Abstract
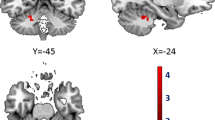
Electroconvulsive therapy (ECT) is the most effective treatment for severe depression and works by applying an electric current through the brain. The applied current generates an electric field (E-field) and seizure activity, changing the brain’s functional organization. The E-field, which is determined by electrode placement (right unilateral or bitemporal) and pulse amplitude (600, 700, or 800 milliamperes), is associated with the ECT response. However, the neural mechanisms underlying the relationship between E-field, functional brain changes, and clinical outcomes of ECT are not well understood. Here, we investigated the relationships between whole-brain E-field (Ebrain, the 90th percentile of E-field magnitude in the brain), cerebro-cerebellar functional network connectivity (FNC), and clinical outcomes (cognitive performance and depression severity). A fully automated independent component analysis framework determined the FNC between the cerebro-cerebellar networks. We found a linear relationship between Ebrain and cognitive outcomes. The mediation analysis showed that the cerebellum to middle occipital gyrus (MOG)/posterior cingulate cortex (PCC) FNC mediated the effects of Ebrain on cognitive performance. In addition, there is a mediation effect through the cerebellum to parietal lobule FNC between Ebrain and antidepressant outcomes. The pair-wise t-tests further demonstrated that a larger Ebrain was associated with increased FNC between cerebellum and MOG and decreased FNC between cerebellum and PCC, which were linked with decreased cognitive performance. This study implies that an optimal E-field balancing the antidepressant and cognitive outcomes should be considered in relation to cerebro-cerebellar functional neuroplasticity.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) affects more than 163 million people, approximately 2% of the world’s population in 2017 [1]. MDD is characterized by persistently depressed mood, anhedonia, impaired cognitive function, and suicidal thoughts [2]. Electroconvulsive therapy (ECT) is one of the most effective treatments along with accelerated transcranial magnetic stimulation, repetitive transcranial magnetic stimulation, Ketamine, and deep brain stimulation for treatment-resistant depressive episodes, which passes a controlled electric current through the brain under general anesthesia, producing substantial improvement in 60 to 80 percent of patients [3]. Despite its effectiveness, ECT may cause cognitive side-effects, including impairment in attention, memory, and executive functioning [4,5,6]. ECT’s mechanisms of cognitive side-effects and antidepressant response are poorly understood. This gap in knowledge limits parameter development to optimize antidepressant benefits and reduce cognitive risk.
ECT promotes changes in how brain cells communicate to normalize aberrant depression-related brain functioning, which is commonly known as neuroplasticity [7]. A wide range of ECT-induced functional connectivity (the strength with which activity in brain regions correlates over time) changes have been implicated in both antidepressant and cognitive outcomes [8, 9]. ECT can normalize dysregulated brain networks in MDD [10], such as the default-mode network, which is involved in self-referential processing including emotion perception [11, 12]. A longitudinal study has demonstrated that ECT modulates the function of the default-mode network, accompanied by improved mood and impaired cognitive function, where the connectivity changes and impaired cognitive function recovered one month after the completion of ECT [17, 18] and functionally [19]. The cerebellum is considered in the pathological model of MDD and the alterations of cerebro-cerebellar connectivity implied neural deficits in depression [20]. Neuroimaging studies have also identified significant cerebellar changes following ECT [21, 22], indicating a potential association between ECT and cerebellar neuroplasticity. Cerebro-cerebellar connectivity changes may be associated with cognitive performance, which implies a potential neural pathway for the mitigation of ECT-induced side-effects [23]. Although increasing evidence has linked the functional connectivity changes to ECT, the mechanisms underlying the relationships between functional neuroplasticity and ECT response, especially the short-term cognition changes, are still unknown.
Research on electric field (E-field) modeling and ECT has tried to link the E-field strength to brain neuroplasticity, with robust associations identified between E-field and structural neuroplasticity [24]. Another study reported that E-field in the temporal lobes is correlated with less optimal ECT outcome [25]. In the context of E-field modeling, the electrode placement determines the geometric shape of the E-field, and the amplitude determines the E-field magnitude [26]. Note that the whole-brain E-field and stimulus amplitude is related (r = 0.7129, p = 6.31 × 10−9). However, with a fixed extracranial amplitude, the ECT “doses” as represented by the intracranial E-field is highly variable due to the anatomic difference in skin, skull, fluid, and brain tissue [27]. The anatomic variability is prominent in older patients with depressive episodes, which can compromise both antidepressant efficacy and safety. E-field is a more accurate depiction of the electric field dose relative to pulse amplitude. It requires pre-ECT anatomic images to achieve the goal of individualized amplitude and reducing the variability of ECT dose, equipment, and expertize. We believe that the investigation of E-field variability will create a more standardized and consistent ECT dosing strategy for treatment with ECT, thus improving the ECT-induced outcomes. We have previously identified a trade-off between amplitude strength on the antidepressant (higher is better) and cognitive outcomes (lower is better) [28]. We also demonstrated that hippocampal neuroplasticity significantly mediated the relationship between E-field strength and antidepressant outcomes, E-field strength was directly related to cognitive side-effects [29]. Nevertheless, previous ECT E-field investigations limited analyses to the structural changes induced by ECT. Functional neuroplasticity is also a key element in ECT investigations, as it may reflect the brain’s capability to restructure itself by forming new neural connections. To date, no study has examined the relationship between E-field strength, functional neuroplasticity, and clinical outcomes.
In this work, we shift the focus from structural neuroplasticity to functional neuroplasticity, and from localized analysis to whole-brain analysis. Via the fully automated independent component analysis (ICA) framework [E-Field modeling The Simulation of Non-Invasive Brain Stimulation (SimNIBS) toolbox was used for E-field modeling to generate a subject-specific anatomically realistic volume conductor model [35]. Via a combination of the FSL toolbox and the SPM 12 toolbox, T1- and T2-weighted images were segmented into skin, bone, eyes, cerebral spinal fluid, ventricles, and gray and white matter. The segmented tissue compartments were meshed into a head model using Gmsh, and ECT electrodes were added to the head mesh in either RUL or received BT configuration, stimulated with 600, 700, or 800 mA as per arm assignment. The voltages and electric fields that correspond to the stimulation configuration were calculated throughout the head mesh. Based on the electrode placement (BT or RUL) and the amplitude (600, 700, or 800 mA) from the last treatment of the ECT series, we calculated the whole-brain E-field strength (Ebrain). Ebrain was measured as the 90th percentile of E-field magnitude across the whole brain. Ebrain at 90th percentile is standard based on previous E-field investigations [29, 36]. Ebrain at 90th percentile is strongly correlated with those calculated at other percentiles: 50th (r = 0.95), 75th (r = 0.99), 85th (r = 1.0), and 95th (r = 1.0). The QC resting-state fMRI data were analyzed via the Neuromark framework which provides a robust estimation of functional networks across subjects [ The code of the Neuromark framework and the Neuromark template have been released and integrated into the group ICA Toolbox (GIFT, https://trendscenter.org/software/gift/), which can be downloaded and used directly by users worldwide. Other MATLAB codes of this study can be obtained from the corresponding author. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (Lond, Engl). 2018;392:1789. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim 2016;2:1–20. Trifu S, Sevcenco A, Stănescu M, Drăgoi A, Cristea M Efficacy of electroconvulsive therapy as a potential first‑choice treatment in treatment‑resistant depression (Review). Exp Ther Med. 2021; 22. https://doi.org/10.3892/etm.2021.10716. Martin D, Katalinic N, Hadzi-Pavlovic D, Ingram A, Ingram N, Simpson B, et al. Cognitive effects of brief and ultrabrief pulse bitemporal electroconvulsive therapy: A randomised controlled proof-of-concept trial. Psychol Med. 2020;50:1121–8. Porter RJ, Baune BT, Morris G, Hamilton A, Bassett D, Boyce P et al. Cognitive side-effects of electroconvulsive therapy: what are they, how to monitor them and what to tell patients. BJPsych Open 2020; 6. https://doi.org/10.1192/bjo.2020.17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biol Psychiatry. 2010;68:568–77. Bouckaert F, Sienaert P, Obbels J, Dols A, Vandenbulcke M, Stek M, et al. ECT: Its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT. 2014;30:143–51. Fu Z, Sui J, Espinoza R, Narr K, Qi S, Sendi MSE, et al. Whole-brain functional connectivity dynamics associated with electroconvulsive therapy treatment response. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. https://doi.org/10.1016/j.bpsc.2021.07.004 Wang D, Tian Y, Li M, Dahmani L, Wei Q, Bai T, et al. Functional connectivity underpinnings of electroconvulsive therapy-induced memory impairments in patients with depression. Neuropsychopharmacology. 2020;45:1579–87. Wang J, Wei Q, Wang L, Zhang H, Bai T, Cheng L, et al. Functional reorganization of intra- and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp. 2018;39:1403–11. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. Li W, Mai X, Liu C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front Hum Neurosci. 2014; 8. https://doi.org/10.3389/fnhum.2014.00074. Wei Q, Bai T, Chen Y, Ji G, Hu X, **e W et al. The changes of functional connectivity strength in electroconvulsive therapy for depression: a longitudinal study. Front Neurosci. 2018; 12. https://doi.org/10.3389/fnins.2018.00661. Schutter DJLG, van Honk J. An electrophysiological link between the cerebellum, cognition and emotion: Frontal theta EEG activity to single-pulse cerebellar TMS. Neuroimage. 2006;33:1227–31. Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. Dep** MS, Wolf ND, Vasic N, Sambataro F, Hirjak D, Thomann PA, et al. Abnormal cerebellar volume in acute and remitted major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;71:97–102. Xu LY, Xu FC, Liu C, Ji YF, Wu JM, Wang Y et al. Relationship between cerebellar structure and emotional memory in depression. Brain Behav. 2017; 7. https://doi.org/10.1002/brb3.738. He Y, Wang Y, Chang TT, Jia Y, Wang J, Zhong S, et al. Abnormal intrinsic cerebro-cerebellar functional connectivity in un-medicated patients with bipolar disorder and major depressive disorder. Psychopharmacol (Berl). 2018;235:3187–3200. Ma Q, Zeng LL, Shen H, Liu L, Hu D. Altered cerebellar-cerebral resting-state functional connectivity reliably identifies major depressive disorder. Brain Res. 2013;1495:86–94. Porta-Casteràs D, Cano M, Camprodon JA, Loo C, Palao D, Soriano-Mas C, et al. A multimetric systematic review of fMRI findings in patients with MDD receiving ECT. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021;108:110178. Dep** MS, Nolte HM, Hirjak D, Palm E, Hofer S, Stieltjes B, et al. Cerebellar volume change in response to electroconvulsive therapy in patients with major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;73:31–35. Wei Q, Ji Y, Bai T, Zu M, Guo Y, Mo Y, et al. Enhanced cerebro-cerebellar functional connectivity reverses cognitive impairment following electroconvulsive therapy in major depressive disorder. Brain Imaging Behav. 2021;15:798–806. Argyelan M, Oltedal L, Deng ZD, Wade B, Bikson M, Joanlanne A, et al. Electric field causes volumetric changes in the human brain. Elife. 2019;8:25. Fridgeirsson EA, Deng ZD, Denys D, van Waarde JA, van Wingen GA. Electric field strength induced by electroconvulsive therapy is associated with clinical outcome. NeuroImage Clin. 2021;30:102581. Lee WH, Deng ZD, Kim TS, Laine AF, Lisanby SH, Peterchev AV. Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: Influence of white matter anisotropic conductivity. Neuroimage. 2012;59:2110–23. Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng. 2015;23:22–31. Abbott CC, Quinn D, Miller J, Ye E, Iqbal S, Lloyd M, et al. Electroconvulsive therapy pulse amplitude and clinical outcomes. Am J Geriatr Psychiatry. 2021;29:166–78. Deng Z, Argyelan M, Miller J, Quinn D. Electroconvulsive therapy, electric field, neuroplasticity, and clinical outcomes. Mol Psychiatry. 2022;27:1676–82. Du Y, Fu Z, Sui J, Gao S, **ng Y, Lin D et al. NeuroMark: an automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders. NeuroImage Clin. 2020; 28. https://doi.org/10.1016/j.nicl.2020.102375. Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–34. Baron IS. Delis-Kaplan executive function system. Child Neuropsychol. 2004;10:147–52. Swanson J. The Delis-Kaplan executive function system: a review. Can J Sch Psychol. 2005;20:117–28. Youssef NA, Ravilla D, Patel C, Yassa M, Sadek R, Zhang LF, et al. Magnitude of reduction and speed of remission of suicidality for low amplitude seizure therapy (Lap-st) compared to standard right unilateral electroconvulsive therapy: a pilot double-blinded randomized clinical trial. Brain Sci. 2019;9:99. Saturnino GB, Antunes A, Thielscher A. On the importance of electrode parameters for sha** electric field patterns generated by tDCS. Neuroimage. 2015;120:25–35. Lee WH, Lisanby SH, Laine AF, Peterchev AV. Minimum electric field exposure for seizure induction with electroconvulsive therapy and magnetic seizure therapy. Neuropsychopharmacology. 2017;42:1192–1200. Fu Z, Iraji A, Sui J, Calhoun VD. Whole-brain functional network connectivity abnormalities in affective and non-affective early phase psychosis. Front Neurosci. 2021; 15. https://doi.org/10.3389/fnins.2021.682110. Tu Y, Fu Z, Mao C, Falahpour M, Gollub RL, Park J et al. Distinct thalamocortical network dynamics are associated with the pathophysiology of chronic low back pain. Nat Commun. 2020; 11. https://doi.org/10.1038/s41467-020-17788-z. Fu Z, Sui J, Turner JA, Du Y, Assaf M, Pearlson GD et al. Dynamic functional network reconfiguration underlying the pathophysiology of schizophrenia and autism spectrum disorder. Hum Brain Mapp. 2020; hbm.25205. Fu Z, Iraji A, Turner JA, Sui J, Miller R, Pearlson GD et al. Dynamic state with covarying brain activity-connectivity: On the pathophysiology of schizophrenia. Neuroimage 2021; 224. https://doi.org/10.1016/j.neuroimage.2020.117385. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA J Am Med Assoc. 1994;271:1004–10. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. Cheng W, Rolls E, Gong W, Du J, Zhang J, Zhang XY, et al. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry. 2021;26:3992–4003. Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci USA. 2009;106:16841–6. Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. Scott BW, Wojtowicz JM, Burnham WMI. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–6. Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–.e5. Nogueira AB, Nogueira AB, Veiga JCE, Teixeira MJ. Letter: human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Neurosurgery. 2018;83:E133–E137. Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483–e483. Van Den Bossche MJA, Emsell L, Dols A, Vansteelandt K, De Winter FL, Van, et al. Hippocampal volume change following ECT is mediated by rs699947 in the promotor region of VEGF. Transl Psychiatry. 2019;9:1–7. Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. Takamiya A, Chung JK, Liang KC, Graff-Guerrero A, Mimura M, Kishimoto T. Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis. Br J Psychiatry. 2018;212:19–26. Gbyl K, Videbech P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;138:180–95. Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19:329–38. Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci USA. 2012;109:5464–8. Jonckheere J, Deloulme J, Dall’Igna G, Stimulation NC-B, 2018 undefined. Short-and long-term efficacy of electroconvulsive stimulation in animal models of depression: The essential role of neuronal survival. Elsevier https://www.sciencedirect.com/science/article/pii/S1935861X18302845 (accessed 19 May 2022). Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. Mueller KD, Koscik RL, LaRue A, Clark LR, Hermann B, Johnson SC, et al. Verbal fluency and early memory decline: results from the Wisconsin registry for Alzheimer’s prevention. Arch Clin Neuropsychol. 2015;30:448–57. Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61:1213–25. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. Habas C. Functional Connectivity of the Cognitive Cerebellum. Front Syst Neurosci. 2021; 15. https://doi.org/10.3389/fnsys.2021.642225. Cano M, Lee E, Cardoner N, Martínez-Zalacaín I, Pujol J, Makris N, et al. Brain volumetric correlates of right unilateral versus bitemporal electroconvulsive therapy for treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2019;31:152–8. Sartorius A, Demirakca T, Böhringer A, Clemm von Hohenberg C, Aksay SS, Bumb JM, et al. Electroconvulsive therapy induced gray matter increase is not necessarily correlated with clinical data in depressed patients. Brain Stimul. 2019;12:335–43. Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, Voshaar RO, et al. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res - Neuroimaging. 2013;214:197–203. Segi-Nishida E. Exploration of new molecular mechanisms for antidepressant actions of electroconvulsive seizure. Biol Pharm Bull. 2011;34:939–44. Miller J, Jones T, Upston J, Deng Z-D, McClintock SM, Ryman S, et al. Ictal theta power as an electroconvulsive therapy safety biomarker. J ECT. 2022;38:88–94. This work was supported by National Institutes of Health (R01MH118695, R01EB020407, R01MH117107, U01MH111826, and R61MH125126), the National Science Foundation (2112455), the National Institute of Mental Health Intramural Research Program (ZIAMH002955), and the China Natural Science Foundation (82022035). ZF, CCA, SMMcC, and VDC designed the study; ZF and CCA performed the data analysis; ZF, JS, JM, Z-DD, and SMMcC wrote the paper. All authors contributed to the results interpretation and discussion. The authors declare no competing interests. Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Fu, Z., Abbott, C.C., Miller, J. et al. Cerebro-cerebellar functional neuroplasticity mediates the effect of electric field on electroconvulsive therapy outcomes.
Transl Psychiatry 13, 43 (2023). https://doi.org/10.1038/s41398-023-02312-w Received: Revised: Accepted: Published: DOI: https://doi.org/10.1038/s41398-023-02312-wNeuromark framework and functional network connectivity
Code availability
References
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Competing interests
Additional information
Supplementary information
Rights and permissions
About this article
Cite this article