Abstract
Pain and anxiety comorbidities are a common health problem, but the neural mechanisms underlying comorbidity remain unclear. We propose that comorbidity implies that similar brain regions and neural circuits, with the lateral septum (LS) as a major candidate, process pain and anxiety. From results of behavioral and neurophysiological experiments combined with selective LS manipulation in mice, we find that LS GABAergic neurons were critical for both pain and anxiety. Selective activation of LS GABAergic neurons induced hyperalgesia and anxiety-like behaviors. In contrast, selective inhibition of LS GABAergic neurons reduced nocifensive withdrawal responses and anxiety-like behaviors. This was found in two mouse models, one for chronic inflammatory pain (induced by complete Freund’s adjuvant) and one for anxiety (induced by chronic restraint stress). Additionally, using TetTag chemogenetics to functionally mark LS neurons, we found that activation of LS neurons by acute pain stimulation could induce anxiety-like behaviors and vice versa. Furthermore, we show that LS GABAergic projection to the lateral hypothalamus (LH) plays an important role in the regulation of pain and anxiety comorbidities. Our study revealed that LS GABAergic neurons, and especially the LSGABAergic-LH circuit, are a critical to the modulation of pain and anxiety comorbidities.
Similar content being viewed by others
Introduction
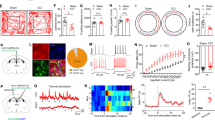
Chronic pain is a thorny medical problem, with the current treatment measures failing to achieve satisfactory results [1]. Chronic pain is often accompanied by several mental diseases or abnormalities, of which anxiety is prominent [2, A, B The distribution pattern of the LS GABAergic projection. A Schematics and timeline of antegrade virus (AAV2/9-DIO-EGFP) tracing. B Schematic summary of mapped recipient brain regions of LS projection fibers with the distribution pattern from whole-brain tracing. C–O By using chemogenetic manipulation, LS GABAergic projections to HDB, LH, PAG were indirectly activated in behavioral experiments. C Experimental scheme of virus injections and behavior tests. Schematics of the stereotaxic surgery targeting the (D) LS-HDB, (H) LS-LH, and (L) LS-PAG circuits. Coronal sections illustrating hM4Di mCherry-positive HDB, LH and PAG neurons at the injection site. Scale bar, 100 μm. E–G Behavioral results for LS-HDB activation. E Withdrawal threshold of the hind paw measured with the von Frey test. F OFT (Left) total distance and (Right) percentage of time in center. G EPM (Left) total entries in arms and (Right) time in open arms. I–K Same as (E–G) but for LS-LH activation. M–O Same as (E–G) but for LS-PAG activation. *P < 0.05, **P < 0.01. ns, no significant difference (P > 0.05). Data are presented as the means ± SEM. For further details of statistical data analysis see Table S1. DM, dorsomedial hypothalamus; HDB, horizontal limb of the diagonal band; HPC, hippocampus; LH, lateral hypothalamus; LPO and MPO, lateral and medial preoptic area; NAc, nucleus accumbens; PAG, periaqueductal gray; VDB, vertical limb of the diagonal band; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
Activation of LS projection induces comorbid pain and anxiety behavior in naïve mice
Different subregions of LS, such as the dorsal and ventral parts, are known to play different roles in the regulation of emotion and other related behaviors [20]. We speculated that these differences might also be reflected in the differences in the anatomical projection targets (i.e., downstream brain regions) associated with the distinct LS subpopulations. To examine the differences in the downstream projection of different LS subregions, we injected anterograde vectors expressing EGFP and mCherry into the dorsal and ventral LS, respectively (Fig. S8A, B). These experiments revealed obvious differences in the brain-wide distribution of the downstream projections between the two subregions of LS. The projection of dorsal LS tends to HDB, LH, VTA et al. while the projection of ventral LS tends to DM, VMH and other brain regions (Fig. S8C, D).
Next, we investigated the role of the different LS projection pathways in pain and anxiety regulation. In selected known target regions of LS projections, we bilaterally injected a retrograde virus vector of a Cre recombinant enzyme (Retro-AAV-Cre) into HDB, LH, PAG, medial preoptic area (MPOA), ventral HPC (vHPC) and VTA, and we also injected a Cre-dependent chemogenetic vector (AAV2/9-DIO-hM3Dq-mCherry) into the bilateral LS (Fig. S9A, E, I; Fig. S10A, E, I) for the selective chemogenetic activation of LS projection neurons. Three weeks after full expression of the virus in the LS, intraperitoneal injection of CNO (2 mg/kg) activates LS projection. We then assessed OFT and EPM testing behaviors. We observed activation of LS projections to HDB and LH reduced time spent in OFT center, EPM open arms and entries in open arms; The projection of LS to HDB decreased the total distance in OFT, but the projection of LS to LH did not affect the total distance (Fig. S9C, D, G, H). Interestingly, the activation of the LS to LH projection had an effect on the pain threshold, the projection of LS to HDB did not change the pain threshold (Fig. S9B, F). The activation of LS projections to PAG also reduced pain thresholds but did not induce anxiety-like behavior (Fig. S9J–L). Chemogenetic activation of the MPOA-projecting, vHPC-projecting, and VTA-projecting LS neurons had no effect on the pain threshold or anxiety behaviors (Fig. S10A–L).
However, we could not fully distinguish the specific roles of the HDB-, LH- and PAG-projecting LS neurons in the regulation of pain and anxiety using the neural circuit construction method described above. To overcome this limitation and to obtain more convincing evidence, C57BL/6 mice were infused with AAV2/1-Cre into the LS, which could anterograde transport from the neuronal cell body to the axon terminal and then across the monosynapse into its projecting neurons, and AAV2/9-DIO-hM4Di-mCherry into the HDB, LH and PAG (Fig. 5C, D, H, L). Through the above circuit construction and with the intraperitoneal injection of CNO (2 mg/kg), the HDB-, LH- and PAG-projecting LS neurons were indirectly activated. The results showed that activation of the neural projection from LS to HDB and to LH triggered anxiety-like behavior including decreased exploring time in the center and total distance in the OFT (Fig. 5F, J) and reduced the number of times and time into the open arm (Fig. 5G, K). The results of von Frey stimulation also showed that activation of neural projection from the LS to LH and PAG neurons induced hyperalgesia (Fig. 5I, M). Activation of LS projection to HDB did not affect the mechanical pain threshold in mice (Fig. 5E). We found that activation of neural projection from the LS to PAG neurons displayed no anxiety-like behaviors in the EPM and OFT tests (Fig. 5N, O). These findings are further confirmation of the remarkable functional differences of the HDB-, LH-, and PAG-projecting subpopulations of LS GABAergic neurons in the regulation of anxiety and pain.
To determine whether these divergent projections originate from different subsets of LS neurons or reflect collateral projections from the same LS population, we performed simultaneous retrograde tracing from the 3 target regions using fluorescent labels of different color. After injection of Retro-AAV-hSyn- expressing mCherry (red), EGFP (green) and mTagBFP-3XFlag (blue) into the HDB, LH and PAG, respectively (Fig. S11A, B), we found strong expression of mCherry, EGFP and mTagBFP-3XFlag in the LS. The expression of mCherry and mtagBFP-3xFlag partially overlapped with EGFP (46.19% and 20.65%), but little overlap between mCherry and mTagBFP-3XFlag (12.78%) (Fig. S11C, D). These experiments suggest that the HDB-, LH - and PAG -projecting LS neurons form largely distinct subpopulations.
Activity of HDB-, LH- and PAG-projecting LS neurons mediates anxiety-like behavior
Above we demonstrated how LS neuron activity affected the behavior of the animals. We next determined whether the activity of the HDB-, LH - and PAG -projecting LS neurons would change in response to noxious stimuli or anxiety behaviors. First, C57BL/6 mice were injected with AAV2/9-DIO-GCaMP6s into the HDB, LH or PAG, and AAV2/1-Cre into the LS. An optic-fiber cannula was implanted in the brain of the animal over the HDB, LH or PAG. We used mice of the CFA model to induce comorbid pain and anxiety behaviors. A 3-day CFA conditioning was conducted, followed by the von Frey, OFT and EPM tests combined with simultaneous monitoring of neuronal activity in the HDB, LH or PAG using position-synchronized in vivo fiber photometry recordings (Fig. S12A). The green fluorescence signals observed in the LS projection areas verified that the AAV2/1-Cre vector crossed the synapse at the LS axon terminals in HDB, LH, and PAG and helped the expression of GCaMP6s in the soma of HDB, LH or PAG neurons (Fig. S12B, F, J).
Mechanical stimulation inhibited calcium signaling in HDB, LH and PAG neurons (Fig. S12C, G, K). The position-synchronized in vivo calcium recording revealed that CFA mice exhibited significant inactivation of GCaMP6s activity following emotional stress exposure of HDB, LH neurons monosynaptically innervated by GABAergic LS projections (Fig. S12D, E, H, I). Calcium signal in PAG was responsive to EMP test, but not to OFT test (Fig. S12L, M). The above results indirectly reflect that mechanical pain stimulation, OFT and EPM test can activate the GABAergic neurons in LS.
Notably, above studies have shown that the LSGABAergic-LH circuit played an importance role in the regulation of comorbid pain and anxiety. To further establish a functional link between the LS and LH, ChR2-mCherry was virally expressed in LH neurons that were the monosynaptic targets of axon projections originated from LS GABAergic neurons (Fig. S13A). In vivo electrophysiological recordings showed that blue laser pulses inhibited LH neurons firing in a time-locked manner (Fig. S13B, C). In CFA model animals exhibiting comorbidity of pain and anxiety behaviors, we next used a genetically encoded GABA sensor (AAV2/9-DIO-iGABASnFR) to determine the dynamics of GABA release in and around the LH from LS projection axon terminals (Fig. S13, D). There were large increases in GABA release around the LH from LS during von Frey stimulation, center of OFT and open-arm exploration of EPM (Fig. S13E–G). These findings represent strong evidence that the LSGABAergic-LH circuit exhibits robust activation during exposure to nocifensive stimulation and it constitutes a key part of the neuronal substrate that functionally mediates anxiety-like behavior in CFA mice.
Discussion
Chronic pain and psychiatric disorders, such as depression and anxiety, are frequently encountered together, each rendering the patient’s satisfactory recovery of the other condition mutually more difficult [21, 22], even though most findings have detected a common neuronal basis for these pathological states [5]. Addressing this context, we report several major findings: (i) chronic pain and anxiety interacted with each other, and pain threshold was positively correlated with the time in the center and open arms in mice; (ii) both chronic pain and anxiety increased excitability of LS GABAergic neurons; (iii) optogenetic/chemogenetic activation of LS GABAergic neurons lowered the pain threshold and induced anxiety-like behavior in naïve mice, and pain and anxiety shared the same cellular subsets regulated in LS; (iv) optogenetic/chemogenetic inhibition of LS GABAergic neurons ameliorated hyperalgesia and anxiety-like behavior in a mouse model of CFA or CRS; (v) CFA or CRS increased inhibitory synaptic input to LH from LS GABAergic neurons; and (vi) chemogenetic activation of LH-projecting LS not only decreased pain threshold but also induced anxiety-like behavior in naïve mice. Together, these results indicate that the activation of LS GABAergic or LH-projecting LS neurons plays a critical role in the comorbidity of pain and anxiety (Fig. S14).
Clinical studies have found that pain and anxiety may interact with each other, and even aggravate each other [5]. In this study, we did find an association between pain and anxiety: in chronic inflammatory pain, anxiety correlated with lowered pain threshold in mice. A similar correlation was found in the CRS model, although the change in the pain threshold there was not as pronounced as in CFA mice. In view of this finding, we propose that pain perception and emotional anxiety are probably processed in part by shared brain networks. The c-Fos protein, expressed by an immediate early gene, is generally used in neurons as a marker for firing activation [23]. Using whole-brain c-Fos staining, we demonstrated that both CFA and CRS activated LS neurons, a necessary condition for LS GABAergic neurons to play a role in the regulation of the comorbidity of pain and anxiety, including in CFA and CRS models. Moreover, with use of the TetTag-hM3Dq system, we were able to verify the active involvement of pain- and anxiety-responsive LS neurons in the modulation of comorbid pain and anxiety behaviors.
Under physiological conditions, the LS receives topographically organized inputs from the cortex and HPC and maintains appropriate excitability. LS is reciprocally connected with thalamic and midbrain structures and regulates appropriate emotional expression [24,25,26]. However, our fiber photometry recording showed that GABAergic neurons in LS were abnormally hyperactivated in pathological conditions, and the enhanced inhibitory LS output to downstream brain regions lead to the development of pain and anxiety. In fact, the activation/inhibition of GABAergic neurons in LS by using multiple manipulation methods resulted in enhanced/diminished occurrence of pain and anxiety comorbidities.
Other studies, however, have implied an anxiogenic role for the LS. One study found that activation of the type 2 CRF receptor (Crfr2) marks a subset of LS GABAergic neurons, which connected with the anterior hypothalamus and enhanced stress-induced anxiety-like behavior [19]. In addition, oxytocin receptor (OXTr) expression in a subset of LS neurons also exerted anxiogenic effects, mainly by inhibiting HDB neurons via inhibitory GABAergic projections to the HDB [27]. Our study is consistent with these findings but goes further by refining the previous observations with the newly revealed role of LS to HDB projection in the regulation of anxiety, but not in pain regulation. More importantly, we also revealed that chemogenetic activation of LH-projecting LS neurons facilitated the mechanical pain threshold and induced anxiety-like behavior in naïve mice. Additionally, fiber photometry recordings indicated that in animals exposed to pain and anxiety-provoking environments, such as the von Frey stimuli, EPM, or OFT, LH-projecting LS neurons, overall, represent pain and anxiety-related features, and this representation is used by the animal to guide pain and anxiety-related behavior. The PAG is another well-validated site for analgesia [28, 29]. In this study, we traced LS GABAergic projections to the dorsolateral part of the PAG and found that the functionally activated LSGABAergic-PAG circuit induced pain, but no anxiety. In other words, distinct LS populations engaged differently in the regulation of pain and anxiety at the circuit level. These findings suggest that one or the other distinct LS GABAergic neuron subpopulation and its respective projection targets in other brain regions, such as the LH, HDB and PAG, may be the neuronal mechanism underlying some form of sensory or emotional dysfunctions.
In fact, we used retrograde tracing technology to show that distinct sets of LS neurons projected to HDB, LH or PAG. Traditionally, the LS has been divided into three main divisions based on their distinct major afferents and efferents. Our anterograde tracing experiment also revealed differences between dorsal and ventral LS projections to downstream brain regions. Different LS projections determined their functional difference, we only focused on the overall role of LS GABAergic neurons in pain and anxiety comorbidities. The differences in function and network connections between LS subregions associated with distinct mechanisms of pain and anxiety processing need to be further explored in the future.
According to an early theory, the LS has an important part in the septohippocampal system. This theory conceptualized the system as a network of emotion and affect processing nodes [19, 30,31,32]. Past studies have focused on the role of vHPC projections to LS in the regulation of anxiety [33]. However, to explore the function of LS projections to the ventral HPC, we failed to find a wanted effect on anxious and pain behavior. It is possible that the projection from hippocampus to LS is involved in the regulation of anxiety, and conversely, the projection from LS to hippocampus has no regulatory effect on anxiety, or there are differences in the participation of the projection from LS subregions to hippocampus in the regulation of anxiety. In this study, the participation of the projection from whole LS in the regulation of anxiety will eventually counteract the effect of each other. Differences in LS subregion projection to the downstream, and its role in the regulation of pain and anxiety will be the subject of our study. Manipulation of the other two pathways, LS-MPOA and LS-VTA circuits had no effect on both anxiety and pain.
In classical lesions of the septum, including both its medial and lateral subdivisions, animals exhibit “septal rage,” marked by increased aggressiveness, hyperactivity, and hyperdefensive behaviors [34]. Reversible inactivation of the LS yielded similar phenotypes [35, 36]. These and other data have led to a prevailing view that LS output is anxiolytic, i.e., dampens fear or anxiety. However, other studies have implied an anxiogenic role for the LS [6]. This study did not distinguish the subregions of LS, and studied the role of overall LS in pain and anxiety, and LS activation induced the occurrence of pain and anxiety comorbidities. It remains to be determined whether a persistent anxiolytic pathways exists or if persistence is rather a unique property of circuits that elevate anxiety.
In conclusion, our findings reveal the critical role of LS GABAergic neurons in the regulation of pain and anxiety comorbidities, and establish the role of GABAergic LS-to-HDB, LH-to-PAG, and LS-to- LH projections in the regulation of anxiety, pain, and both, respectively. Furthermore, strategies targeting neuronal circuits that include LH-projecting LS GABAergic neurons have therapeutic potential for pain and anxiety disorders.