Abstract
Pattern recognition receptors (PRRs) and interferons (IFNs) serve as essential antiviral defense against SARS-CoV-2, the causative agent of the COVID-19 pandemic. Type III IFNs (IFN-λ) exhibit cell-type specific and long-lasting functions in auto-inflammation, tumorigenesis, and antiviral defense. Here, we identify the deubiquitinating enzyme USP22 as central regulator of basal IFN-λ secretion and SARS-CoV-2 infections in human intestinal epithelial cells (hIECs). USP22-deficient hIECs strongly upregulate genes involved in IFN signaling and viral defense, including numerous IFN-stimulated genes (ISGs), with increased secretion of IFN-λ and enhanced STAT1 signaling, even in the absence of exogenous IFNs or viral infection. Interestingly, USP22 controls basal and 2′3′-cGAMP-induced STING activation and loss of STING reversed STAT activation and ISG and IFN-λ expression. Intriguingly, USP22-deficient hIECs are protected against SARS-CoV-2 infection, viral replication, and the formation of de novo infectious particles, in a STING-dependent manner. These findings reveal USP22 as central host regulator of STING and type III IFN signaling, with important implications for SARS-CoV-2 infection and antiviral defense.
Similar content being viewed by others
Introduction
Sensing of “non-self” is a key feature of innate immunity and underlies the recognition of viruses, bacteria, and fungi, but also plays important roles in cancer and auto-immune diseases [1, 2]. Pattern recognition receptors (PRRs), like Toll-like receptors (TLRs), Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene 1 protein (RIG-I)-like receptors (RLRs) are essential components of innate immune signaling and selectively recognize pathogen-associated molecular patterns (PAMPs). Dedicated PRRs, like TLR3, RIG-I, Melanoma differentiation-associated protein 5 (MDA5), and Cyclic GMP-AMP synthase (cGAS)-Stimulator of interferon genes protein (STING) recognize viral double-stranded RNA (dsRNA) and dsDNA, and are important sensors for infections with RNA and DNA viruses, as well as infections with retroviruses [1,2,3]. While TLR3 recognizes dsRNA in endosomes, the prototypical RLRs, RIG-I and MDA5, sense cytosolic dsRNAs, and cGAS-STING detects viral dsDNA [1,2,3,4]. STING is activated either directly via viral dsDNA, through the STING agonist 2′3′-cGAMP generated by the cyclic GMP-AMP synthase cGAS upon detection of viral dsDNA, or indirectly via RIG-I and MDA5 [5]. Activated STING interacts with TANK-binding kinase 1 (TBK1) and activates interferon regulatory factor (IRF) 1, -3, and -7 and Nuclear factor-κB (NF-κB), leading to the initiation of anti-viral and inflammatory transcriptional programs, including interferon-stimulated genes (ISGs) and interferons (IFNs) [5,6,7,8].
IFNs are secreted cytokines with important roles in immunity and anti-viral responses. IFN signaling relies on Janus kinase-Signal transducer and activator of transcription (JAK-STAT) activation, phosphorylation of STAT1/2, and the induction of ISG and IFN gene expression that influence viral replication [9, 10]. Although the vast majority of cell types can be triggered to express type I (IFN-α, -β, -ε, -κ and -ω) and type III (IFN-λ1, -λ2, -λ3 and -λ4) IFNs, the expression of IFN-specific receptors is cell type-restricted and determines IFN responses. For example, type I IFN receptor (IFNAR) is ubiquitously expressed in many tissues, whereas expression of type III IFN receptor IFNLR1 is mainly limited to epithelial cells, e.g. the gastro-intestinal and respiratory epithelium [6,7,8, 11]. Although type I and type III IFNs induce similar ISG signatures, type I IFNs generally trigger a more rapid increase and decay of ISG expression [7]. In addition, IFN-λs have been described to be the first-in-line defense against viral infections and might inhibit viral spread without triggering inflammatory responses, depending on IFN-λ receptor expression [7, 12, 13].
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the pandemic Coronavirus disease 2019 (COVID-19) and belongs to the human coronaviruses (HCoV) that also include SARS-CoV and MERS-CoV [14]. In many patients with severe COVID-19, SARS-CoV-2 infection induces the secretion of highly pro-inflammatory cytokines through cGAS-STING and NF-κB-mediated signaling [15, 16]. Type I and III IFNs are important regulators of host viral defense against SARS-CoV-2 [6,7,8, 11, 17, 18], but at the same time, SARS-CoV-2 evades immune recognition via IFN and ISG suppression [10, 19]. Prolonged expression of low basal levels of type I and III IFNs might prime host responses against virus infection, including SARS-CoV-2 [20,21,22,23]. Although type III IFNs restrict SARS-CoV-2 infection in intestinal and airway epithelial cells [18, 24,25,26,27,28] and STING agonism reduces SARS-CoV-2 infection [29,30,31,32], context-dependent damaging effects of type III IFNs on airway epithelia during viral infections have been described as well [33, 34].
Innate immunity, PRRs, and IFN signaling are closely regulated by ubiquitination, both by the host machinery as well as through viral E3 ligases and deubiquitinating enzymes (DUBs) that hijack the host ubiquitin machinery [35]. STING, RIG-I, TLR3, and TBK1 are positively and negatively regulated by differential modification of polyubiquitin chains, including K11-, K27-, K48- and K63-linked chains [36, 37], by a variety of E3 ligases, such as TRIM56 [38], TRIM32 [39], MUL1 [40], AMFR [41], RNF5 [42] and TRIM29 [44]. The interplay and functional consequences of ubiquitin modifications are complex and include proteasomal degradation as well as stabilization of protein-protein interactions. Importantly, IFNs and anti-viral signaling are also heavily regulated by DUBs, like USP13 [45], USP35 [46], and Cylindromatosis (CYLD) [47].
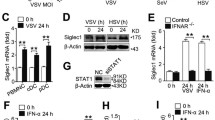
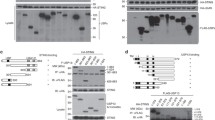
Ubiquitin-specific peptidase 22 (USP22) is a DUB that is part of the deubiquitination module of the Spt-Ada-Gcn5-acetyltransferase (SAGA) complex, through which it regulates transcription via the control of histone H2A K119 and H2B K120 monoubiquitination (H2AK119ub1 and H2BK120ub1, respectively) [48,49,50]. Recently, additional USP22 substrates have emerged, with important roles in cell fate regulation and programmed cell death [51,52,53]. Interestingly, USP22 has mostly been associated with IFN signaling and ISG expression upon viral infection [ Substrate-specific deubiquitination is a crucial determinant of ubiquitin homeostasis and regulates receptor activation and internalization, proteasomal degradation, and transcription. For the ubiquitin-specific protease USP22, both transcriptional and extranuclear targets have been identified. As part of the DUB module of the SAGA complex, USP22 regulates transcriptional elongation via H2AK119ub1 and H2BK120ub1 [48,49,50]. Up till now, the spectrum of target genes regulated by USP22 remains largely unclear, partially due to organism-, cell- and context-dependent redundancy in alternative DUBs that might compensate for loss of USP22 [56]. We previously reported that CRISPR/Cas9-mediated knockout (KO) of USP22 in the human colon carcinoma cell line HT-29 affects RIPK3 ubiquitination during necroptosis without inducing major changes in RIPK1, RIPK3, and MLKL gene expression [53], suggesting gene-specific regulation of USP22. To identify the spectrum of USP22-regulated genes, we profiled USP22-dependent changes in gene expression in the hIEC line HT-29. Quantification of alterations in gene expression in two independent HT-29 USP22 KO single cell clones revealed a marked alteration in gene expression, with 401 genes upregulated and 182 downregulated (Fig. 1A and Supplemental Fig. 1A). Loss of USP22 expression was accompanied by changes in H2Bub1, but not H2Aub1 (Supplemental Fig. 1B, C). Among the top-50 differentially regulated genes, 30 were up- and 20 downregulated, with an adjusted P-value of <0.05 (Fig. 1B). Genes upregulated in both USP22 KO clones (#16 and #62) compared to control (non-human target: NHT) HT-29 cells include genes that encode for proteins involved in growth and differentiation, like Transforming Growth Factor β-1 (TGFB1), Tumor-associated calcium signal transducer 2 (TACSTD2) and Tyrosine-protein kinase Mer (MERTK) and the cytosolic RNA- and DNA sensor DExD/H-Box Helicase 60 (DDX60). Downregulated genes include USP22, mitochondrial adenylate kinase 4 (AK4) that is involved in the regulation of mitochondrial function and ATP production [57], and regenerating islet-derived protein 4 (REG4), a carbohydrate-binding lectin that has been identified as marker for deep crypt secretory cells (DSCs) that acts as niche for Lgr5-positive stem cells in the colon [58]. Differential regulation of gene expression, as well as loss of USP22 expression, was also demonstrated by independent qRT-PCR of the USP22-dependent upregulated genes TGFB1, SLFN5, TGM2, and DDX60, as well as downregulation of USP22, CXCR4, and AK4 (Fig. 1C), confirming the quality of the microarray. A. Volcano plot showing the differential gene expression patterns of two independent single-cell HT-29 USP22 CRISPR/Cas9 KO clones (#16 and #62) compared to CRISPR/Cas9 control (NHT) HT-29 cells. Color code represents the log2 fold change compared to NHT. B Heatmap of the top-50 differentially regulated genes between HT-29 USP22 KO single clones #16 and #62 and the NHT control. Color coding represents the row-wise scaled (Z-score) RNA intensities. Genes are sorted according to their log2 fold change, compared to NHT. C Basal mRNA expression levels of the indicated genes were determined in control and two independent USP22 KO HT-29 single clones using qRT-PCR. Gene expression was normalized against 28S mRNA and is presented as x-fold mRNA expression compared to NHT. Mean and SD of three independent experiments in triplicate are shown. *P < 0.05; **P < 0.01, ***P < 0.001. Next, gene-set enrichment analysis was performed on USP22-regulated genes to investigate if certain gene ontology (GO) terms are specifically regulated by USP22. Interestingly, GO analysis revealed an enrichment of genes linked to type I and II IFN signaling, as well as regulation of viral genome replication and several other viral processes, such as the regulation of viral genome replication, response to virus, response to IFN-γ-, and IFN-γ-mediated signaling pathways in USP22 KO HT-29 cells as compared to control NHT HT-29 cells (Fig. 2A). Interestingly, the GO terms of genes that are strongly downregulated are enriched in mitochondrial translation and gene expression, ribosomal and ribonucleoprotein complex biogenesis and the processing of tRNA, rRNA, and ncRNA (Fig. 2A). A Bar plot showing the top-20 regulated GO terms in two independent single-cell HT-29 USP22 CRISPR/Cas9 KO clones (#16 and #62) compared to control (NHT) HT-29 cells. Color code represents the number of annotated genes within each gene set. B Heatmap of differentially expressed genes contributing to the GO terms response to type I IFN (left) and type II signaling (right). Color code represents the log2 fold change compared to NHT. Note that due to lack of annotation and overlap** ISGs between type I/II and type III IFNs, response to type III IFN as GO term was not included. C Basal mRNA expression levels of GO- enriched genes related to IFN signaling in control (NHT) and two independent USP22 KO HT-29 single clones using qRT-PCR. Gene expression was normalized against 28S mRNA and is presented as x-fold mRNA expression compared to NHT. Mean and SD of three independent experiments in triplicate are shown. *P < 0.05; **P < 0.01, ***P < 0.001, n.s. not significant. D Western blot analysis of basal MX1, IRF9, ISG56, ISG20, and USP22 expression levels in control and USP22 KO HT-29 cells (clone USP22 KO #62). GAPDH served as loading control. Representative blots of at least two different independent experiments are shown. Since previous studies suggest controversial roles of USP22 in IFN signaling [79], differences in GO term enrichment might be functionally irrelevant. IFN-λ mostly exhibits long-term signaling effects and plays important roles in SARS-CoV-2 infection in airway epithelial and gastro-intestinal cells and organoids and has been shown to critically control antiviral defense [18, 24,25,26,27,28]. The susceptibility towards SARS-CoV-2-infections is determined by USP22-mediated regulation of STING. STING is described as a mediator of IFN-λ1 production in HT-29 cells, and during viral infection in primary human macrophages in a Ku70-dependent manner [80, 81]. We furthermore demonstrate for the first time that in the absence of viral infections or exogenous IFN, loss of USP22 expression resulted in basal and 2’3’-cGAMP-induced STING ubiquitination in hIECs. In addition, loss of STING expression decreased IFN/ISG signaling that occurred under conditions of USP22 deficiency, suggesting that STING acts as a physical scaffold for USP22-dependent ubiquitin modifications. STING ubiquitination serves different physiological roles, including determining protein stability, mediating protein-protein interactions, and cellular localization [38,39,40,41,42,44,45,46,47]. Recently, cGAS-STING activity has emerged as regulator of immunopathology in COVID-19, highlighting the relevance of adequate STING regulation [82]. STING ubiquitination enables the STING-TBK1 interaction upon cGAS-mediated recognition of cytosolic DNA and is generally associated with activation of ISG expression [71]. Until now, USP22-mediated STING ubiquitination has only been described upon viral infection and upon ectopic overexpression. For example, overexpressed USP22 modifies ectopically expressed STING with HA-tagged K27 ubiquitin upon HSV-1 infection in HEK293T cells [83]. At least three independent experiments in duplicates are shown. All primers were purchased at Eurofins (Hamburg, Germany). Primer sequences are shown in Supplementary Table 1. To quantify global changes in gene expression, RNA was isolated as described above, followed by a DNase digest upon RNA binding using the peqGOLD DNase Digest Kit, according to the manufacturer’s instructions. Samples were processed and gene expression was profiled at the DKFZ Genomics and Proteomics Core Facility (Heidelberg, Germany) using the Affymetrix human Clariom S array. Raw.CEL files were processed with the oligo R package [84] and normalized intensities were obtained after RMA normalization. Genes with differential expression between NHT control and USP22 KO have been identified using the linear model-based approach limma R package [85]. An adjusted P-value <0.05 was considered significant. Gene-set enrichment analysis was performed with gage R package [86] using the MSigDB [87] as gene set repository. An adjusted P-value < 0.05 was considered significant. Cells were seeded in 2 ml cell culture medium and supernatant was collected after 66 h, centrifuged at 300 x g, 4 °C for 5 min. and frozen in liquid nitrogen. Samples were analyzed using the LEGENDplex™ Human Anti-Virus Response Panel multiplex assay (BioLegend, San Diego, CA, USA) following the manufacturer’s protocol. The analysis was performed with the BD FACSVerse™ flow cytometer (BD Biosciences, San Jose, CA, USA). At least 300 events were acquired per analyte. Data were analyzed with the LEGENDplex v.8 software (BioLegend). Appropriate cells were seeded two days before sample collection. Cell culture media were replaced and incubated for 24 h after which supernatants were collected, centrifuged at 300 x g, 4 °C for 5 min. and frozen in liquid nitrogen. For quantification of IFN-β, VeriKine-HSTM Human IFN Beta Serum ELISA Kit was used according to the manufacturer’s protocol (PBL Assay Science, Piscataway, United States). IFN-β concentrations were calculated using a 4-parameter fit. Quantification of biologically active type I and type III IFNs in cell culture supernatants was done as previously described [88]. Briefly, HEK-Blue IFN-α/β or HEK-Blue IFN-λ (Invivogen) reporter cells were seeded (50,000 cells/well) in the presence of 20 µl of supernatants from indicated cell lines and incubated overnight. Reporter expression was detected with Quanti-Blue Solution (Invivogen) according to the manufacturer’s protocol. The indicated cell lines were seeded two days before lysis and treated as indicated, or left untreated. Lysis was done on ice using RIPA lysis buffer (50 mM Tris–HCl pH 8, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 150 mM MgCl2, 0.5% sodium deoxycholate), with phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM β-glycerophosphate, 5 mM sodium fluoride), protease inhibitor cocktail (Roche, Grenzach, Germany), 0.1% sodium dodecyl sulfate (SDS) and Pierce Universal Nuclease (Thermo Fisher Scientific) for 30 min., followed by centrifugation at 18000 x g for 25 min. at 4 °C. Protein concentrations of the cell lysates were measured using the BCA Protein Assay Kit from Pierce™, according to the manufacturer’s instructions. For Western blot detection, 20-40 µg of the lysates were boiled in Laemmli loading buffer (6x Laemmli: 360 nM Tris Base pH 6.8, 30% glycerol, 120 mg/ml SDS, 93 mg/ml dithiothreitol (DTT), 12 mg/ml bromophenol blue) at 95 °C for 5 min., followed by Western blot analysis. The following antibodies were used: rabbit anti-STING (13647S, Cell Signaling Beverly, MA, USA), rabbit anti-phospho-STAT1 (9167L, Cell Signaling), mouse anti-STAT1 (9176S, Cell signaling), rabbit anti-USP22 (#ab195298, Abcam), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5G4cc, HyTest, Turku, Finland), mouse anti-Vinculin (#V9131-100UL, Merck), rabbit anti-TBK1 (ab40676, Abcam), rabbit anti-phospho-TBK1 (ab109272, Abcam), rabbit anti-Histone H2B (#07-371, Merck), mouse anti-Ubiquityl-Histone H2B (#05-1312, Merck), rabbit anti-p65 (sc-372X, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), rabbit anti-phospho-p65 (3033 S, Cell Signaling), mouse anti-IRF3 (sc-33641, Santa Cruz), rabbit anti-phospho-IRF3 (4947S, Cell Signaling), rabbit anti-RIG-I (3743S, Cell Signaling), rabbit anti-MDA5 (5321S, Cell Signaling), rabbit anti-TLR3 (6961S, Cell Signaling), mouse anti-ISG56 (PA3-848, Thermo scientific), rabbit anti-MX1 (37849S, Cell Signaling), rabbit anti-IRF9 (76684S, Cell Signaling), rabbit anti-ISG20 (PA5-30073, Thermo scientific), rabbit anti-γ-H2AX (phospho Ser139) (NB100-384, Novus Biologicals) and mouse anti-NF-κB p52 (05-361, Millipore). Secondary antibodies labeled with horseradish peroxidase (HRP) were used for detection with enhanced chemiluminescence (Amersham Bioscience, Freiburg, Germany). HRP-conjugated goat anti-mouse IgG (ab6789, Abcam) was diluted at 1:10000 and HRP-conjugated goat anti-rabbit IgG (ab6721, Abcam) was diluted 1:30,000 in 5% milk powder in PBS with 0.2% Tween 20 (PBS-T). When necessary, membranes were stripped using 0.4 M NaOH for 10 min., followed by 1 h of blocking and incubation with a second primary antibody. Representative blots of at least two independent experiments are shown. When detected on separate membranes, only one representative loading control is shown for clarity. Scans of uncropped Western blots are shown in the Supplementary Information. The indicated cell lines were seeded 24 or 48 h prior to stimulation in P/S-free cell culture medium. For stimulation, culture medium was removed and cell lines were permeabilized by incubation with digitonin buffer (50 mM HEPES, 100 mM KCl, 3 mM MgCl2, 0.1 mM dithiothreitol, 85 mM sucrose, 0.2% bovine serum albumin, 1 mM ATP, 5 µg/ml Digitonin) pH 7 in the presence or absence of 10 µg/ml 2’3’-cGAMP for 10 min. at 37 °C. After incubation, the permeabilization buffer was replaced with P/S-free cell culture medium and further incubated at 37 °C/5% CO2 for the indicated time points. The indicated HT-29 cells were seeded 24 h prior to treatment in sterile 6-well plates (Greiner). For each well, two µg of ISD (Invivogen) were pre-mixed with OptiMEM and, after 5 min. incubation at room temperature, mixed with premixed Lipofectamine2000-OptiMEM at a ratio of 3:1, according to the manufacturer’s instructions. After incubation for 15 min. at room temperature, the indicated transfection mixes were added to the cells in P/S free medium. Cell lysis with RIPA or RNA lysis buffer was performed after 24 h. For stimulation with poly(I:C), the indicated HT-29 cells were seeded as described above and for each well, 2 µg of poly(I:C) was mixed with 20 µl LyoVec (Invivogen), incubated for 15 min. at room temperature to allow the formation of lipid-RNA complexes. The transfection mix was then added to the indicated HT-29 cells in P/S free medium at a 1:20 volume ratio and incubated for 24 h, after which cells were processed for Western blot or RNA isolation. Indicated cell lines were seeded 24 h prior to treatment with 1 µg/ml anti-IFNAR2 antibody (clone MMHAR-2, PBL Assay Science). After 24 h, cells were analyzed using Western Blotting. As positive control, cells were pre-treated with 1 µg/ml anti-IFNAR2 antibody for 1 h, followed by 0.05 ng/ml recombinant human IFN-β for 1 h. Ubiquitinated proteins were enriched using GST-tagged tandem-repeated ubiquitin-binding entities (TUBEs) [89], as described before [53]. Briefly, the indicated cell lines were seeded 48 h prior to lysis and/or treatment, harvested in NP-40 lysis buffer (50 mM NaCl, 20 mM Tris pH 7.5, 1% NP-40, 5 mM EDTA, 10% Glycerol) supplemented with 25 mM NEM, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 0.5 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail and Pierce Universal Nuclease on ice for 30 min. GST-TUBE beads were washed once with NP-40 buffer and incubated with 3 mg of protein lysate over night at 4 °C. Beads were washed four times with NP-40 buffer, followed by elution of ubiquitinated proteins by boiling in 2x Laemmli loading buffer at 96 °C for 6 min. Ubiquitinated proteins were analyzed using Western blot analysis. SARS-CoV-2 (strain BavPat1/2020) was obtained from the European Virology Archive and amplified in Vero E6 cells and used at passage 3. Virus titers were determined by TCID50 assay. Caco-2 cells were infected using a MOI of 1 virus particle per cell. Medium was removed from Caco-2 cells and virus was added to cells for 1 h at 37 °C. Viral supernatants were removed, infected cells were washed once with PBS, and media was added back to the cells. Virus infection was monitored 24 h post-infection. Vero E6 cells were seeded (20,000 per well) in 96-well plates 24 h prior to infection. A volume of 100 µl of viral supernatant from the indicated SARS-CoV-2-infected Caco-2 cells was added to the first well. Seven 1:10 dilutions were made (all samples were performed in triplicate). Infections were allowed to proceed for 24 h. At 24 h post infection (hpi), cells were fixed in 2% paraformaldehyde (PFA) for 20 min. at room temperature. PFA was removed and cells were washed twice in PBS and permeabilized for 10 min. at room temperature in 0,5% Triton-X/PBS. Cells were blocked in a 1:2 dilution of LI-COR blocking buffer (LI-COR, Lincoln, NE, USA) for 30 min. at room temperature. Infected cells were stained with 1:1000 diluted anti-dsRNA (J2) for 1 h at room temperature, washed three times with 0.1% PBT-T, followed by incubation with secondary antibody (anti-mouse CW800) and DNA dye Draq5 (Abcam, Cambridge, UK), diluted 1:10,000 in blocking buffer and incubated for 1 h at room temperature. Cells were washed three times with 0.1% PBS-T and imaged in PBS on a LI-COR imager. At 24 hpi, RNA was extracted from infected or mock-treated Caco-2 cells using the Qiagen RNAeasy Plus Extraction Kit (Qiagen, Hilden, Germany). For quantifying the SARS-CoV-2 genome abundance in mock and infected samples, cDNA was generated using 250 ng of RNA with the iSCRIPT reverse transcriptase (BioRad, Hercules, CA, USA), according to the manufacturer’s instructions. qRT-PCR was performed using iTaq SYBR green (BioRad) following the instructions of the manufacturer and normalized on TBP. Primers were ordered at Eurofins, Luxemburg, and are listed in Supplementary Table 1. Cells were seeded on iBIDI glass bottom 8-well chamber slides. At 24 h post-infection, cells were fixed in 4% paraformaldehyde (PFA) for 20 min. at room temperature (RT). Cells were washed and permeabilized in 0.5% Triton-X for 15 min. at RT. Primary antibody SARS-CoV NP (Sino biologicals MM05) was diluted in phosphate-buffered saline (PBS) and incubated for 1 h at RT. Cells were washed in 1X PBS three times and incubated with secondary antibodies goat-anti mouse Alexa Fluor 568 and DAPI for 45 min. at RT. Cells were washed in 1X PBS three times and maintained in PBS. Cells were imaged by epifluorescence on a Nikon Eclipse Ti-S (Nikon). Significance was assessed using Student’s t-test (two-tailed distribution, two-sample, equal variance) using Microsoft Excel, unless indicated otherwise. P-values < 0.05 are considered significant (*P < 0.05; **P < 0.01; ***P < 0.001, n.s.: not significant). Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Sjoerd J. L. van Wijk (s.wijk@kinderkrebsstiftung-frankfurt.de). All unique reagents generated in this study are available from the corresponding author without restriction.Results
Profiling USP22-mediated gene expression in HT-29 hIECs
Loss of USP22 specifically enriches for genes involved in IFN signaling and response to viral infection
Gene expression profiling
Gene expression profiling analysis
Multiplex quantification of cytokine secretion
IFN ELISA
Detection of type I and type III IFN production
Western Blot analysis
Stimulation of STING with 2’3’-cGAMP
PRR stimulation with poly(I:C) and ISD
Blockage of type I/II IFN signaling
Tandem Ubiquitin Binding Entity (TUBE) pull-down analysis
SARS-CoV-2 infection
TCID50 virus titration
Quantification of viral RNA
Indirect Immunofluorescence Assay
Statistical analysis
Resource availability
Materials availability
Data availability
Microarray data are available on Gene Expression Omnibus under the accession number GSE190036.
References
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–20.
Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6:291.
Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity 2007;27:370–83.
Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–10.
Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69.
Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4:914–24.
Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of Type I and Type III interferons. Immunity 2019;50:907–23.
Kotenko SV, Rivera A, Parker D, Durbin JE. Type III IFNs: Beyond antiviral protection. Semin Immunol. 2019;43:101303.
Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–25.
Park A, Iwasaki A. Type I and Type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–8.
Ye L, Schnepf D, Staeheli P. Interferon-lambda orchestrates innate and adaptive mucosal immune responses. Nat Rev Immunol. 2019;19:614–25.
Forero A, Ozarkar S, Li H, Lee CH, Hemann EA, Nadjsombati MS, et al. Differential activation of the transcription factor IRF1 underlies the distinct immune responses elicited by Type I and Type III interferons. Immunity 2019;51:451–64 e6.
Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, et al. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 2017;46:875–90 e6.
Fung TS, Liu DX. Human Coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–57.
Neufeldt CJ, Cerikan B, Cortese M, Frankish J, Lee J-Y, Plociennikowska A, et al. SARS-CoV-2 infection induces a proinflammatory cytokine response through cGAS-STING and NF-κB. Commun Biol. 2022;5:45.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation, and intervention. Nat Rev Immunol. 2020;20:363–74.
Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49.
Stanifer ML, Kee C, Cortese M, Zumaran CM, Triana S, Mukenhirn M, et al. Critical role of Type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells. Cell Rep. 2020;32:107863.
Triana S, Metz-Zumaran C, Ramirez C, Kee C, Doldan P, Shahraz M, et al. Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut. Mol Syst Biol. 2021;17:e10232.
Erlandsson L, Blumenthal R, Eloranta ML, Engel H, Alm G, Weiss S, et al. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr Biol. 1998;8:223–6.
Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193.
Stewart WE 2nd, Gosser LB, Lockart RZ Jr. Priming: a nonantiviral function of interferon. J Virol. 1971;7:792–801.
Kuri T, Zhang X, Habjan M, Martínez-Sobrido L, García-Sastre A, Yuan Z, et al. Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation. J Gen Virol. 2009;90:2686–94. Pt 11
Felgenhauer U, Schoen A, Gad HH, Hartmann R, Schaubmar AR, Failing K, et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem. 2020;295:13958–64.
Stanifer ML, Guo C, Doldan P, Boulant S. Importance of Type I and III interferons at respiratory and intestinal barrier surfaces. Front Immunol. 2020;11:608645.
Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, et al. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94:e00985–20.
Busnadiego I, Fernbach S, Pohl MO, Karakus U, Huber M, Trkola A, et al. Antiviral Activity of Type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS-CoV-2. mBio. 2020;11:e01928–20.
Lamers MM, van der Vaart J, Knoops K, Riesebosch S, Breugem TI, Mykytyn AZ, et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021;40:e105912.
Zhu Q, Zhang Y, Wang L, Yao X, Wu D, Cheng J, et al. Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system. Antivir Res. 2021;187:105015.
Liu W, Reyes HM, Yang JF, Li Y, Stewart KM, Basil MC, et al. Activation of STING signaling pathway effectively blocks human coronavirus infection. J Virol. 2021;95:e00490–21.
Li M, Ferretti M, Ying B, Descamps H, Lee E, Dittmar M, et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6:eabi9007.
Humphries F, Shmuel-Galia L, Jiang Z, Wilson R, Landis P, Ng S-L, et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci Immunol. 2021;6:eabi9002.
Broggi A, Ghosh S, Sposito B, Spreafico R, Balzarini F, Lo Cascio A, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020;369:706–12.
Major J, Crotta S, Llorian M, McCabe TM, Gad HH, Priestnall SL, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 2020;369:712–7.
Isaacson MK, Ploegh HL. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5:559–70.
van Huizen M, Kikkert M. The role of atypical ubiquitin chains in the regulation of the antiviral innate immune response. Front Cell Dev Biol. 2019;7:392.
Heaton SM, Borg NA, Dixit VM. Ubiquitin in the activation and attenuation of innate antiviral immunity. J Exp Med. 2016;213:1–13.
Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y, et al. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 2010;33:765–76.
Zhang J, Hu MM, Wang YY, Shu HB. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem. 2012;287:28646–55.
Ni G, Konno H, Barber GN. Ubiquitination of STING at lysine 224 controls IRF3 activation. Sci Immunol. 2017;2:eaah7119.
Wang Q, Liu X, Cui Y, Tang Y, Chen W, Li S, et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 2014;41:919–33.
Zhong B, Zhang L, Lei C, Li Y, Mao AP, Yang Y, et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity 2009;30:397–407.
**ng J, Zhang A, Zhang H, Wang J, Li XC, Zeng M-S, et al. TRIM29 promotes DNA virus infections by inhibiting innate immune response. Nat Commun. 2017;8:945.
Fenech EJ, Lari F, Charles PD, Fischer R, Laétitia-Thézénas M, Bagola K, et al. Interaction map** of endoplasmic reticulum ubiquitin ligases identifies modulators of innate immune signalling. eLife. 2020;9:e57306.
Sun H, Zhang Q, **g Y-Y, Zhang M, Wang H-Y, Cai Z, et al. USP13 negatively regulates antiviral responses by deubiquitinating STING. Nat Commun. 2017;8:15534.
Zhang J, Chen Y, Chen X, Zhang W, Zhao L, Weng L, et al. Deubiquitinase USP35 restrains STING-mediated interferon signaling in ovarian cancer. Cell Death Differ. 2021;28:139–55.
Zhang L, Wei N, Cui Y, Hong Z, Liu X, Wang Q, et al. The deubiquitinase CYLD is a specific checkpoint of the STING antiviral signaling pathway. PLOS Pathog. 2018;14:e1007435.
Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, et al. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102–11.
Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, et al. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell. 2008;29:92–101.
Zhang XY, Pfeiffer HK, Thorne AW, McMahon SB. USP22, an hSAGA subunit, and potential cancer stem cell marker, reverses the polycomb-catalyzed ubiquitylation of histone H2A. Cell Cycle 2008;7:1522–4.
Kosinsky RL, Helms M, Zerche M, Wohn L, Dyas A, Prokakis E, et al. USP22-dependent HSP90AB1 expression promotes resistance to HSP90 inhibition in mammary and colorectal cancer. Cell Death Dis. 2019;10:911.
Kosinsky RL, Zerche M, Saul D, Wang X, Wohn L, Wegwitz F, et al. USP22 exerts tumor-suppressive functions in colorectal cancer by decreasing mTOR activity. Cell Death Differ. 2020;27:1328–40.
Roedig J, Kowald L, Juretschke T, Karlowitz R, Ahangarian Abhari B, Roedig H, et al. USP22 controls necroptosis by regulating receptor-interacting protein kinase 3 ubiquitination. EMBO Rep. 2021;22:e50163.
Cai Z, Zhang MX, Tang Z, Zhang Q, Ye J, **ong TC, et al. USP22 promotes IRF3 nuclear translocation and antiviral responses by deubiquitinating the importin protein KPNA2. J Exp Med. 2020;217:e20191174.
Liu Q, Wu Y, Qin Y, Hu J, **e W, Qin FX, et al. Broad and diverse mechanisms used by deubiquitinase family members in regulating the type I interferon signaling pathway during antiviral responses. Sci Adv. 2018;4:eaar2824.
Atanassov BS, Mohan RD, Lan X, Kuang X, Lu Y, Lin K, et al. ATXN7L3 and ENY2 coordinate activity of multiple H2B deubiquitinases important for cellular proliferation and tumor growth. Mol Cell. 2016;62:558–71.
Lanning NJ, Looyenga BD, Kauffman AL, Niemi NM, Sudderth J, DeBerardinis RJ, et al. A mitochondrial RNAi screen defines cellular bioenergetic determinants and identifies an adenylate kinase as a key regulator of ATP levels. Cell Rep. 2014;7:907–17.
Sasaki N, Sachs N, Wiebrands K, Ellenbroek SI, Fumagalli A, Lyubimova A, et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA. 2016;113:E5399–407.
Dietlein N, Wang X, Correa ER, Erbil B, Lipka DB, Begus-Nahrmann Y, et al. Loss of Usp22 enhances histone H2B monoubiquitination and stimulates intracellular and systemic interferon immunity. bioRxiv. 2021:2021.04.09.439190.
Hong A, Lee JE, Chung KC. Ubiquitin-Specific Protease 22 (USP22) positively regulates RCAN1 protein levels through RCAN1 de-ubiquitination. J Cell Physiol. 2015;230:1651–60.
Basters A, Knobeloch KP, Fritz G. USP18—a multifunctional component in the interferon response. Biosci Rep. 2018;38:BSR20180250.
Gough DJ, Messina NL, Clarke CJP, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 2012;36:166–74.
Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc Natl Acad Sci USA. 2009;106:9373–8.
Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77.
Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–8.
Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. The DNA damage response induces IFN. J Immunol. 2011;187:5336–45.
Ramachandran S, Haddad D, Li C, Le MX, Ling AK, So CC, et al. The SAGA Deubiquitination Module Promotes DNA Repair and Class Switch Recombination through ATM and DNAPK-Mediated gammaH2AX Formation. Cell Rep. 2016;15:1554–65.
Li C, Irrazabal T, So CC, Berru M, Du L, Lam E, et al. The H2B deubiquitinase Usp22 promotes antibody class switch recombination by facilitating non-homologous end joining. Nat Commun. 2018;9:1006.
Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 2006;24:93–103.
Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009;461:788–92.
Wang Z, Zhu L, Guo T, Wang Y, Yang J. Decreased H2B monoubiquitination and overexpression of ubiquitin-specific protease enzyme 22 in malignant colon carcinoma. Hum Pathol. 2015;46:1006–14.
Lee JS, Shukla A, Schneider J, Swanson SK, Washburn MP, Florens L, et al. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell 2007;131:1084–96.
**ao T, Kao CF, Krogan NJ, Sun ZW, Greenblatt JF, Osley MA, et al. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol Cell Biol. 2005;25:637–51.
Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–8.
Martin-Sancho L, Lewinski MK, Pache L, Stoneham CA, Yin X, Becker ME, et al. Functional landscape of SARS-CoV-2 cellular restriction. Mol Cell. 2021;81:2656–68 e8.
Fonseca GJ, Thillainadesan G, Yousef AF, Ablack JN, Mossman KL, Torchia J, et al. Adenovirus evasion of interferon-mediated innate immunity by direct antagonism of a cellular histone posttranslational modification. Cell Host Microbe. 2012;11:597–606.
Zhu B, Zheng Y, Pham AD, Mandal SS, Erdjument-Bromage H, Tempst P, et al. Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol Cell. 2005;20:601–11.
Zhang S, Wang J, Cheng G. Protease cleavage of RNF20 facilitates coronavirus replication via stabilization of SREBP1. Proc Natl Acad Sci USA. 2021;118:e2107108118.
Stanifer ML, Pervolaraki K, Boulant S. Differential Regulation of Type I and Type III Interferon Signaling. Int J Mol Sci. 2019;20:1445.
Sui H, Zhou M, Imamichi H, Jiao X, Sherman BT, Lane HC, et al. STING is an essential mediator of the Ku70-mediated production of IFN-λ1 in response to exogenous DNA. Sci Signal. 2017;10:eaah5054.
Chen J, Markelc B, Kaeppler J, Ogundipe VML, Cao Y, McKenna WG, et al. STING-dependent interferon-λ1 induction in HT29 cells, a human colorectal cancer cell line, after gamma-radiation. Int J Radiat Oncol Biol, Phys. 2018;101:97–106.
Di Domizio J, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603:145–51.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8.
Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinforma. 2010;26:2363–7.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 2009;10:161.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–50.
Bojkova D, Rothenburger T, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. SARS-CoV-2 Omicron variant virus isolates are highly sensitive to interferon treatment. Cell Discov. 2022;8:42.
Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep. 2009;10:1250–8.
Acknowledgements
The authors thank the members of the van Wijk lab for advice, discussions and support during the study, Dr. M. Bewerunge-Hudler and her team from the Genomics and Proteomics Core Facility, German Cancer Research Center/DKFZ, Heidelberg, Germany, for help and support with performing the microarray analysis and Christina Hugenberg for proofreading. S.J.L.v.W. is supported by the Deutsche Forschungsgemeinschaft (DFG) (WI 5171/1-1, FU 436/20-1 and project-ID 259130777-SFB 1177), the Deutsche Krebshilfe (70113680), the Frankfurter Stiftung für krebskranke Kinder and the Dr. Eberhard and Hilde Rüdiger Foundation. M.B. is supported by the DFG-CRC 850 subprojects C9 and Z1, CRC1479 (Project ID: 441891347- S1), CRC 1160 (Project Z02), CRC1453 (Project ID 431984000-S1) and TRR167 (Project Z01), the German Federal Ministry of Education and Research by MIRACUM within the Medical Informatics Funding Scheme (FKZ 01ZZ1801B and EkoEstMed–FKZ 01ZZ2015 for G.A.). S.B. was supported by DFG project numbers 415089553 (Heisenberg program), 240245660 (SFB1129), 278001972 (TRR186), and 272983813 (TRR179), the state of Baden-Württemberg (AZ: 33.7533.-6-21/5/1), the BMBF (01KI20198A) and within the Network University Medicine - Organo-Strat COVID-19. M.L.S. was supported by the BMBF (01KI20239B) and DFG project 416072091.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
RK performed experiments and analyzed data with help from JR, SS, and LK, MLS and SB performed SARS-CoV-2 infections and accompanying experiments, gene expression analysis was performed by GA and MB, RS provided access and support with the LEGENDplex analysis. MB, DB, and JCJr provided the Caco-2 and HCoEpiC cells and expertise. RK and SJLvW conceived the project and wrote the manuscript. All authors have read, commented, and agreed on the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Mauro Piacentini
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karlowitz, R., Stanifer, M.L., Roedig, J. et al. USP22 controls type III interferon signaling and SARS-CoV-2 infection through activation of STING. Cell Death Dis 13, 684 (2022). https://doi.org/10.1038/s41419-022-05124-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05124-w
- Springer Nature Limited
This article is cited by
-
USP22 regulates APL differentiation via PML-RARα stabilization and IFN repression
Cell Death Discovery (2024)