Abstract
Rationale
Genetic studies have proved the involvement of Tuberous sclerosis complex subunit 2 (Tsc2) in aortic aneurysm. However, the exact role of macrophage Tsc2 in the vascular system remains unclear. Here, we examined the potential function of macrophage Tsc2 in the development of aortic remodeling and aortic aneurysms.
Methods and results
Conditional gene knockout strategy combined with histology and whole-transcriptomic analysis showed that Tsc2 deficiency in macrophages aggravated the progression of aortic aneurysms along with an upregulation of proinflammatory cytokines and matrix metallopeptidase-9 in the angiotensin II-induced mouse model. G protein-coupled receptor 68 (Gpr68), a proton-sensing receptor for detecting the extracellular acidic pH, was identified as the most up-regulated gene in Tsc2 deficient macrophages compared with control macrophages. Additionally, Tsc2 deficient macrophages displayed higher glycolysis and glycolytic inhibitor 2-deoxy-D-glucose treatment partially attenuated the level of Gpr68. We further demonstrated an Tsc2-Gpr68-CREB network in macrophages that regulates the inflammatory response, proteolytic degradation and vascular homeostasis. Gpr68 inhibition largely abrogated the progression of aortic aneurysms caused by Tsc2 deficiency in macrophages.
Conclusions
The findings reveal that Tsc2 deficiency in macrophages contributes to aortic aneurysm formation, at least in part, by upregulating Gpr68 expression, which subsequently drives proinflammatory processes and matrix metallopeptidase activation. The data also provide a novel therapeutic strategy to limit the progression of the aneurysm resulting from Tsc2 mutations.
Similar content being viewed by others
Introduction
Aortic aneurysm (AA) is a life-threatening aortic disease, characterized by a progressive dilatation of the aorta. A majority of AAs are located at two distinct aneurysm-prone regions, the abdominal aorta and thoracic aorta involving the ascending aorta. Small-sized AAs are mostly asymptomatic, whereas large-sized AAs often lead to aortic rupture and sudden death when the diameters are more than 6.0 cm in thoracic aortic aneurysm (TAA) and more than 5.0 cm in abdominal aortic aneurysm (AAA) [1, 2]. Although great progress has been achieved in surgical techniques including endovascular aneurysm repair, there is still no effective pharmacological treatments for patients with small AAs or contradictions to surgery [3]. Thus, it is important to expand our knowledge of the mechanisms involved in AAs development and progression.
Although TAA and AAA have different aetiologies and pathogeneses, accumulating experimental evidence propose the strong role of a chronic immune/inflammatory process in both TAA and AAA evocation and progression [3, 4]. Likewise to AAA, histological studies demonstrate the presence of large amounts of inflammatory cell infiltrates, particularly macrophages, in the media and adventitia of thoracic aortic wall [4]. Infiltrating macrophages contribute to the degradation of extracellular matrix and death or dysfunction of vascular smooth muscle cells (SMCs) by releasing a range of proteolytic enzymes, including matrix metalloproteinases (MMPs), oxidation-derived free radicals and proinflammatory cytokines (TNF-α, IL-1β, and IL-6), which induce arterial wall destruction and subsequent aorta dilatation [3, 4].
Tuberous sclerosis complex subunit 1 (Tsc1) and 2 (Tsc2), which encode the proteins hamartin and tuberin, respectively, form a heterodimeric complex. Tsc1 enhances Tsc2 function by stabilizing Tsc2. Tsc complex participates in a wide range of cellular functions and acts as a tumor growth suppressor [5]. Studies have shown that Tsc2 is a catalytic GTPase-activating protein (GAP) and directly inhibits Rheb small GTPase upstream of mTOR [6, 7]. Inactivating mutations in Tsc1 or Tsc2 give rise to the tuberous sclerosis. Patients with tuberous sclerosis have a higher incidence of AAs compared with the general population [8]. However, the underlying molecular mechanisms are poorly understood. Recent studies have reported the role of smooth muscle Tsc1/Tsc2 in the pathogenesis of AAs [9, 10]. Tsc1 deletion in smooth muscle cells results in a degradative smooth muscle cell phenotype and progressive TAA [9]. In addition, descending aortic aneurysm is associated with subintimal smooth muscle cell proliferation in a child with a Tsc2 mutation [10]. Whether the deficiency of macrophage Tsc2 is directly involved in aneurysm formation is still a mystery. Emerging evidence indicates that macrophage Tsc2 orchestrates inflammatory response, implying that macrophage Tsc2 may be closely related to vascular homeostasis [11, 12]. Thus, we aimed to evaluate the role of macrophage Tsc2 in vascular inflammation and aneurysm pathogenesis.
In this study, we demonstrate that myeloid Tsc2 depletion increased susceptibility to AAs formation and elastin degradation in the angiotensin II-induced mouse model. An evaluation for the underlying mechanism of exacerbating vascular inflammation and extracellular matrix remodeling in myeloid-specific Tsc2 deficient mice leads to the identification of the macrophage G protein-coupled receptor 68 (Gpr68) as a key molecule involved in AAs formation.
Results
Myeloid-specific deletion of Tsc2 exacerbates AngII (angiotensin II) infusion–induced aneurysm development
Animals with myeloid-specific disruption of Tsc2 were sacrificed and their aortas compared with controls. Conditional myeloid deletion of Tsc2 in an ApoE–/– background induced swollen paws and tails (Fig. S1A and B), which was accordance with a previous report [11]. However, Tsc2MKOApoE–/– mice are viable and fertile with uncompromised vasculature development. Systemic infusion of AngII via osmotic mini-pumps into ApoE–/– mice for 4 weeks has been repeatedly demonstrated to cause aneurysmal pathology in both thoracic and abdominal aortas [13]. Subsequently, to investigate whether macrophage Tsc2 is crucial to maintain vasculature homeostasis, we crossed myeloid-specifc Tsc2MKO with hyperlipidemic ApoE–/– mice and established AngII-induced murine aortic aneurysm model. The absence of Tsc2 expression on myeloid cells in Tsc2MKOApoE–/– mice was confirmed by RT-PCR and western blot analysis in peritoneal macrophages (Fig. S1C and D).
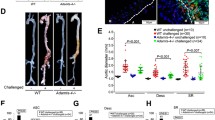
After 4 weeks of AngII (1000 ng/kg/min) infusion, systolic blood pressure increased similarly in conscious Tsc2MKOApoE–/– and Tsc2WTApoE–/– mice, but no significant difference in body weight between the two groups was observed (Table S1). Morphologically, the aortas of saline-infused Tsc2MKOApoE–/– mice did not differ from those of saline-infused controls (Fig. 1A). No spontaneous aortic dilations and aneurysms were observed in the saline-infused mice (Fig. 1A). Although AngII treatment resulted in vascular expansion in both genotypes, Tsc2MKOApoE–/– mice exhibited significant increases in the maximal descending thoracic and abdominal aortic diameter compared to Tsc2WTApoE–/– mice (Fig. 1A–C). As well, both the incidence of AAA and TAA were markedly increased in Tsc2MKOApoE−/− mice (83.3% and 55.6%, respectively) than in Tsc2WTApoE–/– mice (47.1% and 11.8%, Fig. 1B, Table S2).
Tsc2MKOApoE−/− and Tsc2WTApoE−/− mice were infused with saline or AngII for 28 d. A Representative images of aortic specimens from saline or AngII-infused Tsc2WTApoE−/− and Tsc2MKOApoE–/– mice. B Incidence of AAA and TAA in AngII-infused Tsc2WTApoE–/– and Tsc2MKOApoE–/– mice (Tsc2WTApoE–/– + saline, n = 10; Tsc2MKOApoE–/– + saline, n = 10; Tsc2WTApoE−/− + AngII, n = 17; Tsc2MKOApoE–/– + AngII, n = 18). C Comparison of maximal diameters in the ascending thoracic (Asc), descending thoracic (Desc), and suprarenal abdominal (Abd) aortic segments measured with vernier caliper (n = 10). *P < 0.05, vs. Tsc2WTApoE–/– mice infused with AngII. #P < 0.05, vs. Tsc2WTApoE–/– mice infused with saline.
Myeloid-specific deletion of Tsc2 promotes AngII infusion–induced aortic structural and composition changes
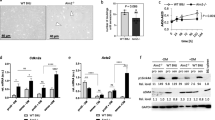
Elastin fragmentation is considered crucial for aortic wall dilation and can be detected by Verhoeff-Van Giessen (VVG) staining. Histological analysis of the suprarenal aorta with VVG staining showed that elastin fibers were disrupted in AngII-infused arteries (Fig. 2A, B). No obvious elastin breakage was identified in aortic sections from saline-infused Tsc2MKOApoE−/– and Tsc2WTApoE–/– mice. Notably, disruption and degradation of medial elastic lamina were more severe in AngII-infused Tsc2MKOApoE–/– mice compared with Tsc2WTApoE–/– mice (Fig. 2A, B).
A Representative hematoxylin and eosin (H&E) and Verhoeff-van Gieson (VVG) staining of the abdominal aortic sections in Tsc2WTApoE–/– and Tsc2MKOApoE–/– mice with or without AngII infusion. Red arrow indicated elastin break. B Quantifcation of elastin breaks per section in aortic cross sections harvested from AngII infused mice (n = 10). C Quantification of IL-6, IL1-β, MCP-1, and Mmp9 mRNA expression in abdominal aorta harvested from Tsc2WTApoE–/– and Tsc2MKOApoE–/– mice with or without AngII infusion for 28 d (n = 5). D Western blot analysis of IL-6, IL1-β, MCP-1, and Mmp9 expression in abdominal aorta harvested from AngII-infused Tsc2WTApoE–/– and Tsc2MKOApoE–/– mice (n = 4). E Representative images of IL1-β expression by F4/80+ macrophages in abdominal aortic tissues from AngII-infused Tsc2MKOApoE–/– and Tsc2WTApoE–/– mice (n = 5). *P < 0.05, vs. Tsc2WTApoE–/– mice infused with AngII. #P < 0.05, vs. Tsc2WTApoE–/– mice infused with saline.
We then checked the expression of several proinflammatory cytokines and matrix metalloproteinase-9 (Mmp9), which have been repeatedly demonstrated to contribute to the pathogenesis of AAs in the aortas [14]. Tsc2 deletion in myeloid cells significantly increased the expression of IL-6 and IL1-β but not MCP-1 and Mmp9 expression in the saline-infused aortas compared with control mice (Fig. 2C). In addition, AngII augmented the expression of proinflammatory cytokines IL-6, IL1-β and MCP-1 in the presence as well as in absence of Tsc2 (Fig. 2C and D). Consistently, the aortas from Tsc2MKOApoE−/− mice infused with AngII displayed a marked upregulation of Mmp9 expression (Fig. 2C and D). Furthermore, double immunofluorescence analysis detected increased F4/80+ macrophages infiltrating into aortas expressed IL1-β in the aortas from AngII-infused Tsc2MKOApoE−/− mice (Fig. 2E).
Moreover, Tsc2 deletion in myeloid cells decreased the relative contents of collagen and smooth muscle cells but augmented macrophage infiltration in the aortic wall, as determined by Masson’s trichrome staining and immunostaining (Fig. 3A). As proinflammatory macrophages could enhance the phenotypic changes of smooth muscle cells [44] and purchased from BoShang Bio-tech Company (**an, China). sh-Gpr68 or sh-NC (2 × 107 TU per mouse) was injected into the mice through the tail vein before AngII infusion. Randomization and allocation concealment were performed. All experimental protocols conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the animal ethics committee of Shandong University.
Angiotensin II (AngII) were from Sigma Aldrich (St. Louis, MO, USA). Primary antibodies used include: Gpr68 (Cat#NBP2-93067, Cat#NLS1195) was purchased from Novus Biologicals (Littleton, USA). Tsc2 (Cat#4308 S), CREB (Cat#9197 S), phosphorylated CREB (Cat#9198 S), vimentin (Cat#5741 S), IL-6 (Cat#12912 S), MCP-1 (Cat#41987 S) and β-actin (Cat#3700 S) were purchased from Cell Signaling Technology (Danvers, USA). Mmp9 (Cat#ab38898), α-SMA (Cat#ab5694), sm22α (Cat#ab14106) and IL-1β (Cat#ab234437) were purchased from Abcam (Cambridge, UK). F4/80 (Cat#MCA497) were purchased from BioRad (Hercules, USA). Secondary antibodies used for western blot were from ZSGB-BIO (Bei**g, China). Secondary antibodies used for immunofluorescence staining were purchased from Abcam (Cambridge, UK).
Determination of blood pressure
Systolic blood pressure (SBP) was measured noninvasively on conscious mice using a noninvasive tail-cuff system (Natsume, Tokyo, Japan). Measurements were recorded at the same time of day throughout the study. Systolic blood pressure was measured at least five times, and the mean was used for each mice.
Angiotensin II infusion
To induce aortic aneurysms formation, all mice received angiotensin II (1000 ng/kg/min) or saline by micro-osmotic pumps (Alzet model 2004; Durect, Cupertino, CA, USA) as we previously described [45]. All mice received regular laboratory chow diet throughout the study. Mice that died immediately after the osmotic pumps implantation were excluded from the present study. At 28 days after the osmotic pumps implantation, mouse aortas were harvested, and aneurysms were quantified. We measured the diameter of the ascending, descending thoracic, and abdominal aortic segments of the extracted aortas. An aneurysm was defined as ≥50% enlargement of the external diameter compared to aortas from saline-infused control mice. Necropsy was performed to confirm aortic rupture during AngII infusion. Operators responsible for the experimental procedures and data analysis were blinded to group allocation. The number of animals that received AngII infusion was determined based on prior experimentation in our lab.
Histology and immunohistochemistry
Paraffin-embedded aortic sections (5 μm thick) were subjected to hematoxylin and eosin (H&E) and Verhoeff-Van Gieson Elastin staining (Sigma-Aldrich) for morphological assessment and detection of elastic fiber integrity, respectively. For immunohistochemical staining, sections were incubated with primary antibodies overnight at 4 °C, followed by secondary antibody before visualization with diaminobenzidine (Gene Tech, Shanghai, China). In the double immunofluorescence analysis, appropriate secondary antibodies (Alexa Fluor 488 and 594) were employed. DAPI was used for nuclei staining. Images were observed with a fluorescence microscope (Olympus, Tokyo, Japan).
Cell cultures
Three days before collecting peritoneal macrophages, mice were intraperitoneally injected with 1 mL of 3.0% thioglycollate medium. Cells were further perfused from the peritoneal cavity and then cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS).
Assays of oxygen consumption rate and extracellular acidification rate
Peritoneal macrophages were seeded at 4×104 cells per well in Seahorse XF 96-well culture microplates. The cellular oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were assessed using the Seahorse XFe 96 Extracellular Flux Analyzer (Agilent Technologies, CA, USA) according to the manufacturer’s instructions. OCR and ECAR were determined using Seahorse XF Cell Mito Stress Test Kit and Seahorse XFe Glycolysis Stress Test Kit, respectively. Results were collected with Wave software (Agilent Technologies, CA, USA). OCR is indicated in pmol/minute and ECAR in mpH/minute.
Transwell migration assay
Bone marrow-derived macrophages (BMDMs) were isolated from long bones and washed with PBS. Cells were starved 24 h prior to assay set up. 1×104 cells/200 μl were suspended in medium without FBS and seeded into the upper chamber of transwell plates (Corning, USA) with 8 μm pore filters. The lower chamber was added with complete medium (containing 10% FBS). Following 12 h incubation at 37 °C, cells that migrated to the lower surface were stained with crystal violet. Migrated cells per field was quantified under a microscope.
Real-time polymerase chain reaction (RT-PCR)
Total RNAs were extracted using TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using the PrimeScript reverse transcriptase reagent kit (Takara, Japan). All samples were amplified using SYBR Green-based RT-PCR. The mRNA levels were normalized to those of β-actin. Primer sequences for real-time quantification are listed in Table S4.
Western blot assay
Total proteins were extracted from cultured cells or tissues with RIPA buffer. Proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore). Membranes were incubated with specific antibodies. After further incubation with horseradish peroxidase-conjugated secondary antibodies, bound primary antibodies were detected by chemiluminescence (Millipore, USA). The intensity of bands was quantified with ImageJ software (NIH, Bethesda, MD, USA).
Gelatin zymography
Mmp9 activities were determined by a Mmp gelatin zymography kit (GenMed Scientific Inc., USA) according to the manufacturer’s instructions. In brief, the supernatant of macrophages culture medium was collected and mixed with sample buffer dye then separated by 10% SDS-PAGE gels containing 0.1% gelatin. The gels were further washed with renaturing buffer and incubated with zymography reaction buffer. Finally, the gels were stained with Coomassie Brilliant Blue.
Microarray gene expression analysis
Total RNA was extracted from peritoneal macrophages in AngII-infused Tsc2MKOApoE−/− and Tsc2WTApoE−/− mice. The RNA concentration and integrity were determined by Agilent 2100 RNA nano 6000 assay kit (Agilent Technologies, CA, USA). The transcriptome sequencing experiments were performed by BoShang Bio-tech Company (**an, China). The differentially expressed genes were identified with a fold-change of >2.0 and P value < 0.05 between two groups.
Statistical analysis
Statistical analysis was performed using SPSS16.0 software. Continuous variables were expressed as mean ± SD if normally distributed or median if non-normally distributed. Categorical data were expressed as frequency and percentage. We tested the normality and equal variance using the Kolmogorov-Smirnov test implemented in Prism statistical software (GraphPad, San Diego) before parametric data analysis. Comparison of 2 groups of normally distributed samples was done using unpaired Student’s t tests. Comparison of multiple groups of normally distributed samples was done using ANOVA, and Tukey was performed as a post test. Nonnormally distributed data were analyzed with Mann–Whitney test or the Kruskal-Wallis test, as appropriate. Categorical data were analyzed by chi-square test or Fisher’s exact test. P < 0.05 was considered statistically significant.
Data availability
The experimental data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pinard A, Jones GT, Milewicz DM. Genetics of thoracic and abdominal aortic diseases. Circulation Res. 2019;124:588–606.
Quintana RA, Taylor WR. Cellular mechanisms of aortic aneurysm formation. Circulation Res. 2019;124:607–18.
Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16:225–42.
Postnov A, Suslov A, Sobenin I, Chairkin I, Sukhorukov V, Ekta MB, et al. Thoracic aortic aneurysm: blood pressure and inflammation as key factors in the development of aneurysm dissection. Curr Pharm Des. 2021;27:3122–7.
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90.
Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34.
Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat cell Biol. 2003;5:578–81.
Salerno AE, Marsenic O, Meyers KE, Kaplan BS, Hellinger JC. Vascular involvement in tuberous sclerosis. Pediatr Nephrol (Berl, Ger). 2010;25:1555–61.
Li G, Wang M, Caulk AW, Cilfone NA, Gujja S, Qin L, et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J Clin Investig. 2020;130:1233–51.
Cao J, Gong L, Guo DC, Mietzsch U, Kuang SQ, Kwartler CS, et al. Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/- mice. Hum Mol Genet. 2010;19:1908–20.
Linke M, Pham HT, Katholnig K, Schnöller T, Miller A, Demel F, et al. Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nat Immunol. 2017;18:293–302.
Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696.
Zhao G, Fu Y, Cai Z, Yu F, Gong Z, Dai R, et al. Unspliced XBP1 Confers VSMC Homeostasis and Prevents Aortic Aneurysm Formation via FoxO4 Interaction. Circulation Res. 2017;121:1331–45.
Rabkin SW. The role matrix metalloproteinases in the production of aortic aneurysm. Prog Mol Biol Transl Sci. 2017;147:239–65.
Li P, Wang Y, Liu X, Liu B, Wang ZY, **e F, et al. Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell death Dis. 2020;11:22.
Pan L, Bai P, Weng X, Liu J, Chen Y, Chen S, et al. Legumain is an endogenous modulator of integrin αvβ3 triggering vascular degeneration, dissection, and rupture. Circulation. 2022;145:659–74.
Yoshida Y, Matsunaga N, Nakao T, Hamamura K, Kondo H, Ide T, et al. Alteration of circadian machinery in monocytes underlies chronic kidney disease-associated cardiac inflammation and fibrosis. Nat Commun. 2021;12:2783.
Endo-Umeda K, Kim E, Thomas DG, Liu W, Dou H, Yalcinkaya M, et al. Myeloid LXR (Liver X receptor) deficiency induces inflammatory gene expression in foamy macrophages and accelerates atherosclerosis. Arteriosclerosis, thrombosis, Vasc Biol. 2022;42:719–31.
Cao Q, Cui X, Wu R, Zha L, Wang X, Parks JS, et al. Myeloid deletion of α1AMPK exacerbates atherosclerosis in LDL receptor knockout (LDLRKO) mice. Diabetes. 2016;65:1565–76.
Wiley SZ, Sriram K, Liang W, Chang SE, French R, McCann T, et al. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J: Off Publ Federation Am Societies Exp Biol. 2018;32:1170–83.
Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol (Baltim, Md: 1950). 2010;185:6413–9.
Cui H, Chen Y, Li K, Zhan R, Zhao M, Xu Y, et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur heart J. 2021;42:4373–85.
Ariyoshi W, Okinaga T, Chaweewannakorn W, Akifusa S, Nisihara T. Mechanisms involved in enhancement of matrix metalloproteinase-9 expression in macrophages by interleukin-33. J Cell Physiol. 2017;232:3481–95.
Huang CK, Dai D, **e H, Zhu Z, Hu J, Su M, et al. Lgr4 Governs a Pro-Inflammatory Program in Macrophages to Antagonize Post-Infarction Cardiac Repair. Circulation Res. 2020;127:953–73.
Sahu S, Ganguly R, Raman P. Leptin augments recruitment of IRF-1 and CREB to thrombospondin-1 gene promoter in vascular smooth muscle cells in vitro. Am J Physiol Cell Physiol. 2016;311:C212–224.
Yang H, Zhou T, Sorenson CM, Sheibani N, Liu B. Myeloid-derived tsp1 (thrombospondin-1) contributes to abdominal aortic aneurysm through suppressing tissue inhibitor of metalloproteinases-1. Arteriosclerosis, thrombosis, Vasc Biol. 2020;40:e350–66.
Wiemer-Kruel A, Mayer H, Ewert P, Martinoff S, Eckstein HH, Kriebel T, et al. Congenital lymphatic malformation and aortic aneurysm in a patient with TSC2 mutation. Neuropediatrics. 2020;51:57–61.
Pan H, O’Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol (Baltim, Md: 1950). 2012;188:3658–66.
Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834.
Wang J, Filippakis H, Hougard T, Du H, Ye C, Liu HJ, et al. Interleukin-6 mediates PSAT1 expression and serine metabolism in TSC2-deficient cells. Proc Natl Acad Sci USA. 2021;118:e2101268118.
Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77.
Aoki H, Mogi C, Hisada T, Nakakura T, Kamide Y, Ichimonji I, et al. Proton-sensing ovarian cancer G protein-coupled receptor 1 on dendritic cells is required for airway responses in a murine asthma model. PloS One. 2013;8:e79985.
Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008;48:417–23. discussion 424
McBride OM, Joshi NV, Robson JM, MacGillivray TJ, Gray CD, Fletcher AM, et al. Positron emission tomography and magnetic resonance imaging of cellular inflammation in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51:518–26.
Tsuruda T, Hatakeyama K, Nagamachi S, Sekita Y, Sakamoto S, Endo GJ, et al. Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arteriosclerosis, thrombosis, Vasc Biol. 2012;32:1410–7.
Oeing CU, Jun S, Mishra S, Dunkerly-Eyring BL, Chen A, Grajeda MI, et al. MTORC1-regulated metabolism controlled by TSC2 limits cardiac reperfusion injury. Circulation Res. 2021;128:639–51.
Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98.
Naghavi M, John R, Naguib S, Siadaty MS, Grasu R, Kurian KC, et al. pH heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis. 2002;164:27–35.
Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J allergy Clin Immunol. 2004;113:610–9.
Liu CL, Liu X, Wang Y, Deng Z, Liu T, Sukhova GK, et al. Reduced Nhe1 (Na(+)-H(+) exchanger-1) function protects ApoE-deficient mice from Ang II (angiotensin II)-induced abdominal aortic aneurysms. Hypertension (Dallas, Tex: 1979). 2020;76:87–100.
Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal. 2013;25:2263–71.
Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, et al. GPR68 senses flow and is essential for vascular physiology. Cell. 2018;173:762–75.e716.
Shi J, Hua L, Harmer D, Li P, Ren G. Cre driver mice targeting macrophages. Methods Mol Biol (Clifton, NJ). 2018;1784:263–75.
Yang M, Mailhot G, Birnbaum MJ, MacKay CA, Mason-Savas A, Odgren PR. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J Biol Chem. 2006;281:23598–605.
Liang ES, Bai WW, Wang H, Zhang JN, Zhang F, Ma Y, et al. PARP-1 (Poly[ADP-Ribose] Polymerase 1) inhibition protects from Ang II (Angiotensin II)-induced abdominal aortic aneurysm in mice. Hypertension (Dallas, Tex: 1979). 2018;72:1189–99.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (Grant no. 81900420, 81900397), Shandong Provincial Natural Science Foundation of China (Grant no. ZR201808120047, ZR201709250018, ZR2021MH126); China Postdoctoral Science Foundation (Grant no. 2021M702008, 2019M652396) and the Postdoctoral Innovation Program of Shandong Province (202003007).
Author information
Authors and Affiliations
Contributions
EL designed this study. XL and YL drafted the manuscript. RY and XD edited and revised the manuscript. XL, YL, RY and XD performed the experiments, interpreted the results of the experiments, and prepared the Figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study was reviewed and approved by the animal ethics committee of Shandong University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Dr Yufang Shi
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Liu, Y., Yang, Rx. et al. Loss of myeloid Tsc2 predisposes to angiotensin II-induced aortic aneurysm formation in mice. Cell Death Dis 13, 972 (2022). https://doi.org/10.1038/s41419-022-05423-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05423-2
- Springer Nature Limited