Abstract
Background
We sought to define the frequency of antibiotic resistance over time in a collection of invasive GBS isolates derived from infant early-onset disease (EOD), late-onset disease (LOD), and late-late onset disease (LLOD).
Methods
A multicenter retrospective review of infants born from 1970 to 2021 with GBS isolated from blood, cerebrospinal fluid, synovial fluid, cellulitis, or bone. All isolates were serotyped and antimicrobial susceptibility testing performed using disk diffusion.
Results
The most common serotypes in our 2017 isolates were III (n = 1112, 55.1%), Ia (n = 445, 22%), Ib (n = 182, 9%) and II (n = 146, 7.2%). A total of 945 (46.8%) isolates were from infants with EOD, 976 (48.3%) from LOD, and 96 (4.75%) from LLOD. All isolates were penicillin-susceptible. Compared to strains isolated <2000, strains isolated ≥2000 showed significantly greater frequency of erythromycin (4.0% to 32.3%, P < 0.0001) and clindamycin (1.5% to 17.5%, P < 0.0001) resistance. Year of isolation (≥2000) and serotype V were significantly associated with erythromycin and/or clindamycin resistance.
Conclusions
We document a rapid and significant increase in clindamycin and erythromycin resistance. As clindamycin may be considered in severely penicillin-allergic women needing GBS intrapartum prophylaxis, obstetricians, pediatricians, and neonatologist should be aware of this disturbing trend.
Impact
-
Group B streptococcal strains isolated from infants with invasive infection have become more resistant to second-line antibiotics over time.
-
In this epidemiologic study of 2017 group B streptococci isolated from 1970 to 2021, penicillin susceptibility remained uniform; however, resistance to erythromycin and clindamycin increased significantly over time across all capsular serotypes. Clindamycin resistance exceeded 20% by 2010 in most serotypes.
-
While penicillin remains the treatment of choice for group B streptococcal infant disease, pediatricians and neonatologists should be aware of the high prevalence of resistance to clindamycin, a recommended alternative drug used for intrapartum-antibiotic prophylaxis in penicillin-allergic women.
Similar content being viewed by others
Background
Streptococcus agalactiae (Group B Streptococcus, GBS) is a common cause of neonatal and young infant sepsis and meningitis, constituting a significant source of morbidity and mortality in this population worldwide. First recognized in the 1970s as the most common cause of invasive disease in young infants, GBS still represents an important public health problem, with an estimated 2000 infant cases per year in the United States1. Based on distinct clinical and epidemiological features, invasive GBS infant disease historically has been categorized as early-onset disease (EOD, birth through age 6 days), late-onset disease (LOD, age7 through 89 days), and late-late onset disease (LLOD, age 90 days or more)2,3. Widespread implementation of universal GBS screening during late pregnancy and use of intrapartum antibiotic prophylaxis (IAP) since the early 2000s has been associated with a reduction in EOD by more than 80%, but LOD or LLOD incidence is unchanged1. By contrast, invasive GBS disease in non-pregnant adults typically with underlying co-morbidities or age ≥65 years has increased over this interval1,4,5,6,7.
GBS is classified into ten serotypes (Ia, Ib, II-IX), determined by structural differences in capsular polysaccharides (CPS)8,9. Serotype III remains the most common serotype in infant disease, causing 47% of EOD and 73% of LOD3. The antimicrobial agent of choice for treatment and prevention of invasive GBS infection in both infants and adults is penicillin. Erythromycin, clindamycin, and vancomycin are second-line treatment options for patients with severe penicillin allergy. The widespread use of IAP as well as the rise in adult invasive disease cases raises concern for potential emergence of antibiotic resistance in GBS4,5,10,11,12.
Although GBS remains susceptible to penicillin, contemporary studies from North America have reported relatively high rates of resistance to erythromycin (16–50%), clindamycin (5 to 42%), and tetracycline (>80%)1,13. Studies of GBS antibiotic resistance in China have shown even higher rates with nearly two out of three strains demonstrating resistance to erythromycin or clindamycin and >90% with resistance to tetracycline14,15. Similar to the United States, multiple studies originating in Europe have reported relatively high rates of resistance to erythromycin (12–36%), clindamycin (3–28%), and tetracycline (>85%)16,17,18,19,20. GBS resistance to clindamycin has been identified by the Centers for Disease Control and Prevention (CDC) as a “concerning” threat given that >40% of invasive infections are now caused by clindamycin-resistant strains21. Thus, understanding the trends and epidemiological (clinical and bacterial strain) features associated with antibiotic resistance in GBS is crucial in our management and prevention strategies for GBS disease.
Few studies have examined the temporal trends of antibiotic resistance in GBS owing to a relative absence of large GBS strain collections spanning multiple decades. The Active Bacterial Core Surveillance (ABCs) of the Centers for Disease Control and Prevention (CDC) has performed population-based surveillance for multiple bacterial pathogens including GBS. However, despite beginning GBS surveillance in 1997, routine GBS antibiotic resistance phenoty** has been limited to the past decade22. In an effort to better understand the temporal emergence and serotype contributions to antibiotic resistance in GBS, we examined more than 2000 individual GBS strains derived from infants with invasive infant disease over a 50-year period (1970–2021). Our study represents one of the only studies to describe the emergence of antimicrobial resistance in GBS over such an extended time interval.
Methods
Bacterial growth and susceptibility testing
We performed an epidemiological assessment using 2017 GBS isolates from infants with invasive infection from 1970 to 2021. The strains were collected from multiple institutions within the Texas Medical Center and internationally under a protocol approved by the Institutional Review Board at Baylor College of Medicine. Age at onset of an infection as well as body site(s) and year of GBS isolation was obtained from the medical record. All strains were minimally passaged and stored at −80 °C. Seroty** of isolates was performed after receipt from hospital laboratories by capillary precipitin method; all non-typeable isolates also were tested by latex agglutinins (Statum Serum Institute, Copenhagen, Denmark). The Clinical and Laboratory Standards Institute (CLSI) protocol was followed for antimicrobial susceptibility testing using disk diffusion23. Briefly, individual GBS strains were grown on Mueller-Hinton Agar (MHA) with 5% sheep blood (BBL; ThermoFisher) at 37 °C supplemented with 5% CO2 following the placement of antimicrobial discs (BD Sensi-Disc; ThermoFisher) using a standard dispenser to ensure proper spacing between disks. Tested antibiotics included penicillin (10 U), tetracycline (30 μg), clindamycin (2 μg), erythromycin (15 μg), and levofloxacin (5 μg). Zones of inhibition were measured using a digital caliper following 18 h of growth and recorded as susceptible, intermediate, or resistant according to the CLSI breakpoints23.
Study definitions
EOD was defined as isolation of GBS from blood or cerebrospinal fluid (CSF) culture obtained before age 7 days, LOD as isolation of GBS from blood, CSF, joint fluid, bone, lymph node, cellulitis aspirate or wound between age 7 and 90 days and LLOD as isolation of GBS from blood, CSF, joint, or spleen after age 89 days.
Results
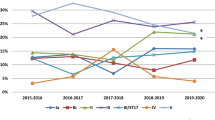
A total of 2017 invasive infant GBS isolates were examined from 1970 to 2021 (Fig. 1). The most common GBS serotypes in our collection were III (n = 1112, 55.1%), Ia (n = 445, 22%), Ib (n = 182, 9%) and II (n = 146, 7.2%); all other serotypes accounted for less than 100 isolates each and six strains were non-typeable (Table 1). Interestingly, serotype IV and V infant infections, first noted in the late 1970s, proportionately increased during the last two decades with a concomitant decrease in serotype Ia (Fig. 1).
A total of 945 (46.9%) isolates were from infants with EOD, 976 (48.4%) from LOD, and 96 (4.8%) from LLOD. Serotypes Ia and III accounted for most of the early- and late-onset disease in our collection (Table 1). The most frequent disease manifestation was bacteremia (sepsis) without meningitis, (n = 1243, 61.6%) followed by meningitis (n = 696, 34.5%) (Table 1). Other focal site infections were uncommon and occurred exclusively among infants with LOD, while bone and joint infections virtually disappeared after 2010.
All isolates were susceptible to penicillin. Given the reported rates of erythromycin, clindamycin, and tetracycline resistance in contemporary GBS strains11, we focused the remainder of our analysis on these antibiotics. To improve our ability to identify important temporal and serotype-specific trends, we analyzed resistance frequencies by decade (Fig. 2). Distinct changes in the frequency of resistance to erythromycin and clindamycin among all serotypes were observed beginning in the year 2000 (Fig. 2, vertical dashed lines). Overall, we detected an increase in resistance to erythromycin from 4% (55/1373) prior to 2000 to 32.3% (208/644) in 2000 or after (Fig. 2, Table 2). After 2000, the highest rates of erythromycin resistance were observed for serotype II (25/33, 75.8%) and serotype V (29/44, 65.9%) strains. A similar pattern was observed for clindamycin (Fig. 2), with an increase to 17.5% (113/644) after 2000 from 1.5% (20/1373) prior to that year (Table 2). Consistent with erythromycin resistance and potentially explained by a common within-serotype genetic mechanism, the highest rates of clindamycin resistance were observed in serotypes II (60.6%, 20/33) and V (61.4%, 27/44) GBS strains causing infant disease after the year 2000 (Table 2). Irrespective of the year of isolation, the overall rates of erythromycin and clindamycin resistance were significantly greater than would otherwise be expected for serotypes II, IV, and V and significantly less than expected for serotype III (Table 2).
Resistance to tetracycline remained high over the 5 decades (Fig. 2, Table 2). However, in contrast to erythromycin and clindamycin, we observed an overall decrease in the rate of tetracycline resistance from 94.0% (1291/1373) prior to 2000 to 88.5% (570/644) in 2000 or later (Table 2). Surprisingly, the overall decline was driven by decreased rates of resistance in serotypes III (94.3% to 87.3%) and IV (100% to 50%). However, only 26 serotype IV isolates were identified during the study interval, 21 isolated after 2010 (Fig. 1, table), making comparisons between defined decides inaccurate. The overall rate of tetracycline resistance among serotype IV GBS strains (14/26, 53.8%) was significantly lower than expected (Table 2, P < 0.0001, Fisher’s exact).
Finally, we sought to identify factors predictive for GBS resistance phenotypes using multiple logistic regression. We used the year of isolation (pre-/post-2000) and capsular serotype as independent variables. Given the strong associations between serotype and age at disease onset and disease type (Table 1), our analysis did not include disease variables. Dependent variables included resistance to erythromycin, clindamycin, and tetracycline. Inasmuch as genetic elements have commonly been identified in GBS conferring both erythromycin and clindamycin resistance (e.g., ermB)24, we also included combined erythromycin and clindamycin resistance as a dependent variable. For each resistance phenotype, isolation after 2000 was predictive of resistance and greatest for the erythromycin, clindamycin, and erythromycin and clindamycin phenotypes (Table 3). However, among the capsular serotypes only serotype V was predictive of resistance to either erythromycin or clindamycin or both of these antibiotics (Table 3).
Discussion
Using 2017 GBS strains derived from invasive infant disease spanning 50 years, we describe the emergence of erythromycin and clindamycin resistance. The expansive temporal distribution of our unique collection demonstrated the distinct emergence of erythromycin and clindamycin resistance beginning in the late 1990s in all major GBS serotypes. Our findings have important clinical implications. The rapid increased frequency of resistance directly coincides with the widespread use of macrolide antibiotics beginning in the 1990s—a resistance pattern akin to what has been observed for Streptococcus pneumoniae25. We did not find any isolates with resistance to penicillin, emphasizing continued support for recommendations for use of penicillin in maternal IAP and treatment of invasive infant GBS disease. However, the marked increase in resistance to second-line antibiotics such as erythromycin and clindamycin (>60% for GBS serotypes II and V) are of great concern given the potential use of clindamycin in pregnant women with severe penicillin allergy. Interestingly, the mis-diagnosis of penicillin allergy26 in the general population and the resulting overuse of second-line antibiotics is likely a contributing factor in the rapidly increasing frequency of erythromycin and clindamycin resistance in GBS and other pathogens.
In addition to having direct clinical implications, our data provide a foundation for the future study of the mechanisms of antibiotic resistant GBS clone emergence. Several studies have sought to identify key genetic factors that contributed to GBS emergence in humans. Recently, an extensive analysis from diverse animals demonstrated bacterial adaptation through gene gain/loss among GBS strains was specific to the host27. The study confirmed previous findings that overall antimicrobial resistance was more frequent in GBS isolates from human origin28. Interestingly, the evidence supporting antibiotic resistance associated with GBS expansion within humans was associated with a strong signal for genes conferring erythromycin resistance27. Consistent with this concept, a key finding in our analysis was the emergence of macrolide resistance over time in all of the GBS serotypes examined. In our collection, clindamycin resistance mirrors erythromycin resistance, with increase over time. Based on previous GBS serotype V strain population-based studies24, it is likely that erm resistance gene elements (e.g., ermB) are also contributing concomitantly to clindamycin resistance and may be on shared mobile genetic elements. More detailed analyses of GBS serotype-specific populations may provide new insights into common features contributing to the continued rise in antibiotic resistance. Further, continued surveillance combined with molecular genetic studies are warranted to determine the effects, if any, on novel clone emergence and the pathogenesis of GBS lineages over time.
GBS strains have largely been considered resistant to tetracycline owing to the presence of tet resistance gene elements (most frequently tetM). Our analysis shows that resistance to tetracycline has remained high and well preserved since the 1970s. These data are consistent with findings described by Da Cunha et al. suggesting proliferation of tetracycline resistant clones as a result of widespread tetracycline use28. Intriguingly, the rise of serotype IV GBS strains in our and US population-based strain surveillance collections11 have been accompanied by decreased rates of tetracycline resistance. However, the overall decline in the frequency of tetracycline resistance in our collection was driven by decreases within the serotype III population (Table 2).
Strength and limitations
Our study has notable strengths and limitations. The primary strength lies in the large GBS strain collection spanning 50 years. No single study has examined antibiotic resistance phenotypes in GBS over such a time span. Despite all GBS strains being derived from invasive infant disease, the frequency of resistance identified is likely to be reflective of strains derived from adult invasive infections given that adult and infant invasive strains likely originate from the same genetic pool29. A few limitations are also worthy of mention. Our collection originated from multiple institutions primarily within the Texas Medical Center which limits our ability to generalize to the general population of the United States. Overall, our population differs slightly from national-level surveillance serotype distribution and specifically in the higher frequency of serotype III strains in our strain collection that may affect overall frequency of antimicrobial resistance. In addition, characteristics of infant cases apart from age at disease onset and body site afflicted are incomplete limiting potential secondary analyses.
Conclusions
In summary, our analysis better defines the temporal emergence of antimicrobial resistance in GBS isolates from invasive infant disease. Continued overuse of second line antibiotics, in particular macrolides and clindamycin, for empiric therapy may have implications beyond increased rates of resistance that clinicians should recognize when making treatment choices.
Data availability
Data are available from the corresponding author upon request.
References
Nanduri, S. A. et al. Epidemiology of invasive early-onset and late-onset group B Streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 173, 224–233 (2019).
Raabe, VN, Shane, AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr. 2019;7. https://doi.org/10.1128/microbiolspec.GPP3-0007-2018
Madrid, L. et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 65, S160–S172 (2017). suppl_2.
Phares, C. R. et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA 299, 2056–2065 (2008).
Fernandez, M., Hickman, M. E. & Baker, C. J. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrob. Agents Chemother. 42, 1517–1519 (1998).
Castor, M. L. et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect. Dis. Obstet. Gynecol. 2008, 727505 (2008).
Francois Watkins, L. K. et al. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern. Med. 179, 479–488 (2019).
Cieslewicz, M. J. et al. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 73, 3096–3103 (2005).
Edwards, M. S. & Baker, C. J. Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41, 839–847 (2005).
Borchardt, S. M. et al. Frequency of antimicrobial resistance among invasive and colonizing Group B streptococcal isolates. BMC Infect. Dis. 6, 57 (2006).
McGee, L. et al. Multistate, population-based distributions of candidate vaccine targets, clonal complexes, and resistance features of invasive group B streptococci within the United States, 2015–2017. Clin. Infect. Dis. 72, 1004–1013 (2021).
Murdoch, D. R. & Reller, L. B. Antimicrobial susceptibilities of group B streptococci isolated from patients with invasive disease: 10-year perspective. Antimicrob. Agents Chemother. 45, 3623–3624 (2001).
Flannery, D. D. et al. Antimicrobial susceptibility profiles among neonatal early-onset sepsis pathogens. Pediatr. Infect. Dis. J. 41, 263–271 (2022).
Guo, D. et al. Neonatal colonization of group B streptococcus in China: Prevalence, antimicrobial resistance, serotypes, and molecular characterization. Am. J. Infect. Control. 46, e19–e24 (2018).
Wu, B. et al. Phenotypic and genetic differences among group B Streptococcus recovered from neonates and pregnant women in Shenzhen, China: 8-year study. BMC Microbiol. 19, 185 (2019).
Hays, C. et al. Changing epidemiology of group B streptococcus susceptibility to fluoroquinolones and aminoglycosides in France. Antimicrob. Agents Chemother. 60, 7424–7430 (2016).
Imperi, M. et al. Invasive neonatal GBS infections from an area-based surveillance study in Italy. Clin. Microbiol. Infect. 17, 1834–1839 (2011).
Kadanali, A., Altoparlak, U. & Kadanali, S. Maternal carriage and neonatal colonisation of group B streptococcus in eastern Turkey: prevalence, risk factors and antimicrobial resistance. Int. J. Clin. Pract. 59, 437–440 (2005).
Plainvert, C. et al. Multidrug-Resistant Hypervirulent Group B Streptococcus in Neonatal Invasive Infections, France, 2007–2019. Emerg. Infect. Dis. 26, 2721–2724 (2020).
Capanna, F. et al. Antibiotic resistance patterns among group B Streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med. Wkly 143, w13778 (2013).
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. U.S. Department of Health and Human Services; 2019.
Active Bacterial Core Surveillance; Centers for Disease Control and Prevention. Accessed 7 June 2022, https://www.cdc.gov/abcs/bact-facts-interactive-dashboard.html
Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. Clinical Laboratory Standards Institute; 2018.
Cubria, M. B. et al. Population genomics reveals distinct temporal association with the emergence of ST1 serotype V Group B streptococcus and macrolide resistance in North America. Antimicrob. Agents Chemother. 66, e0071421 (2022).
Olesen SW, et al. The distribution of antibiotic use and its association with antibiotic resistance. Elife. 2018;7
Shenoy, E. S., Macy, E., Rowe, T. & Blumenthal, K. G. Evaluation and management of penicillin allergy: a review. JAMA 321, 188–199 (2019).
Richards VP, et al. Population gene introgression and high genome plasticity for the zoonotic pathogen Streptococcus agalactiae. Mol. Biol. Evol. 2019.
Da Cunha, V. et al. Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat. Commun. 5, 4544 (2014).
Teatero, S. et al. Clonal Complex 17 Group B Streptococcus strains causing invasive disease in neonates and adults originate from the same genetic pool. Sci. Rep. 6, 20047 (2016).
Author information
Authors and Affiliations
Contributions
E.M.S., M.A.S.I., M.R., T.M., H.H., M.E., C.J.B., A.R.F.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. E.M.S., M.A.S.I., C.J.B., A.R.F.: Drafting the article or revising it critically for important intellectual content. E.M.S., M.A.S.I., C.J.B., A.R.F.: Final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabroske, E.M., Iglesias, M.A.S., Rench, M. et al. Evolving antibiotic resistance in Group B Streptococci causing invasive infant disease: 1970–2021. Pediatr Res 93, 2067–2071 (2023). https://doi.org/10.1038/s41390-022-02375-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02375-3
- Springer Nature America, Inc.