQ (50 μM), 3-MA (10 mM), or MG132 (10 μM) for 10 h before harvesting. g HCT116 p53+/+ and p53−/− cells treated with or without CQ (50 μM) for 10 h, followed by western blot analysis of ATIC expression. Data were presented as mean ± SD. **P < 0.01, ***P < 0.001.
To gain mechanistic insight into p53-mediated inhibition of ATIC activity, we found that p53−/− HCT116 cells express more ATIC protein than their p53+/+ counterparts and that Nutlin3 decreased ATIC protein without affecting its mRNA levels (Figs. 4e and S4g). Chloroquine (CQ), a lysosome inhibitor, could block ATIC degradation (Fig. 4f). Furthermore, ATIC’s half-life was significantly shortened when treated with Nutlin3 (Fig. S4h). Consistent with these findings, ATIC-K266Q and ATIC-K266R had markedly shorter and longer, half-lives, respectively (Fig. S4i). Moreover, a significant difference was not observed at protein level of ATIC when treated with CQ in HCT116 p53+/+ and HCT116 p53−/− cells (Fig. 4g) and degradation through ATIC acetylation by p53 was interdicted (Figs. 4g and S4j). Thus, these results suggest that p53 promotes ATIC degradation through acetylation by KAT2B, which consequently suppresses ATIC activity.
Acetylation of ATIC is correlated to colorectal tumorigenesis
We next explored the effect of ATIC K266 acetylation on tumor growth. To this end, CRCs were induced in Trp53fl/fl or VP mice by AOM/DSS, followed by intraperitoneal injection with adeno-associated vectors (AAV) encoding WT-ATIC, ATIC-K266R, ATIC-K266Q or a control empty vector and ATIC expression was confirmed by immunohistochemistry (IHC) and western blot (Figs. 5a, 5b, S5a and S5b). Ectopic expression of ATIC-K266R promoted more rapid tumor growth compared with that of WT-ATIC in both Trp53fl/fl and VP mice (Figs. 5a and 5c). We found that Trp53 KO mice showed significantly enhanced tumor growth; even so, delivery of ATIC-K266Q inhibited tumor growth compared with WT-ATIC even in VP mice (Figs. 5a and 5c). Human CRC cells expressing ATIC-K266R also proliferated faster than those expressing WT-ATIC (Fig. S5c). However, K266Q mutant still significantly inhibited cell proliferation even in p53-deficient CRC cells (Fig. S5c). Interestingly, when p53 was stably expressed in HCT116 cells, the WT-ATIC exhibited inhibiting effect like K266Q mutant on cell proliferation (Fig. S5d). However, the K266R mutant still strongly accelerated cell proliferation (Fig. S5d), indicating that p53-induced highly acetylated ATIC had a similar effect of the mimicked K266Q mutant on suppressing tumor growth and cell proliferation. Furthermore, the K266Q mutant led to a greatly reduction of ATIC enzyme activity and purine nucleotide levels (IMP, AMP, and GMP), while the K266R mutant showed the opposite effects compared with those with K266Q mutant (Figs. 5d, 5e, S5e and S5f). Interestingly, exogenous expression of ATIC-K266Q partially promote tumor growth compared with AAV-Ctrl (Figs. 5a, 5c and S5c). This implies that other post‐translational modifications may also play a role in regulating ATIC activity.
a A Scheme for the AOM/DSS-induced colon cancer model in Trp53fl/fl and VP mice (top). Each mouse received two intraperitoneal injections of the indicated virus during the second processing of DSS treatment. Typical images of colon tumors expressing the indicated ATIC proteins 98 days after AOM/DSS treatment. b ATIC immunohistochemistry (IHC) in representative sections of tumor tissues from a. Scale bar, 20 μm. c Colon tumor numbers in mice from a, n = 6. d ATIC activity and ATP levels were measured in tumor tissues from a, n = 6. e Relative purine nucleotide levels were analyzed by LC-MS/MS in CRC tumors from Trp53fl/fl and VP mice and normalized to per mg tumor tissue, n = 3. f–h HCT116 cells with stable expression the indicated vectors were injected subcutaneously into BALB/c nude mice, n = 6. Tumor size was measured every 3 days f. Tumors were dissected and photographed (g) and weighed on day 18 (h). i Western blot analysis of ATIC acetylation in tumor tissues from each group, as in f, n = 3. j ATIC activity was measured in tumor tissues from f, n = 6. Data were presented as mean ± SD.
Taken together, these results demonstrate that ATIC acetylation plays an important role in the regulation of CRC growth.
Having shown the importance of ATIC acetylation inhibits CRC growth, we next explored the role of ACOX1 in this process. Overexpression of ACOX1 significantly inhibited tumor growth and tumor weights in both p53 WT and KD cells (Fig. 5f–h). Western blot analysis confirmed that ATIC acetylation significantly increased when overexpression of ACOX1 (Fig. 5i). Meanwhile, ACOX1 overexpression also effectively inhibited ATIC activity in mouse xenograft tumor tissues (Fig. 5j). Together, these findings suggest that ACOX1-mediated ATIC acetylation is critical for tumor suppression.
Targeting ATIC suppresses colorectal tumorigenesis
Cpd14 is a small-molecule inhibitor that prevents the homodimerization and activation of ATIC (Fig. 6a) and thereby inhibits the proliferation of MCF-7 breast cancer cells and HCT116 cells [27, 28]. We found that Cpd14 also dramatically suppressed tumor growth rates and total tumor burden in AOM/DSS-induced CRC and ApcMin/+ mice. This was more pronounced VP and AP mice than in control mice (Figs. 6b–d and S6b-d), indicating that p53-deficient tumors were more sensitive to ATIC inhibition. Cpd14 treatment also more significantly reduced ATIC activity and ATP levels in VP and AP mice (Figs. 6e and S6e). Of note, Cpd14 treatment resulted changes in purine nucleotide pools that included the accumulation of AICAR and the depletion of IMP, XMP, AMP, and ATP, all of which were consistent with the intended inhibition of ATIC (Fig. 6f).
a A scheme of Cpd14 treatment during AOM/DSS-induced Trp53fl/fl and VP mouse CRC model (left). The chemical structure of Cpd14 (right). b Typical images of colon tumors from Control and Cpd14-treated mice 42 days after Cpd14 treatment. c Colon tumor numbers in mice from a, n = 6 or 8. d Tumor inhibition rate for Cpd14-treated mice relative to control mice in p53 WT and p53-deficient mice, n = 6 or 8. e ATIC activity (left) and ATP levels (right) in tumor tissues from AOM/DSS-induced CRC mouse model, n = 6. f Purine metabolite abundance were determined by LC-MS/MS in AOM/DSS-induced CRC mouse treated with Cpd14 or not, n = 3. g–i Schematic depicting experimental setup. Nude mice bearing HCT116 tumors were intraperitoneally administered 5-Fluorouracil (5-FU) and Cpd14, either alone or in combination, n = 6. Tumor size was measured every 3 days (g). Tumors were dissected and photographed (h) and weighed on day 18 (i). Data were presented as mean ± SD.
We also evaluated the potential synergy between Cpd14 and 5-Fluorouracil (5-FU) on cell-derived xenografts (CDX) in BALB/c nude mice (Fig. 6g). Treatment with either compound alone was effective in reducing the growth of both p53 WT and p53 KD cells. The combination of Cpd14 and 5-FU provided no additional benefit over 5-FU alone in p53 WT cells but did prove effective in p53 KD cells suggesting that p53 knockdown tumor cells were more sensitive to combination treatment (Fig. 6g–i).
To further verify the above results, we next determined whether inhibiting ATIC by AAV2-Atic shRNA delivery could delay CRC. This experiment was designed as illustrated in Fig. S6f and immunohistochemistry (IHC) and western blot analysis confirmed the KD of Atic (Figs. S6g and S6h). Essentially identical to the degree of CRC growth inhibition obtained with Cpd14 treatment, Atic KD diminished tumor burden by ~85% in VP mice and by ~65% in Trp53fl/fl mice (Figs. S6f, S6i and S6j). The effects on ATIC activity and ATP levels were also more obvious in VP mice (Fig. S6k). In vitro studies also revealed directly that ATIC KD significantly repressed the proliferation of human CRC cells which reduced ATIC enzyme activity and ATP levels (Figs. S6l and S6m) with the effects again being more pronounced in p53−/− cells.
ATIC acetylation is downregulated in human CRCs
To further study the potential clinical relevance of the above-described novel p53-KAT2B-ATIC axis, we collected 45 pairs of human CRC samples (T) with adjacent normal colon tissues (N) along with detailed pathological and clinical information (Extended Data Table 8). Among these samples, 30 cases of p53 WT samples for analysis. In addition, p53, ACOX1 and KAT2B proteins were significantly decreased in CRC samples relative to control adjacent normal colonic tissue (Figs. 7a and S7a), which is consistent with the data from TCGA, GEO and Clinical Proteomic Tumor Analysis Consortium (CPTAC) database (Figs. 7b, S7b and S7c). In general, ACOX1 and KAT2B protein levels were positively correlated with p53, whereas ATIC levels were negatively correlated (Fig. 7c). ATIC acetylation levels also positively correlated with p53, ACOX1, and KAT2B (Fig. 7d) and significantly decreased in CRC samples (Fig. 7a). We further explored the association between these genes’ expression and patient prognosis in human carcinomas by using data from TCGA database. Interestingly, we found that patients with high-level expression of peroxisomal FAO genes had favorable survival (Fig. 7e). Importantly, this association was only observed in TP53 wild-type patients, but not TP53-mutant patients, status (Fig. 7e). These data indicate that, as in the case of the murine AOM/DSS and APCmin/+ CRC model, ATIC acetylation plays a critical role in human CRC pathogenesis.
a Relative protein levels of p53, ACOX1, KAT2B, ATIC. The results shown are from the same tissues shown in Fig. S7a. Signal intensity of these proteins were quantified by Image J, and then normalized to β-actin band intensity. b ACOX1, KAT2B, ATIC expression in CRC tumors and normal colorectal tissues. The raw data from TCGA. c Correlation between the expression levels of p53 and ACOX1, KAT2B, ATIC, as determined by Pearson’s r analysis. The results shown are from the same tissues shown in Fig. S7a. d Correlation between the expression levels of Ac-ATIC and p53, ACOX1, KAT2B, as determined by Pearson’s r analysis. The results shown are from the same tissues shown in Fig. S7a. e Kaplan–Meier analysis of overall survival (OS) in CRC patients according to the expression of peroxisomal FAO. P values were calculated by log-rank test. The raw data was from TCGA. f Schematic illustration of the mechanistic model for the role of p53-mediated peroxisomal β-oxidation-ATIC axis in colorectal tumorigenesis. Data were presented as mean ± SD.