Abstract
Epithelial to mesenchymal transition (EMT) is a dynamic process that drives cancer cell plasticity and is thought to play a major role in metastasis. Here we show, using MDA-MB-231 cells as a model, that the plasticity of at least some metastatic breast cancer cells is dependent on the transcriptional co-regulator CBFβ. We demonstrate that CBFβ is essential to maintain the mesenchymal phenotype of triple-negative breast cancer cells and that CBFβ-depleted cells undergo a mesenchymal to epithelial transition (MET) and re-organise into acini-like structures, reminiscent of those formed by epithelial breast cells. We subsequently show, using an inducible CBFβ system, that the MET can be reversed, thus demonstrating the plasticity of CBFβ-mediated EMT. Moreover, the MET can be reversed by expression of the EMT transcription factor Slug whose expression is dependent on CBFβ. Finally, we demonstrate that loss of CBFβ inhibits the ability of metastatic breast cancer cells to invade bone cell cultures and suppresses their ability to form bone metastases in vivo. Together our findings demonstrate that CBFβ can determine the plasticity of the metastatic cancer cell phenotype, suggesting that its regulation in different micro-environments may play a key role in the establishment of metastatic tumours.
Similar content being viewed by others
Introduction
The triple-negative sub-type of breast cancer is a highly aggressive cancer for which treatment options are limited [1]. Expression of the RUNX transcription factors in patients with the triple-negative sub-type of breast cancer correlates with a poor prognosis [2, 3]. Emerging evidence suggests that the role of RUNX proteins in breast cancer is dependent on the specific RUNX factor involved and the sub-type of breast cancer cell [4]. In order to consider the RUNX factors as viable targets in breast cancer therapies it is therefore critically important to determine the role of the different factors in different sub-types of breast cancer. Since CBFβ facilitates the function of all three RUNX transcription factors, establishing its role in determining the phenotype of breast cancer cells is essential [5, 6].
Mutations in CBFβ are amongst the most frequently reported for breast cancer tumours, suggesting a tumour suppressor role for CBFβ in ER+ breast cancer [7, 8]. In contrast, we and others have previously shown that expression of RUNX2 and CBFβ contribute to the metastatic phenotype of triple-negative breast cancer cells [9,10,11,12]. In this context it is therefore the maintained expression of RUNX factor activity that promotes their metastatic phenotype.
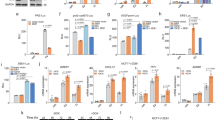
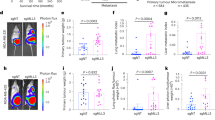
Epithelial to mesenchymal transition (EMT) contributes to the progression of metastatic cancer as it enables cancer cells to become migratory and invasive [5d). We found that 7 out of 11 animals injected with the control cells developed tumours in the hind limbs, with numerous cancer-induced bone lesions detected, compared with three out of seven animals injected with CBFβ-CRISPR (Fig. 5d). In addition, the animals receiving the control cells had higher numbers of skeletal lesions (average = 8.1 lesions/mouse) compared with those receiving CBFβ-CRISPR cells (average = 1.7 lesions/mouse), (Fig. 5d). These data demonstrate that loss of CBFβ reduces the ability of MDA-MB-231 cells to metastasise to bone.
a MDA-MB-231 cells were transplanted into the inguinal mammary fat pad of CD1-Nude females. Data shown at 4 weeks post-transplantation. Data is presented as mean ± SDM (shNS; n = 5; shCBFβ; n = 7). The difference between the two groups is significant (p < 0.05) as determined using an unpaired Student’s t-test. b Growth curves showing knockdown of CBFβ reduces growth rate in 3D culture. MDA-MB-231, MDA-shNS, or MDA-shCBFβ cells were grown on 3D culture and cells were counted every 2 days. c Knockdown of CBFβ inhibits invasion in 3D co-cultures with osteoblasts. 3D cultures of MC-3T3 osteoblasts were grown for 2 months prior to addition of MDA-MB-231 or MDA-shCBFβ cells. Confocal images were taken after 8 days of co-culture. Cells were stained with phalloidin. MDA cells were identified by GFP fluorescence. d CBFβ silencing reduces tumour growth in bone in vivo. MDA-MB-231 control or CBFβ−/− cells were injected i.c. into 6-week old BALB/c nude mice and tumour growth in the hind limbs analysed 26 days later. 3D reconstruction of tumour-bearing tibia showing the presence of osteolytic lesions. The histogram shows the average number of bone tumours per mouse for each cell line as indicated.
Discussion
In this study, we have shown that the RUNX co-regulator CBFβ is essential to drive MDA-MB-231 breast cancer cells through EMT. Maintenance of the mesenchymal phenotype is, at least in part, achieved by regulating the expression of the EMT transcription factor Slug. We also demonstrated that the MET induced by loss of CBFβ is completely reversible by re-expression of CBFβ. These findings are important since they demonstrate that, in principle, regulation of RUNX/CBFβ activity can determine the extent to which triple-negative breast cancer cells differentiate along the epithelial-mesenchymal continuum. In the context of metastasis in vivo this raises the possibility that interactions between cancer cells and the microenvironment influence the activity of RUNX/CBFβ, thereby shifting their phenotype toward the epithelial state and enabling the cells to colonise the new niche. Indeed, the loss of CBFβ significantly reduced the capacity of metastatic cancer cells to invade osteoblast cultures in vitro and to form osteolytic lesions in vivo.
Previous work has shown that about two thirds of the RUNX1 transcriptome is shared with the RUNX2 transcriptome in MCF7 cells [19]. Our finding that CBFβ, RUNX1 and RUNX2 are all necessary for SNAI2 expression suggests that all three factors combine to ensure a sufficient level of Slug is available to maintain the mesenchymal phenotype. This suggests that none of these factors are redundant in this context. This may reflect the need for a threshold level of RUNX factors to be expressed but does not discount the possibility that RUNX1 and RUNX2 also regulate specific subsets of genes. SNAI2 is a well-established EMT transcription factor that appears to be a key target for RUNX transcription factor complexes and is perhaps one of several genes that contribute to the metastatic nature of breast cancer cells [16, 18].
In contrast to these findings, we did not observe significant changes in classical EMT markers when either CBFβ or RUNX1 was depleted in MDA-MB-468 cells. The emerging picture is that RUNX complexes have pleiotropic effects dependent on the cellular context and it is of note that whilst MDA-MB-231 and MDA-MB-468 cells are both “triple-negative” they exhibit different characteristics and gene expression profiles, MDA-MB-231 being of the claudin low sub-type and MDA-MB-468 are basal [32]. Moreover, mutations in RUNX1 and CBFβ are associated with the ER+ sub-type of breast cancer and is therefore predicted to have a tumour suppressor role in this context [7, 8, 33]. Indeed, previous studies have shown that RUNX1 suppresses development of ER+ luminal breast cancer but it is not known how CBFβ contributes in this context [34]. Our finding that depletion of RUNX1 in MDA-MB-231 cells induces MET is in agreement with a previous study in which the expression of miR-378 in MDA-MB-231 cells, which inhibits RUNX1 expression, also resulted in suppression of migration and invasion [35]. However, this is in contrast to the role of RUNX1 in MCF10A and MCF7 cells where RUNX1 expression is required to maintain an epithelial-like phenotype [20, 36]. Taken together these findings suggest that RUNX transcription factors contribute to the epithelial-mesenchymal continuum is a cell-context dependent manner.
Finally, small molecule inhibitors that inhibit the interaction between CBFβ and RUNX have been shown to inhibit colony formation in a basal-like breast cancer cell line [37]. Our findings that CBFβ is essential to maintain the mesenchymal phenotype, and that it contributes to the formation of bone metastases, suggests that in principle inhibiting this complex might maintain metastatic colonies in a less aggressive epithelial state by driving MET. Thus, targeting the RUNX/CBFβ complex in this way might be a viable option to treat a sub-group of triple-negative breast cancer patients.
Methods
Cell lines
Parental MDA-MB-231 expressing GFP were a kind gift from D. Welch, University of Alabama. MDA-MB-231-shCBFβ/RUNX1/RUNX2 were produced using Sure Silencing shRNA plasmids (SABiosciences) as previously described [16]. Lines were authenticated by multiplex-PCR assay using the AmpF/STR system (Applied Biosystems) and confirmed as mycoplasma free. Monolayers were grown in complete medium (DMEM/10% FCS/2 mmol/L L-glutamine/PenStrep 0.4 μg/mL puromycin, 50 μg/mL geneticin, 500 μg/mL hygromycin as required) and maintained in a humidified incubator at 37 °C at an atmospheric pressure of 5% (v/v) CO2/air.
Western blotting
Protein was separated on an SDS–PAGE and transferred to Hybond-C Extra nitrocellulose membrane. Primary antibodies included: β-Tubulin (Abcam, ab6046), Lamin-B1 (Abcam, ab16048), CBFβ (Abcam, ab33516), RUNX1 (Abcam, ab23980), RUNX2 (MBL, D130-3), Snai2 (Cell Signalling, C19G7), FLAG (Sigma, F1804).
For all experiments three biological replicates were performed and densitometry was conducted to calculate average changes using ImageJ software, which is freely available at http://rsb.info.nih.gov/ij/.
Cell scratch assay
Confluent monolayers were scratched on day 0 and medium was changed to serum free. Cells were grown in an AS MDW live cell imaging microscope system at 37 °C 5% CO2 for 48 h. Images were taken every 20 min and 40 views were taken in each well. For all experiments three technical and three biological replicates were performed. Image data analysis was performed using Cell Profile software.
Overlay three-dimensional culture of breast cells
Matrigel (Corning, 354230) was thawed on ice overnight at 4 °C and then spread evenly onto dishes (MatTek P35G-1.0-14-C) or into 24-well plates (Greiner, Bio-one 662892). Cells were resuspended in 3D assay medium (2% Matrigel, 95% DMED, 2%FBS, 1% Pen/Strep, 1% nonessential amino acid, 1% L-glutamine) and plated on to solidified Matrigel. Cells were grown in 5% CO2 humidified incubator at 37 °C. Assay medium was changed every 3 or 4 days. Cells were fixed at day 14. For all experiments three technical and three biological replicates were performed.
Microscopy
2D culture
Images were collected on a Zeiss Axioimager.D2 upright microscope using a 10× objective and captured using a Coolsnap HQ2 camera (Photometrics) through Micromanager software v1.4.23. Specific band pass filter sets for DAPI, FITC and Cy5 were used to prevent bleed through from one channel to the next.
3D culture
Images were collected on a Leica TCS SP5 AOBS inverted and upright confocal microscopes. Images were collected using PMT detectors with the following detection mirror settings; [FITC 494–530 nm; Texas red 602-665 nm; Cy5 640-690 nm] using the [488 nm (20%), 594 nm (100%) and 633 nm (100%)] laser lines, respectively. When it was not possible to eliminate cross-talk between channels, the images were collected sequentially. When acquiring 3D optical stacks the confocal software was used to determine the optimal number of Z sections. Only the maximum intensity projections of these 3D stacks are shown in the results. Images were then processed and analysed using Fiji ImageJ (http://imagej.net/Fiji/Downloads) [14], which is freely available online.
For all experiments three technical and three biological replicates were performed.
Immunofluorescence
For cell grown on coverslips, fixing and permeabilisation was performed in 4% paraformaldehyde (Sigma) and 0.1% Triton-100 (Sigma) before blocking in 1% Bovine Serum Albumin. The cells were then incubated with the primary antibodies overnight at 4 °C at a dilution of 10 µg/µl). pERM (Cell Signalling Technology; Antibody #3141), anti-integrin avβ6 (Abcam, ab97588). Alexafluor secondary antibodies (Invitrogen) were used at a 1/200 dilution. The coverslips were mounted on glass slides using mounting medium with DAPI (Invitrogen P36965). For cells grown in Matrigel, following fixing and permeabilisation as detailed above non-specific staining was blocked using cells were blocked using IF Buffer (7.7 mM NaN3, 0.1% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween-20 in PBS) + 10% goat serum. Cells were stained using Phalloidin for F-actin (Sigma, P1951) and mounted using DAPI (Invitrogen).
For all experiments three technical and three biological replicates were performed.
Mammosphere culture
Mammosphere culture was carried out as previously described [38]. Spheres >50 µm were counted on day 5. For all experiments three technical and three biological replicates were performed.
Quantitative reverse transcription PCR
RNA was extracted using the Qiagen RNAeasy kit according to manufacturer’s instructions and quantified on the Nanodrop spectrophotometer (Thermo). Real time one step qRT-PCR was carried out using the QuantiTect SYBR® Green RT-PCR Kit (Qiagen) according to manufacturer’s instructions before analysis on the 7900 PCR machine (Applied Biosystems). A table of the primers used can be found in Supplementary Table 1. For all experiments three biological replicates were performed.
Inducible Cell line production
Mouse CBFβ-FLAG and ER was ligated into pcDNA3.1/Hygro(-) vector producing pcDNA3.1/Hygro(-)-CBFβ-FLAG-ER. Stable lines were made using this vector and cells were transfected using Lipofectamine according to manufacturer’s instructions (Fig. S1).
Nuclear/Cytoplasmic separation
Cells were resuspended in 400 μl of ice cold Buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF) with the addition of complete mini-EDTA-free protease inhibitor cocktail (Roche) and incubated at 4 °C for 15 min. Cells were lysed by addition of 10% NP-40 (Sigma) before centrifuging at 4 °C and removal of the cytoplasmic extracts in the supernatant. The pellet was then resuspended in ice cold Buffer B (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 1 mM PMSF) containing protease inhibitors and vortexed vigorously for 45 min at 4 °C. Nuclear proteins were collected from supernatant following centrifugation at 4 °C. For all experiments three biological replicates were performed.
Invasion assay
Matrigel Matrix (Corning, 354230) was diluted to final concentration of 300 μg/mL in cold coating buffer (0.01 M Tris (pH8.0), 0.7% NaCl) before being added to invasion chambers (Corning Cat, 353097) and left to set overnight at 37 °C. 2 × 104 cells were added to each chamber in serum free medium and 0.75 mL complete medium was added to the wells. Cells were allowed to invade 24 h in cell culture incubator. Invading cells were fixed and permeabilised with 4% PFA (Electron Microscopy Sciences, 15713-S) and 0.1% Triton (Sigma). Non-Invading cells were removed using a cotton swab. Cells were stained with Crystal violet solution. For all experiments three technical and three biological replicates were performed.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as previously described [39]. ChIP-PCR was performed using Quantitect SYBR green (Qiagen). The primers used can be found in Supplementary Table 1. For all experiments three biological replicates were performed.
CRIPSR-Cas9 mediated gene deletion
CBFβ and RUNX1 gene knockout was performed using a double nickase CRISPR-Cas9 strategy as described previously [31]. Guide-RNA sequences were designed using E-CRISP to minimise off target effects [24]. Cells were Fluorescence-activated cell sorted (FACS) for GFP-Cas9 expression 48 h after transfection and grown up from single colonies prior to genomic DNA PCR and western blot screening.
Mammary fat pad xenografts
3 × 106 MDA-MB-231 cells were transplanted in matrigel into the inguinal mammary fat pad of 12 week old CD1-Nude females (Charles River, UK). Mice were randomised to receive shNS or shCBFβ-KO cells to give groups of comparable weight/age. The same investigator (SMM) transplanted all cells into the recipients.
Animals were excluded if they failed to grow a tumour to clinical endpoint, and/or exhibited unrelated general ill health within the duration of the experiment.
Caliper measurements were carried out throughout by technical staff blinded to the expected outcome of the experiment to assess tumour volume which was calculated using the formula ½(length × width2).
This experiment was carried out in dedicated animal facilities under project licence 60/4181 with adherence to the Animal (Scientific Procedures) Act, the European Directive 2010 and local ethical approval (University of Glasgow). No randomisation was required.
Bone tumour growth studies
Tumour growth studies used 6–8 week old female BALB/c nude between 13 and 18.4 g (Charles River, Kent, UK). Experiments were carried out in accordance with local guidelines and with Home Office approval under project licence 70/8799, University of Sheffield, UK. 12 mice per group were injected with 1 × 105 MDA-MB-231 control (2014-8-044) or CBFβ-CRISPR knockout cells (2015-6-010 CRISPR) via the left cardiac ventricle to generate tumours in bone [30]. Mice were randomised to receive control or CBFβ-KO cells to give groups of comparable weight/age. Mice were removed early from the study if they showed luciferase signal in the chest only (indicating a missed injection) or if the mice developed hind limb paralysis within the first 48 h. These parameters were pre-defined before the experiment commenced.
Animals were culled 26 days following tumour cell injection and hind limbs collected for analyses of tumour growth and associated bone lesions in tibiae and femurs.
Analysis of bone lesions
Hind limbs were fixed in 4%PFA and scanned by μCT prior to decalcification in 1%PFA/0.5% EDTA and processing for histological sectioning. μCT analysis was carried out using a Skyscan 1272 × -ray-computed μCT scanner (Skyscan, Aartselar, Belgium) equipped with an x-ray tube (voltage, 50 kV; current, 200uA) and a 0.5-mm aluminium filter. Pixel size was set to 5.99 μm and scanning initiated from the top of the proximal tibia or distal femur. Lytic, tumour-induced bone lesions were counted manually for each bone and performed by a technician being unaware of anticipated outcome of the experiment.
Statistical analysis
Data is represented as mean +/− SD, n = 3 unless otherwise stated.
Statistical significance was measured using parametric testing, assuming equal variance, unless otherwise stated, with standard t-tests for two paired samples used to assess difference between test and control samples. An asterisk (*) indicates 0.01 < P < 0.05; ** indicates 0.001 < P < 0.01; *** indicates P < 0.001; **** indicates P < 0.0001; N indicates 0.05 < P when compared to control.
Power calculations were performed for mammary fat pad experiments. Using 80% power and 95% confidence, 25% practical difference and 15% coefficient of variation we anticipated that 8-10 mice was required for each cohort and so n = 10 animals per cohort were transplanted.
Power calculations were also performed for bone tumour growth assays based on the minimum number of animals required to obtain statistically significant data in a factorial ANOVA design were based on our extensive previous studies: Metastasis is known to develop in the hind limbs of 80–90% of mice injected with control MDA-MB-231 cells, for studies predicted to decrease metastasis (or metastatic lesions) by 70%, a minimum of six mice per group is required to obtain 80% power with 10% error.
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674–90.
Ferrari N, Mohammed ZM, Nixon C, Mason SM, Mallon E, McMillan DC, et al. Expression of RUNX1 correlates with poor patient prognosis in triple negative breast cancer. PLoS ONE. 2014;9:e100759.
McDonald L, Ferrari N, Terry A, Bell M, Mohammed ZM, Orange C, et al. RUNX2 correlates with subtype-specific breast cancer in a human tissue microarray, and ectopic expression of Runx2 perturbs differentiation in the mouse mammary gland. Dis Model Mech. 2014;7:525–34.
Rooney N, Riggio AI, Mendoza-Villanueva D, Shore P, Cameron ER, Blyth K. Runx genes in breast cancer and the mammary lineage. Adv Exp Med Biol. 2017;962:353–68.
Ito Y, Bae S-C, Chuang LSH. The RUNX family: developmental regulators in cancer. Nat Rev Cancer. 2015;15:81–95.
Tahirov TH, Bushweller J. Structure and biophysics of CBFβ/RUNX and its translocation products. Adv Exp Med Biol. 2017;962:21–31.
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–60.
Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9.
Shore P. A role for Runx2 in normal mammary gland and breast cancer bone metastasis. J Cell Biochem. 2005;96:484–9.
Pratap J, Lian JB, Stein GS. Metastatic bone disease: role of transcription factors and future targets. Bone. 2011;48:30–6.
Mendoza-Villanueva D, Deng W, Lopez-Camacho C, Shore P. The Runx transcriptional co-activator, CBFβ, is essential for invasion of breast cancer cells. Mol Cancer. 2010;9:171.
Mendoza-Villanueva D, Zeef L, Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13:R106.
Kim DH, **ng T, Yang Z, Dudek R, Lu Q, Chen YH. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7:1.
Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–54.
Ingthorsson S, Briem E, Bergthorsson JT, Gudjonsson T. Epithelial plasticity during human breast morphogenesis and cancer progression. J Mammary Gland Biol Neoplasia. 2016;21:139–48.
Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–60.
Luo M, Brooks M, Wicha MS. Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr Pharm Des. 2015;21:1301–10.
Chimge NO, Baniwal SK, Little GH, Chen YB, Kahn M, Tripathy D, et al. Regulation of breast cancer metastasis by Runx2 and estrogen signaling: the role of SNAI2. Breast Cancer Res. 2011;13:R127.
Chimge N-O, Frenkel B. The RUNX family in breast cancer: relationships with estrogen signaling. Oncogene. 2013;32:2121–30.
Kulkarni M, Tan TZ, Syed Sulaiman NB, Lamar JM, Bansal P, Cui J, et al. RUNX1 and RUNX3 protect against YAP-mediated EMT, stem-ness and shorter survival outcomes in breast cancer. Oncotarget. 2018;9:14175–92.
Browne G, Taipaleenmäki H, Bishop NM, Madasu SC, Shaw LM, van Wijnen AJ, et al. Runx1 is associated with breast cancer progression in MMTV-PyMT transgenic mice and its depletion in vitro inhibits migration and invasion. J Cell Physiol. 2015;230:2522–32.
Pratap J, Imbalzano KM, Underwood JM, Cohet N, Gokul K, Akech J, et al. Ectopic runx2 expression in mammary epithelial cells disrupts formation of normal acini structure: implications for breast cancer progression. Cancer Res. 2009;69, 6807–14.
Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96.
Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68.
Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18.
Zhu Y, Luo M, Brooks M, Clouthier SG, Wicha MS. Biological and clinical significance of cancer stem cell plasticity. Clin Transl Med. 2014;3:32.
Shore P, Dietrich W, Corcoran LM. Oct-2 regulates CD36 gene expression via a consensus octamer, which excludes the co-activator OBF-1. Nucleic Acids Res. 2002;30:1767–73.
Ferrari N, Riggio AI, Mason S, McDonald L, King A, Higgins T, et al. Runx2 contributes to the regenerative potential of the mammary epithelium. Sci Rep. 2015;5:15658.
Mastro AM, Vogler EA. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer Res. 2009;69:4097–100.
Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7:75571–84.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010;12:R68.
Chimge N-O, Ahmed-Alnassar S, Frenkel B. Relationship between RUNX1 and AXIN1 in ER-negative versus ER-positive Breast Cancer. Cell Cycle. 2017;16:312–8.
van Bragt MP, Hu X, **e Y, Li Z. RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells. Elife. 2014;3:e03881.
Browne G, Dragon JA, Hong D, Messier TL, Gordon JA, Farina NH, et al. MicroRNA-378-mediated suppression of Runx1 alleviates the aggressive phenotype of triple-negative MDA-MB-231 human breast cancer cells. Tumour Biol. 2016;37:8825–39.
Hong D, Messier TL, Tye CE, Dobson JR, Fritz AJ, Sikora KR, et al. Runx1 stabilizes the mammary epithelial cell phenotype and prevents epithelial to mesenchymal transition. Oncotarget. 2017;8:17610–27.
Illendula A, Gilmour J, Grembecka J, Tirumala VSS, Boulton A, Kuntimaddi A, et al. Small molecule inhibitor of CBFβ-RUNX binding for RUNX transcription factor driven cancers. EBioMed. 2016;8:117–31.
Shaw FL, Harrison H, Spence K, Ablett MP, Simões BM, Farnie G, et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia. 2012;17:111–7.
Deng W, Lopez-Camacho C, Tang JY, Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA, et al. Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc Natl Acad Sci 2012;109:1524–9.
Acknowledgements
The Bioimaging Facility microscopes used in this study were purchased with grants from BBSRC, Wellcome and the University of Manchester Strategic Fund. The FACS Aria within the flow cytometry core was purchased with funding from the MRC. We are grateful to Michael Jackson for guidance and assistance with cell sorting and to Anne Fowles for support with the in vivo studies of bone metastasis. Additional funding from The University Alumni Fund (RA) and CRUK (SM & KB (C596/A17196)). We would also like to thank the Core Services and Advanced Technologies at the Cancer Research UK Beatson Institute (C596/A17196), with particular thanks to Biological Services Unit and Histology.
Funding
The work was funded by Breast Cancer Now (2013 May PR018; PS, IH and HH), a Cancer Research UK PhD Studentship (PS and HP), a BBSRC PhD studentship and the Manchester Alumni fund.
Author information
Authors and Affiliations
Contributions
RR performed most of the 3D and gene expression analysis of the knockdown cell lines and contributed to the development of the project. HH generated and analysed the CBFβ CRISPR cell line and contributed to the development of the project. RH performed the initial 3D analysis on CBFβ-knockdown cells. NSA generated and analysed the RUNX1 CRISPR cells. HP assisted with CRISPR and writing the manuscript. WD assisted with ChiP assays. PS performed the osteoblast co-cultures. AM assisted with the 3D bioreactor cultures. PO and SMM performed in vivo experiments. KB performed in vivo experiments and assisted with writing the manuscript. IH assisted with the conception and design of the project, interpretation of the data and preparation of the manuscript. PS conceived and directed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All in vivo studies of bone metastasis were approved by the University of Sheffield project Applications and Amendments (Ethics) Committee and conducted in accordance with UK Home Office Regulations under project license 70/8799.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ran, R., Harrison, H., Syamimi Ariffin, N. et al. A role for CBFβ in maintaining the metastatic phenotype of breast cancer cells. Oncogene 39, 2624–2637 (2020). https://doi.org/10.1038/s41388-020-1170-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1170-2
- Springer Nature Limited
This article is cited by
-
CBFβ promotes colorectal cancer progression through transcriptionally activating OPN, FAM129A, and UPP1 in a RUNX2-dependent manner
Cell Death & Differentiation (2021)