Abstract
Proteasomes are overexpressed in multiple myeloma (MM) and proteasomal inhibitors (PIs) have been widely used for the treatment of MM. PIs are reported to induce MM cell apoptosis but impair necroptosis. In the present study, we found that PIs MG132 and bortezomib induce MM cell pyroptosis, a novel type of cell death, in a GSDME-dependent manner. Lack of GSDME totally blocks PI-induced pyroptosis. Interestingly, we found that Caspase-3/6/7/9 are all involved in pyroptosis triggered by PIs because the specific inhibitor of each caspase ablates GSDME activation. PIs markedly reduce mitochondrial membrane potential. Moreover, PIs disrupt the interaction of Bcl-2 and BAX, induce cytochrome c release from mitochondria to cytosol and activate GSDME. Furthermore, we found that overexpression of an N-terminal portion of GSDME suffices to release cytochrome c from mitochondria and to activate Caspase-3/9, suggesting N-GSDME might penetrate the mitochondrial membrane. Consistent with Bcl-2 inhibition, BAX can induce MM cell pyroptosis in a GSDME-dependent manner. In accordance with these findings, inhibition of Bcl-2 synergizes with PIs to induce MM cell pyroptosis. Therefore, the present study indicates that PIs trigger MM cell pyroptosis via the mitochondrial BAX/GSDME pathway and provides a rationale for combined treatment of MM with Bcl-2 and proteasome inhibitors to increase therapeutic efficiency via induction of pyroptosis.
Similar content being viewed by others
Introduction
The proteasome is a multi-subunit enzyme complex that is highly expressed in multiple cancer cells where it quickly processes unfolded, misfolded, and aged proteins in support of cancer cell survival [1]. Proteasomes have been demonstrated as ideal therapeutic targets for cancers. Proteasome inhibitors (PIs), including bortezomib, carfilzomib and ixazomib, have been widely used to treat multiple myeloma (MM), an incurable malignancy derived from cloned plasma cells [2], but their mechanisms of action are not fully elucidated.
Induction of cancer cell death is a major goal in cancer treatment. In addition to apoptosis, several novel types of cell death have been identified and well documented, such as necroptosis, autophagic cell death, and pyroptosis [3]. PIs have been shown to induce apoptosis and autophagy [4] but impair necroptosis [5] in MM cells, indicating proteasomes may play a specific role in the process of different types of cell death. Pyroptosis is an inflammatory form of programmed cell death firstly reported to be triggered by microbial infection in association with the inflammasome pathways and activation of gasdermin D (GSDMD) [6]. During pyroptosis, the N-terminal domain of GSDMD forms a pore in plasma membrane and leads to the release of interleukin-1β (IL-1β) and interleukin-18 (IL-18) [6]. In addition to inflammation and infections, anti-cancer drugs such as etoposide and doxorubicin can also induce pyroptosis but via other GSDMs such as GSDME [7]. Although it is well known that PIs induce MM cell apoptosis and other forms of cell death, their roles in pyroptosis remain unknown. In the present study, we found that PIs trigger MM cell pyroptosis via BAX/GSDME. We also find that Bcl-2 inhibitors synergize with PIs against MM via pyroptosis.
Materials and methods
Cell culture
MM cell lines including RPMI-8226, OCI-My5, LP-1, KMS11, JJN-3, and OPM-2 were obtained from American Tissue and Cell Collection (ATCC, https://www.atcc.org) or from Dr. Aaron Schimmer, University of Toronto, Canada. All MM cells were cultured in Iscove’s Modified Dulbecco’s Media (IMDM). All media were supplemented with 10% FBS (TransGen Biotech Co., Ltd, Bei**g, China), 100 U/mL penicillin and streptomycin. All cells were grown at 37 °C in a 5% CO2 incubator.
Primary cells from patients with B-cell acute lymphocytic leukemia (B-ALL)
Primary cells were donated by patients with B-ALL from the Second Affiliated Hospital of Guangzhou Medical University with written consent for research purposes and approved by the Review Board and Ethics Committee of Guangzhou Medical University. After isolation with Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO, USA), cells were cultured in IMDM supplemented with fetal bovine serum (ExCell Bio, Shanghai, China) and appropriate antibiotics (Sigma-Aldrich).
Plasmids
The pWPI-GSDME and human pWPI -GSDME-D270A were kindly provided by Dr. Feng Shao (National Institute of Biological Sciences, Bei**g, China) [7]. GSDME-N and BAX were cloned from myeloma cell line OCI-My5. To construct the N-GSDME-T6E variant, codon 6 encoding threonine (T) was mutated to encode glutamic acid (E) by using a site-directed mutagenesis kit (TransGen Biotech). All genes were subcloned into a pCDH-CMV vector carrying a Flag tag.
Antibodies and reagents
Anti-caspase-1 (Cat. #2225 S) and anti-IL-1β (Cat. #12703 S) were from Cell Signaling Technology. Anti-caspase-3 (Cat. #19677-1-AP), anti-caspase-6 (Cat. #10198-1-AP), anti-caspase-7 (Cat. #27155-1-AP), anti-caspase-8 (Cat. #66093-1-Ig), anti-caspase-9 (Cat. #10380-1-AP), anti-BAX (Cat. #50599-2-Ig), anti-Bcl2 (Cat. #60178-1-Ig), anti-cytohrome c (Cyto c) (Cat. #10993-1-AP), anti-VDAC1 (Cat. #55259-1-AP), anti-β-actin (Cat. #20536-1-AP), and anti-GAPDH (Cat. #10494-1-AP) were obtained from Proteintech Group (Wuhan, China). Anti-DFNA5/GSDME (Cat. #ab215191), Z-VEID-FMK (Cat. #ab142025), and anti-GSDMB (Cat. #ab215729) were obtained from Abcam. Anti-GSDMA (Cat. #PA5-24813) and Anti-GSDMC (Cat. #PA5-24259) were from Invitrogen. Anti-GSDMD (Cat. #NBP2-33422) was from Novus Biologicals. MG132 (Cat. #S2619), Bortezomib (BZ) (Cat. #S1013), Venetoclax (Cat. #S8048), Z-DEVD-FMK (Cat. #S7312), and Z-LEHD-FMK (Cat. #S7313) were obtained from Selleck Chemicals. Hoechst 33342 (Cat. #C1026) and propidium iodide (Cat. #ST511) were purchased from Beyotime Institute of Biotechnology (Nantong, China). Lipopolysaccharide (LPS, Cat. #L4391), Nigericin (Cat. #HY-100381), and Calcein AM (Cat. #17783) were provided by Sigma-Aldrich. MitoTracker Red CMX Ros (Cat. #M7512) was purchased from Invitrogen.
CRISPR-Cas9 knockout (KO)
The KO cell lines were generated by using CRISPR/Cas9 technology. The CRISPR design tools at Benchling (https://benchling.com) were used to identify single-guide RNA (sgRNA) and two sgRNAs of GSMDE (5ʹ-GTATAACTCAATGACACCGT-3ʹ and 5ʹ-GAGAAGTGTGGTGGCATCGT-3ʹ) were cloned into the gRNA-expression plasmid LentiCRISPR v2-Puro (Addgene.org) as described previously [8].
Generation of stable cell lines
Forty-eight hours after infection with virus containing genes of interest the cells were sorted by flow cytometry (BD Biosciences, FACS Aria III) and further cultured in IMDM containing puromycin (MedChemExpress, Shanghai, China).
LDH release assay
Lactate dehydrogenase (LDH) in culture media was measured by using the LDH Cytotoxicity Assay Kit (Beyotime) as instructed by the manufacturer.
The enzyme-linked immunosorbent assay (ELISA)
Mature IL-18 (Cat. #DL180) and IL-1β (Cat. #DLB50) in culture media was measured by using the ELISA kit from R&D Systems according to the instructions of the manufacturer.
Microscopy analysis
The morphology of pyroptotic cells were captured using a Zeiss AxioVert A1 microscope.
Calcein AM/Propidium Iodide (PI) staining
Celllines of interest were treated with MG132 or BZ as required followed by staining with Calcein AM (4 μg/ml) and PI (30 μg/ml) for 30 min in dark and visualized by fluorescence microscopy (Zeiss AxioVert A1).
Immunoblotting (IB)
Cell lysates from cells of interest were fractionated by SDS-PAGE, the isolated proteins were then transferred to polyvinylidenedifluoride membranes. The blots were subjected to IB analysis against appropriate antibodies as described previously [9].
Cytochrome c (Cyto c) release assay
Culture media from cells of interest were collected and clarified. The supernatants were then subjected to measurement of the Cyto c concentrations by using the Cyto c ELISA Kit (Cat. #E0594h, EIAab Science Inc., Wuhan, China).
Mitochondrial membrane potential (MMP) assay
Cells of interest were treated with MG132 or BZ before analysis of MMP by using the Mitochondrial Membrane Potential Assay Kit with TMRE (Cat. #C2001S, Beyotime) as instructed by the manufacturer. MMP was analyzed by fluorescence microscopy (Zeiss AxioVert A1).
Mitochondria isolation
Mitochondria were isolated by using the Cell Mitochondria Isolation Kit (Cat. #C3601, Beyotime) as instructed by the manufacturer.
Statistics
Statistical difference between the control and the experimental groups was analyzed by Student’s t test. P < 0.05 was considered statistically significant. All experiments were repeated at least 3 times independently.
Results
PIs trigger myeloma cell pyroptosis
Inhibition of proteasomes has been found to induce MM cell apoptosis and autophagy [4], but impairs necroptosis [5]. To find out whether PIs induce MM cell pyroptosis, MM cells were treated with typical PIs MG132 and BZ. We found some MM cell lines displayed balloon-like morphology and the nuclei were forced to one side of the cell and the other side was clear (Fig. 1a), a typical early feature of pyroptosis [7]. Moreover, pyroptotic cells were increased upon the concentrations of PIs (Fig. 1b). To further characterize cell membrane integrity of these dying/dead MM cells upon proteasomal inhibition, OCI-My5 and OPM-2 cells were treated with MG132 or BZ for 24 h at low concentrations, followed by Calcein AM/propidium iodide staining [10] and measurement of LDH in culture media [7]. The results showed that most of the cells remained Calcein AM positive, but some were balloon-like and propidium iodide positive (Supplementary Fig. 1), indicating the plasma membranes of these cells were disrupted, which was confirmed by increased LDH levels in cell culture media (Fig. 1c). All these findings suggest that PIs led to MM cell pyroptosis.
a OCI-My5 and OPM-2 cells were treated with MG132 or BZ at indicated concentrations. Phase-contrast imaging assays were performed. Arrowheads indicated balloon-like pyroptotic cells, scale bar: 20 μm. b Statistic analyses of pyroptotic cells after MG132 and BZ treatment from (a). c The measurement of LDH in culture medium after MG132 and BZ treatment. Results were representative of at least three independent experiments. Error bars represented standard error of the mean (s.e.m). Student’s t test, **P < 0.01, ***P < 0.001, against the control (0).
PIs activate GSDME in association with Caspase-3 but not Caspase-1
Given pyroptosis depends on the characteristic cleavage and activation of GSDM proteins [11], we next measured the expression profiles of five GSDMs including GSDM-A, -B, -C, -D, and -E in MM cell lines. We found that GSDM-A and -B were expressed in LP1 cells only (1 of 6), while GSDM-C, -D, and -E were found in most of MM cells (Fig. 2a). Subsequent studies showed that GSDM-D and –E but not GSDM-A, -B and -C were cleaved by PIs (Fig. 2b). However, the cleavage of GSDMD generated a fragment with a molecular weight of 43 kDa, a hallmark of apoptotic activation [12]. In contrast, GSDME was cleaved to generate a characteristic product of 35 kDa, the activated N-terminal fragment that oligomerizes to form a pore in the plasma membrane [7]. These results suggest PIs might induce GSDME-dependent pyroptosis in MM cells. To confirm this hypothesis, two typical MM cell lines OCI-My5 and OPM-2 were further treated with LPS + Nig (a typical trigger for classic GSDMD-dependent pyroptosis) [13], or MG132 or BZ. The subsequent IB assays showed that, as expected, LPS + Nig resulted in cleavage of GSDMD to a p35 product, along with activated Caspase-1 and mature IL-1β, hallmarks of GSDMD-dependent pyroptosis (Fig. 2c). In contrast, in a concentration-dependent manner, both MG132 and BZ resulted in cleavage of GSDMD to a p43 product that indicated cell apoptosis, and GSDME to a p35 product that indicated cell pyroptosis (Fig. 2d). Interestingly, PIs activated Caspase-3 but not Caspase-1 (Fig. 2c). Moreover, the concentrations of IL-18 and IL-1β were strikingly raised upon the treatment with LPS + Nig but not by either MG132 or BZ (Supplementary Fig. 2), which was consistent with the hypothesis that IL-18 and IL-1β are cleaved by Caspase-1 before they can be released from cytoplasm to culture media. Therefore, PIs induced both GSDMD-dependent apoptosis and GSDME-dependent pyroptosis in MM cells and GSDME might play a critical role in switching MM cells from apoptosis to pyroptosis.
a The expression profile of GSDMs in MM cell lines analyzed by IB. b MM cells were treated with MG132 or BZ as indicated. Cell lysates were subjected to IB assays. c OCI-My5 and OPM-2 cells were treated with LPS + Nigericin (Ni), MG132 or BZ as indicated. Cell lysates were prepared for IB assay. d RPMI-8226, OCI-My5 and OPM-2 cells were treated with increased BZ or MG132 for 24 h, followed by IB assays as indicated.
Caspase-3, -6, -7 and -9 are involved in PI-triggered MM cell pyroptosis
Given caspases are key players in GSDM activation and pyroptosis, we wondered which caspase(s) are involved in PI-induced pyroptosis. To this end, OCI-My5 and OPM-2 cells were treated with a range of MG132 or BZ concentrations. The results showed that both MG132 and BZ markedly cleaved GSDME in a concentration-dependent manner, in association with induced cleavage of Caspase-3, -6, -7 and -9, but not Caspase-8 (Fig. 3a). To find out which caspase(s) are responsible for GSDME activation, specific inhibitors of each caspase were co-treated with MG132 or BZ, followed by IB assays to measure GSDME cleavage. The results showed that all the inhibitors of Caspase-3, -6, -7 and -9 strikingly inhibited the cleavage of each corresponding caspase and GSDME (Fig. 3b–d), suggesting Caspase-3/6/7/9 might participate in PI-induced pyroptosis and activation of GSDME.
a OCI-My5 and OPM-2 cells were treated with MG132 or BZ at increased concentrations. Cell lysates were then prepared for IB assays as indicated. OCI-My5 and OPM-2 cells were treated with MG132 or BZ and zDEVD (b), zVEID (c) or zLEHD (d) as indicated. Cell lysates were prepared for IB assays against specific proteins as indicated.
GSDME is required for PI-induced pyroptosis
The above study found that PIs lead to pyroptotic cleavage of GSDME but not other GSDMs, indicating pyroptosis in MM cells might be GSDME-dependent. To confirm this hypothesis and to check the role of GSDME in PI-induced pyroptosis, RPMI-8226, a typical MM cell line lacking GSDME (Fig. 2a), was treated with MG132 or BZ for 24 h. The observation showed that RPMI-8226 did not present marked pyroptotic morphology (Fig. 4a) and the LDH concentration was not significantly increased (Fig. 4b). However, when wild-type (wt) but not the D270A mutant GSDME was introduced into RPMI-8226 cells by lentivirus, MG132 and BZ triggered a pyroptotic appearance (Fig. 4c). This phenotype was consistent with the concentrations of LDH in these cells (Fig. 4d) and the cleavage of GSDME and Caspase-3 (Fig. 4e), suggesting GSDME is required for pyroptosis in MM cells after inhibition of proteasomes. To further confirm this hypothesis, GSDME was knocked out from OCI-My5 and OPM-2 cells, followed by re-expression wt or D270A GSDME, and treatment of MG132 or BZ. The morphological analyses found that MG132 and BZ induced pyroptosis in both cell lines with re-expression of wtGSDME but not its D270A mutant (Supplementary Fig. 3), which was consistent with the finding that wtGSDME but not D270A mutant was cleaved by PIs, along with Caspase-3 activation (Fig. 4f and Supplementary Fig. 4). The alterations of GSDME/Caspase-3 in these cells were consistent with the LDH release (Fig. 4g and Supplementary Fig. 4d). Therefore, GSDME is required for PI-induced MM cell pyroptosis.
a RPMI-8226 cells lacking GSDME were treated with MG132 or BZ, followed by microscopy analysis. b RPMI-8226 cells were treated with increased MG132 or BZ for 24 h, followed by LDH measurement and analysis. c RPMI-8226 cells were infected with lentiviral GSDME or its D720A mutant. Four days later cells were treated with MG132 or BZ, followed by phase-contrast image analysis. d Cell culture medium from (c) was subjected to LDH measurement. e RPMI-8226 cells were infected with lentiviral GSDME or its D720A mutant. Four days later cells were treated with MG132 or BZ, cell lysates were subjected to IB assays as indicated. f OPM2 and OCI-My5 cells were subjected to knock out of GSDME, followed by re-infection with lentiviral wild-type (WT) or D270A (DA) GSDME. Ninety-six hours later, cells were treated with MG132 for 24 h followed by IB assays. g The cell culture media from (f) were subjected to determine LDH release measurement. Error bars represented standard error of the mean (s.e.m). Scale bar: 20 μm. Student’s t test, ***P < 0.001.
N-GSDME promotes the release of Cyto c from mitochondria and activates Caspase-9/3
The above study showed that more Caspase-3 cleavage was observedin the presence of GSDME (Fig. 4e, f), suggesting GSDME might promote the activation of Caspase-3. To verify this hypothesis, RPMI-8226 cells were infected with lentiviral full-length (F) GSDME, N-GSDME or inactivated N-GSDME-T6E (Fig. 5a). The subsequent assays showed that N-GSDME but not F-GSDME or N-GSDME-T6E activated Caspase-3 and -9 (Fig. 5a), along with increased pyroptotic cells (Fig. 5b) and increased LDH in the culture media (Fig. 5c). This finding suggested that N-GSDME could directly activate Caspase-3/9. Given that Caspase-3/9 activates GSDME (Fig. 3), these findings revealed a positive feedback circuit between caspases and GSDME.
a RPMI-8226 cells were infected with wtGSDME, N-GSDME or N-GSDME-T6E lentivirus for 96 h, followed by IB assays as indicated. Phase-contrast imaging assay (b) and LDH measurement in culture medium (c) were performed on RPMI-8226 cells after treatment as shown in (a). Arrowheads indicated pyroptotic cells, scale bar: 20 μm. d OCI-My5 and OPM-2 cells were treated with MG132 or BZ, followed by isolation of the mitochondrial and cytosol fractions and IB assay as indicated. e The culture media from (d) were subjected to Cyto c determination. f RPMI-8226 cells were infected with indicated lentivirus for 96 h, followed by MG132 or BZ treatment, cells were then subjected isolation of the mitochondrial and cytosol fractions and IB assays. g The culture media from (f) were subjected to Cyto c determination. h RPMI-8226 cells were infected with wtGSDME, N-GSDME or N-GSDME-T6E lentivirus for 96 h, followed by isolation of the cytosol and mitochondrial fractions that were subjected to IB assays as indicated. i The culture media from (h) were subjected to Cyto c determination. Error bars represented standard error of the mean (s.e.m). Student’s t test, **P < 0.05, ***P < 0.001.
Given formation of the Apaf-1/Cyto c complex precedes activation of Caspase-9 and Caspase-9 activation is highly associated with Cyto c release from mitochondria, we next wondered PIs altered Cyto c and GSDME in mitochondria. To this end, we measured the distribution of GSDME and Cyto c in cytosol and mitochondria in OCI-My5 and OPM-2 cells after MG132 or BZ treatment. The results showed that both MG132 and BZ induced cleavage of GSDME, and both F- and N-GSDME could be found in cytosol and mitochondria (Fig. 5d). As expected, Cyto c was strikingly reduced from mitochondria and more was found in cytosol (Fig. 5d), suggesting the outer membrane of mitochondria was impaired. In addition, Cyto c was also found in cell culture media, a hallmark of pyroptosis (Fig. 5e), further suggesting PIs impaired mitochondrial membranes in association with pyroptosis. To further verify this hypothesis, RPMI-8226 cells lacking GSDME were infected with wtGSDME or its D270A mutant, followed by MG132 or BZ treatment. The IB assays showed that MG132 and BZ induced more Cyto c leakage from mitochondria into cytosol in the presence of wt but not D270A GSDME (Fig. 5f). These findings further demonstrated that GSDME cleavage promotes Cyto c release from mitochondria in the presence of PIs. Notably, Cyto c was also found in culture media from PRMI-8226 cells expressing wtGSDME but not its mutant (Fig. 5g), further indicating the impairment of plasma and mitochondria membranes is dependent on GSDME. Lastly, we assayed the distribution of Caspase-9 and Caspase-3 upon GSDME expression. Again, we found that only N-GSDME but not the D720A mutant or full length GSDME increased the activation Caspase-9 and -3 in cytosol (Fig. 5h) and promoted the release of Cyto c in cell culture media (Fig. 5i).
It is reported that mitochondria aggregation contributes to Cyto c release from mitochondria to cytosol [14]. To find out whether proteasome inhibitors induced Cyto c release was associated with mitochondrial aggregation, MM cells treated with MG132 or BZ and then stained with MitoTracker/Hoechst. Subsequent confocal microscopy analyses revealed that mitochondria formed large aggregates in the presence of MG132 or BZ (Supplementary Fig. 5). Therefore, all the above findings suggest that PIs promote MM cell pyroptosis by cleaving GSDME/Caspase-3/9 and promoting Cyto c release from mitochondria.
PIs impair the integrity of mitochondrial membrane by inhibiting Bcl-2 interacting with BAX
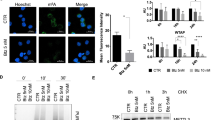
The above study suggests the GSDME is an essential factor in PI-induced pyroptosis, and its activation might further promote Cyto c leaks from mitochondria and activation of Caspase-9/3. Given the leak of Cyto c from mitochondria is a hallmark of mitochondrial impairment [15], we evaluated the effects of PIs on mitochondrial membrane potential (MMP) in MM cells treated with MG132 or BZ. After treatment, cells were stained with Rhodamine B, a mitochondrial probe for MMP [16]. Fluorescence microscopy analyses revealed that pyroptotic cells completely failed to be stained by Rhodamine B (Fig. 6a), suggesting that PIs lead to loss of MMP and PI-induced pyroptosis might be associated with dysfunction of mitochondria.
a OCI-My5 and OPM-2 cells were treated with MG132 or BZ for 24 h, followed by TMRE staining. The MMP was visualized by fluorescence microscope, scale bar: 20 μm. The insets showed higher magnification images of pyroptotic cells. b OCI-My5 and OPM-2 cells were treated with MG132 or BZ, followed by IB assays against proteins as indicated. c OCI-My5 and OPM-2 cells were treated with MG132 or BZ, the cell lysates were then prepared for IP/IB assays as indicated. d OCI-My5 and OPM-2 cells were treated with Venetoclax (Ven) for 24 h. Phase-contrast imaging assay was performed. Arrowheads indicated the pyroptotic cells. Scale bar: 20 μm. The insets showed higher magnification images of pyroptotic cells. e OCI-My5 and OPM-2 cells were treated with Venetoclax for 24 h at increased concentrations, followed by IB assay of cell lystates. f OCI-My5 and OPM-2 cells were treated with Ven, BZ or MG132 as indicated, followed by IB assays.
MMP is an important indicator of mitochondrial integrity which is typically controlled by Bcl-2 family proteins, such as Bcl-2 and BAX [17]. Dissociated from Bcl-2, BAX can translocalize to the mitochondrial membrane and form a pore with BAK, thus releasing Cyto c to activate apoptotic Caspase-9 [18]. Given PIs lead to MMP reduction and GSDME cleavage, we wondered whether PIs affected the stability and interaction between Bcl-2 and BAX. To this end, we first examined the stability of Bcl-2 and BAX proteins after PI treatment. To our surprise, neither MG132 nor BZ stabilized Bcl-2 or BAX (Fig. 6b). Next, we examined the interaction between Bcl-2 and BAX in the presence of PIs. The reciprocal IP/IB assays demonstrated that PIs markedly decreased the interaction between Bcl-2 and BAX (Fig. 6c). Given Bcl-2 inhibitors also disrupt the interaction of Bcl-2 with BAX-like proteins, we wondered whether inhibition of Bcl-2 could also induce pyroptosis. To this end, MM cells were treated with Venetoclax, an approved Bcl-2 inhibitor for the treatment of lymphoma and myeloma [19]. The results showed that Venetoclax induced balloon-like pyroptotic morphology (Fig. 6d) and triggered the cleavage of both GSDME and Caspase-3 (Fig. 6e), suggesting inhibition of Bcl-2 indeed induces MM cell pyroptosis. Furthermore, we found that the Venetoclax strikingly increased the cleavage of both GSDME and Caspase-3 by both MG132 and BZ (Fig. 6f). These pyroptotic indicators were also found in primary cells from patients with B-cell acute lymphocytic leukemia after the treatment of Venetoclax and BZ (Supplementary Fig. 6). Therefore, inhibition of Bcl-2 induces MM cell pyroptosis suggesting that inhibitors of both Bcl-2 and proteasomes could be used for the combined treatment of MM.
BAX induces MM cell pyroptosis in a GSDME-dependent manner
After dissociation from Bcl-2, BAX is able to penetrate mitochondrial membranes and forms a pore with BAK to permit leakage of Cyto c from mitochondria [18]. Therefore, we wondered whether BAX itself was sufficient to induce MM cell pyroptosis. To this end, we knocked out GSDME from MM cell lines OCI-My5 and OPM-2 followed by re-expression of wt- or D270A GSDME. These cells were then subjected to overexpression of BAX by lentivirus. The subsequent morphological analysis showed that pyroptotic cells were observed in BAX-overexpressing cells (Fig. 7a). BAX also induced pyroptosis in KO cells re-expressing wt- but not D270A GSDME (Fig. 7a), suggesting that GSDME was required for BAX-induced MM cell pyroptosis. This finding was consistent with that overexpression of BAX led to striking cleavage of GSDME in association with Caspase-3 activation (Fig. 7b). Consequent with these findings, BAX markedly promoted LDH release from MM cells in the presence of wt- but not D270A GSDME (Fig. 7c). All these results thus concluded that BAX overexpression is able to trigger MM cell pyroptosis in a GSDME-dependent manner. They also demonstrate that PIs dissociate BAX from Bcl-2 and allow BAX to penetrate the mitochondrial membrane to form a pore that permits Cyto c release into the cytosol, therefore finally inducing MM cell pyroptosis (Fig. 7d).
OCI-My5 and OPM-2 cells were knocked out GSDME followed by infection with WT or D270A GSDME lentivirus. These cells were then infected with BAX lentivirus or empty virus for 96 h. a Phase-contrast imaging assays. Arrowheads indicated pyroptotic cells. Scale bar: 20 μm. b IB assays for the proteins as indicated. c LDH measurement in culture media. d The proposed mechanism of inhibition of proteasomes in promoting myeloma cell pyroptosis.
Discussion
The above studies have demonstrated that PIs trigger MM cell pyroptosis by impairing mitochondria and activating GSDME, specifically, PIs disrupt the interaction between Bcl-2 and BAX, therefore releasing Cyto c and activating Caspase-9/-3/GSDME. Moreover, we also identified that activated GSDME is also a regulator of caspases in promoting MM cell pyroptosis.
The classic pyroptosis pathway is mediated by the inflammatory Caspase-1/4/5/11-GSDMD axis upon bacterial or viral infection [20]. Recent research demonstrated that apoptotic caspases such as Caspase-3, -6, -8, and other GSDM proteins (such as GSDM-A, -B, -C and -E) are also involved in chemical- or granzyme-induced pyroptosis [21]. For example, doxorubicin can induce pyroptosis via Caspase-8/GSDMC [7]. In our study, we found that GSDME but not other GSDMs is essential for PI-induced pyroptosis because both MG132 and BZ, two typical proteasome inhibitors, fail to induce MM cell pyroptosis in the absence of GSDME. Moreover, we found that when GSDME is mutated at D270, a cleaving site for Caspase-3, PIs could not induce MM cell pyroptosis suggesting PI-induced MM cell pyroptosis is GSDME-dependent. In addition to inflammatory caspases, other caspases such as Caspase-6 and caspase-3 mediate cell pyroptosis [22] and the Shao group believed that only caspase-3 is able to cleave GSDME based on cell-free assays [7]. However, in MM cells we found that the specific inhibitors of Caspase-6 or -7 or -9 not only inhibited the activity of each corresponding caspase, but also decreased the cleavage of GSDME. These findings suggest that unlike the cell-free context, in the more complex intracellular milieu, in addition to Caspase-3, other caspases including Caspase-6, -7 and -9 can function in GSDME activation and MM cell pyroptosis. The involvement of Caspase-9 in pyroptosis could be easily understood because Caspase-9 is the upstream enzyme that activates Caspase-3. One of the novelties of the study is that Caspase-6 and -7 are also able to cleave GSDME and involved in pyroptosis.
It is well known that caspases cleave GSDM proteins to initiate pyroptosis, however, in the present study, we found that N-GSDME is sufficient to promote Cyto c release from mitochondria and to activate Caspase-9 and -3, suggesting there might be positive feedback between Caspase-3 and GSDME. Our finding is consistent with the previous report that GSDME localizes to mitochondria [23], and adds mechanistic insight including the specific role of N-GSDME (but not the inactive N-GSDME-T6E variant) in promoting Cyto c release and Caspase-9 and -3 activation. These findings also suggest that in addition to GSDME acting at the plasma membrane to form a pore to finalize pyroptosis, it might also act at the mitochondria membrane to induce mitochondria-derived pyroptosis. Interestingly, a recent study revealed that GSDME induces mitochondrial DNA release from cells during pyroptosis and apoptosis as a damage-associated molecular pattern [24] although the fundamental mechanisms might be different.
The integrity of the mitochondrial membrane is well maintained by Bcl-2 family proteins, in which Bcl-2 binds to BAX and restricts it from penetration of the outer mitochondrial membrane and thereby preventing release of Cyto c [25]. In the present study, we found that PIs dissociate BAX from Bcl-2, in a manner similar to Venetoclax, an approved anti-leukemia and anti-lymphoma drug and a specific Bcl-2 inhibitor. Venetoclax leads to GSDME cleavage and induces MM cell pyroptosis. This finding is consistent with a previous report in that navitoclax, another Bcl-2/Bcl-xL inhibitor, induces colon cancer cell pyroptosis [21]. In our study, we further find that BAX expression is sufficient to cleave GSDME at D270 thereby triggering MM cell pyroptosis. Notably, the combination of Bcl-2 inhibitors and PIs could significantly promote MM cell pyroptosis. This finding is consistent with a previous report in which BAX/BAK is able to induce cancer cell pyroptosis [21]. In the present study, we found that Bcl-2/BAX and GSDME are essential for PI-induced MM cell pyroptosis because PIs inhibit the binding of Bcl-2 to BAX and allow BAX to form a pore in the mitochondrial membrane. Moreover, BAX alone was able to activate Caspase-3/GSDME and MM cell pyroptosis. Interestingly, a previous study claimed that Bcl-2 may suppress GSDMD cleavage at D275 by Caspase-1, -4 or -5 but enhances GSDMD cleavage at D87 in leukemia cells [26]. However, whether Bcl-2 directly alters GSDME in MM cells requires further study. A previous report that finds PIs block GSDMD-induced pyroptosis in epithelial cells [27] is consistent with our study. As shown in Fig. 2, PIs cleave GSDMD at D87, which leads to cell apoptosis [12]. Moreover, cleavage at D87 prevents GSDMD activation at D275 that is essential for classical and non-classical but GSDMD-dependent pyroptosis.
Mitochondrial N-GSDME but not the inactive N-GSDME-T6E variant forms a pore in mitochondria to promote Cyto c leakage from mitochondria thereby activating Caspase-9/3 and triggering cell pyroptosis. Given this finding and other conclusions, we believe that there might be a positive feedback between Caspase-3 and GSDME. Specifically, inhibition of proteasomes enables BAX to penetrate the outer mitochondrial membrane and activate Caspase-3 (and probably other caspases, such as Caspase-6 and Caspase-7 in a similar manner) that further cleaves GSDME at D270, which generates N-GSDME to generate plasma membrane pores for pyroptosis. On the other hand, Caspase-3-cleaved GSDME (N-GSDME) translocalizes to mitochondria and promotes mitochondria-dependent activation of the Cyto c/Caspase-9/Caspase-3 axis and MM cell pyroptosis. This study therefore provides a novel understanding of mitochondrion-derived pyroptosis and highlights a novel role of proteasomes in preventing cell pyroptosis.
In summary, the present study demonstrates that PIs induce MM cell pyroptosis in a mitochondrion-dependent manner, specifically the BAX/GSDME axis. Given Bcl-2/BAX and GSDME are also involved in PI-induced pyroptosis, targeting Bcl-2 and proteasomes might be an ideal combination therapy strategy for myeloma by targeting both apoptotic and pyroptotic pathways. Moreover, BAX or GSDME inducers may also sensitize proteasomal inhibitors for MM treatment.
References
Thibaudeau TA, Smith DM. A practical review of proteasome pharmacology. Pharmacol Rev. 2019;71:170–97.
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327:464–77.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21.
Laussmann MA, Passante E, Dussmann H, Rauen JA, Wurstle ML, Delgado ME, et al. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 2011;18:1584–97.
Ali M, Mocarski ES. Proteasome inhibition blocks necroptosis by attenuating death complex aggregation. Cell Death Dis. 2018;9:346.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103.
Xu Y, Xu M, Tong J, Tang X, Chen J, Chen X, et al. Targeting the Otub1/c-Maf axis for the treatment of multiple myeloma. Blood. 2021;137:1478–90.
Zhang Z, Tong J, Tang X, Juan J, Cao B, Hurren R, et al. The ubiquitin ligase HERC4 mediates c-Maf ubiquitination and delays the growth of multiple myeloma xenografts in nude mice. Blood. 2016;127:1676–86.
Qiu Z, He Y, Ming H, Lei S, Leng Y, **a ZY. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced Injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:8151836.
Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57.
Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–14.e4.
Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–55.e20.
Haga N, Fujita N, Tsuruo T. Mitochondrial aggregation precedes cytochrome c release from mitochondria during apoptosis. Oncogene. 2003;22:5579–85.
Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–33.
Reungpatthanaphong P, Dechsupa S, Meesungnoen J, Loetchutinat C, Mankhetkorn S. Rhodamine B as a mitochondrial probe for measurement and monitoring of mitochondrial membrane potential in drug-sensitive and -resistant cells. J Biochem Biophys Methods. 2003;57:1–16.
Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria–specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–9.
Aluvila S, Mandal T, Hustedt E, Fajer P, Choe JY, Oh KJ. Organization of the mitochondrial apoptotic BAK pore: oligomerization of the BAK homodimers. J Biol Chem. 2014;289:2537–51.
Chen X, Glytsou C, Zhou H, Narang S, Reyna DE, Lopez A, et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 2019;9:890–909.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H, et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 2020;11:281.
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–9.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689.
de Torre-Minguela C, Gómez AI, Couillin I, Pelegrín P. Gasdermins mediate cellular release of mitochondrial DNA during pyroptosis and apoptosis. FASEB J. 2021;35:e21757.
Ku B, Liang C, Jung JU, Oh BH. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011;21:627–41.
Shi CS, Kehrl JH. Bcl-2 regulates pyroptosis and necroptosis by targeting BH3-like domains in GSDMD and MLKL. Cell Death Discov. 2019;5:151.
Griswold AR, Huang HC, Bachovchin DA. The NLRP1 inflammasome induces pyroptosis in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2022;63:2.
Acknowledgements
The author extended many thanks to Prof. Michael F. Moran, The Hospital for Sick Children, University of Toronto, Toronto, Canada, for his critical reading and editing of the manuscript. This project was partly supported by National Natural Science Foundation of China (#82170176, #81970194), by the National Key Research and Development Program of China (#2022YFC2705003), by Guangzhou Medical University Discipline Construction Funds (Basic Medicine) (#JCXKJS2022A05), by Guangzhou Key Discipline of Medicine (Geriatric Medicine, #ZDXK202103).
Author information
Authors and Affiliations
Contributions
XLM designed the study; JPL, YMH, YLC, and YNS conducted experiments; XLM, JPL, and YMH analyzed data; GSH and ZGZ provided key materials. JPL, YMH and XLM wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Jp., He, Ym., Cui, Yl. et al. Proteasomal inhibitors induce myeloma cell pyroptosis via the BAX/GSDME pathway. Acta Pharmacol Sin 44, 1464–1474 (2023). https://doi.org/10.1038/s41401-023-01060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01060-3
- Springer Nature Singapore Pte Ltd.