Abstract
The energy-converting organelles mitochondria and chloroplasts are tightly embedded in cellular metabolism and stress response. To appropriately control organelle function, extensive regulatory mechanisms are at play that involve two-way exchange between the nucleus and mitochondria/chloroplasts. In recent years, our understanding of how mitochondria and chloroplasts provide ‘retrograde’ feedback to the nucleus, resulting in targeted transcriptional changes, has greatly increased. Nevertheless, mitochondrial and chloroplast retrograde signalling have largely been studied independently, and only few points of interaction have been found or proposed. Through reassessment of recent publications, this perspective proposes that two of the most well-studied retrograde signalling pathways in plants, those mediated by ANAC017 and those mediated by phosphoadenosine phosphate (PAP), are most likely convergent and can direct overlap** genes. Furthermore, at least part of this common retrograde response appears targeted towards suppression of programmed cell death (PCD) triggered by organellar defects. The identified target genes are discussed in light of their roles in PCD suppression and amplifying the signalling cascade via positive-feedback loops. Finally, a mechanism is proposed that may explain why the convergence of PAP/ANAC017-dependent signalling appears capable of suppressing some types of PCD lesions, but not others, based on the subcellular location of the initial PCD-inducing dysfunction.
Similar content being viewed by others
Main
The complex endosymbiotic origin of eukaryotic cells and the inherent separation of intracellular compartments necessitates efficient communication between the organelles and the nucleus. The feedback provided by organelles to the nucleus – retrograde signalling – has been described in all eukaryotic kingdoms (animals, fungi and plants), but each kingdom seems to have its proprietary systems.1, 2, 3 Plants also evolved intricate retrograde pathways between chloroplasts and the nucleus.4 Mitochondria and chloroplasts have a key role during normal life in energy metabolism and biosynthesis of important compounds for the cell. However, they can also have key roles in the execution of programmed cell death (PCD) to remove unwanted cells that may produce toxic levels of reactive molecules or are infected by pathogens.5, 6, 7, 8 Therefore, many systems are in place that control organelle numbers, composition and quality, and keep track of their suicidal tendencies.3 This perspective paper proposes that mitochondrial and chloroplast retrograde signalling – or at least some types – are convergent on overlap** target genes. Furthermore, this common response mechanism may help prevent PCD initiation and steer the balance towards cell survival, most likely by suppressing excessive oxidative stress and repairing organelle damage.
The ANAC017 Pathway and PCD
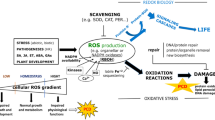
The most clearly understood pathway for mitochondrial retrograde signalling in plants involves activation of the transcription factor ANAC017.9, 10, 11, 12 This activation can be triggered by acute inhibition of mitochondrial function, for example, by antimycin A (complex III).10 Recently it was shown that the ANAC017-dependent signalling pathway is also active when mitochondrial biogenesis is disturbed by genetic defects, for instance when the mitochondrial prohibitin AtPHB3 scaffolding complex is defective, or when mitochondrial/plastid RNA polymerase RpoTmp is impaired.9 ANAC017 has a C-terminal transmembrane domain, which probably anchors it into the endoplasmic reticulum (ER).10, 11 Currently, we have little understanding of how signals from dysfunctional mitochondria reach the ER and activate ANAC017. Most evidence points towards mitochondrial reactive oxygen species (ROS) production, with H2O2 as the most likely mobile signal.10, 13 Inhibitor studies suggested that rhomboid proteases might be involved in release of ANAC017 from the ER.10
Although we know little about the activation of ANAC017, we have a good overview of its downstream target genes, at least 200 in Arabidopsis.9 Many of these encode mitochondrial proteins such as alternative oxidase (AOX1a), alternative NADH dehydrogenases, OPA3 potentially involved in mitochondrial fission and a range of oxidative stress inducible genes with less-defined roles.14, 15, 16 On the basis of recent findings, it appears that one of the functions of the ANAC017 pathway may be to suppress cell death. First, when ANAC017 function is abolished in mitochondrial RNA polymerase rpotmp anac017 double mutants, the plants develop spontaneous lesions.9, 17 This is likely caused at least in part by the lack of ANAC017-dependent induction of AOX1a in rpotmp anac017 double mutants, as rpotmp aox1a double mutants also display similar lesions.18 Although the exact reasons for lesion formation in rpotmp aox1a or rpotmp anac017 mutants are unknown, they are likely caused by spontaneous PCD. In agreement, AOX has been shown extensively to suppress PCD in plants during inhibition of mitochondrial function.Figure 1). It is tempting to speculate that they are in fact directly related or operate in parallel, although a possible mechanism behind this interaction is not clear yet. Many downstream genes appear unique to either ANAC017 or PAP pathways (although the mutants were not compared directly under the same conditions), so their overlap is most likely partial.12 Whether ANAC017/PAP target genes are induced in vivo depends on the circumstances of the cellular dysfunction, most probably the location and type of ROS produced (Figure 2). Evidence to date implicates superoxide and H2O2 produced in chloroplasts and mitochondria, but changes in chloroplastic redox poise per seare sufficient to inactivate SAL1 and increase PAP. It is intriguing that the induction of the PAP pathway can rescue some PCD-causing defects, but plants apparently do not always activate this mechanism: for instance, in the mips1 mutants. Perhaps the needed cross-talk between signalling pathways is not present (yet) to activate the PAP pathway in those cases. Alternatively, overactivation of the PAP pathway may overall be more detrimental than beneficial, resulting in negative selection pressure. Finally and critically, the function of mitochondrial-targeted SAL1 remains to be determined.
References
Ng S, De Clercq I, Van Aken O, Law SR, Ivanova A, Willems P et al. Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth, development, and stress. Mol Plant 2014; 7: 1075–1093.
Chandel NS . Evolution of mitochondria as signaling organelles. Cell Metab 2015; 22: 204–206.
Quiros PM, Mottis A, Auwerx J . Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 2016; 17: 213–226.
Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ . Learning the languages of the chloroplast: retrograde signaling and beyond. Annu Rev Plant Biol 2016; 67: 25–53.
Van Aken O, Van Breusegem F . Licensed to kill: mitochondria, chloroplasts, and cell death. Trends Plant Sci 2015; 20: 754–766.
Shumbe L, Chevalier A, Legeret B, Taconnat L, Monnet F, Havaux M . Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 kinase. Plant Physiol 2016; 170: 1757–1771.
Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K et al. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 2012; 24: 3026–3039.
Kasahara A, Scorrano L . Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol 2014; 24: 761–770.
Van Aken O, Ford E, Lister R, Huang S, Millar AH . Retrograde signalling caused by heritable mitochondrial dysfunction is partially mediated by ANAC017 and improves plant performance. Plant J 2016; 88: 542–558.
Ng S, Ivanova A, Duncan O, Law SR, Van Aken O, De Clercq I et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 2013; 25: 3450–3471.
De Clercq I, Vermeirssen V, Van Aken O, Vandepoele K, Murcha MW, Law SR et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 2013; 25: 3472–3490.
Van Aken O, De Clercq I, Ivanova A, Law SR, Van Breusegem F, Millar AH et al. Mitochondrial and chloroplast stress responses are modulated in distinct touch and chemical inhibition phases. Plant Physiol 2016; 171: 2150–2165.
Huang S, Van Aken O, Schwarzlander M, Belt K, Millar AH . The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol 2016; 171: 1551–1559.
Wang Y, Lyu W, Berkowitz O, Radomiljac JD, Law SR, Murcha MW et al. Inactivation of mitochondrial complex I induces the expression of a twin cysteine protein that targets and affects cytosolic, chloroplastidic and mitochondrial function. Mol Plant 2016; 9: 696–710.
Van Aken O, Zhang B, Carrie C, Uggalla V, Paynter E, Giraud E et al. Defining the mitochondrial stress response in Arabidopsis thaliana. Mol Plant 2009; 2: 1310–1324.
Van Aken O, Giraud E, Clifton R, Whelan J . Alternative oxidase: a target and regulator of stress responses. Physiol Plant 2009; 137: 354–361.
Kuhn K, Richter U, Meyer EH, Delannoy E, de Longevialle AF, O'Toole N et al. Phage-type RNA polymerase RPOTmp performs gene-specific transcription in mitochondria of Arabidopsis thaliana. Plant Cell 2009; 21: 2762–2779.
Kuhn K, Yin G, Duncan O, Law SR, Kubiszewski-Jakubiak S, Kaur P et al. Decreasing electron flux through the cytochrome and/or alternative respiratory pathways triggers common and distinct cellular responses dependent on growth conditions. Plant Physiol 2015; 167: 228–250.
Liu J, Li Z, Wang Y, **ng D . Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. J Exp Bot 2014; 65: 4465–4478.
Vanlerberghe GC, Robson CA, Yip JY . Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 2002; 129: 1829–1842.
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007; 316: 715–719.
Mehrshahi P, Stefano G, Andaloro JM, Brandizzi F, Froehlich JE, DellaPenna D . Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts. Proc Natl Acad Sci USA 2013; 110: 12126–12131.
Brunkard JO, Runkel AM, Zambryski PC . Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci USA 2015; 112: 10044–10049.
Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S et al. Chloroplast stromules function during innate immunity. Dev Cell 2015; 34: 45–57.
Coll NS, Smidler A, Puigvert M, Popa C, Valls M, Dangl JL . The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: functional linkage with autophagy. Cell Death Differ 2014; 21: 1399–1408.
Stael S, Kmiecik P, Willems P, Van Der Kelen K, Coll NS, Teige M et al. Plant innate immunity—sunny side up? Trends Plant Sci 2015; 20: 3–11.
Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M . Plant organellar calcium signalling: an emerging field. J Exp Bot 2012; 63: 1525–1542.
Triantaphylides C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M et al. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 2008; 148: 960–968.
Lee KP, Kim C, Landgraf F, Apel K . EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 2007; 104: 10270–10275.
Mullineaux PM, Baker NR . Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol 2010; 154: 521–525.
Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K . Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 2007; 104: 672–677.
Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem 2012; 287: 11717–11729.
Carmody M, Crisp PA, d'Alessandro S, Ganguly D, Gordon M, Havaux M et al. Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol 2016; 171: 1734–1749.
Baruah A, Simkova K, Apel K, Laloi C . Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol Biol 2009; 70: 547–563.
Gordon MJ, Carmody M, Albrecht V, Pogson B . Systemic and local responses to repeated HL stress-induced retrograde signaling in Arabidopsis. Front Plant Sci 2012; 3: 303.
Bruggeman Q, Mazubert C, Prunier F, Lugan R, Chan KX, Phua SY et al. Chloroplast activity and 3'phosphadenosine 5'phosphate signaling regulate programmed cell death in Arabidopsis. Plant Physiol 2016; 170: 1745–1756.
Bruggeman Q, Prunier F, Mazubert C, de Bont L, Garmier M, Lugan R et al. Involvement of Arabidopsis hexokinase1 in cell death mediated by myo-inositol accumulation. Plant Cell 2015; 27: 1801–1814.
Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011; 23: 3992–4012.
Bruggeman Q, Garmier M, de Bont L, Soubigou-Taconnat L, Mazubert C, Benhamed M et al. The polyadenylation factor subunit CLEAVAGE AND POLYADENYLATION SPECIFICITY FACTOR30: a key factor of programmed cell death and a regulator of immunity in Arabidopsis. Plant Physiol 2014; 165: 732–746.
Chan KX, Mabbitt PD, Phua SY, Mueller JW, Nisar N, Gigolashvili T et al. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc Natl Acad Sci USA 2016; 113: E4567–E4576.
Van Aken O, Whelan J . Comparison of transcriptional changes to chloroplast and mitochondrial perturbations reveals common and specific responses in Arabidopsis. Front Plant Sci 2012; 3: 281.
Meng PH, Raynaud C, Tcherkez G, Blanchet S, Massoud K, Domenichini S et al. Crosstalks between myo-inositol metabolism, programmed cell death and basal immunity in Arabidopsis. PLoS One 2009; 4: e7364.
Aviv DH, Rusterucci C, Holt BF 3rd, Dietrich RA, Parker JE, Dangl JL . Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J 2002; 29: 381–391.
Li Y, Chen L, Mu J, Zuo J . LESION SIMULATING DISEASE1 interacts with catalases to regulate hypersensitive cell death in Arabidopsis. Plant Physiol 2013; 163: 1059–1070.
Jabs T, Dietrich RA, Dangl JL . Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996; 273: 1853–1856.
Maxwell DP, Wang Y, McIntosh L . The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 1999; 96: 8271–8276.
Zhang B, Van Aken O, Thatcher L, De Clercq I, Duncan O, Law SR et al. The mitochondrial outer membrane AAA ATPase AtOM66 affects cell death and pathogen resistance in Arabidopsis thaliana. Plant J 2014; 80: 709–727.
Hortensteiner S, Krautler B . Chlorophyll breakdown in higher plants. Biochim Biophys Acta 2011; 1807: 977–988.
Pattanayak GK, Venkataramani S, Hortensteiner S, Kunz L, Christ B, Moulin M et al. Accelerated cell death 2 suppresses mitochondrial oxidative bursts and modulates cell death in Arabidopsis. Plant J 2012; 69: 589–600.
Chrobok D, Law SR, Brouwer B, Linden P, Ziolkowska A, Liebsch D et al. Dissecting the metabolic role of mitochondria during developmental leaf senescence. Plant Physiol 2016; 172: 2132–2153.
Zwack PJ, Robinson BR, Risley MG, Rashotte AM . Cytokinin response factor 6 negatively regulates leaf senescence and is induced in response to cytokinin and numerous abiotic stresses. Plant Cell Physiol 2013; 54: 971–981.
Zwack PJ, De Clercq I, Howton TC, Hallmark HT, Hurny A, Keshishian EA et al. Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress. Plant Physiol 2016; 172: 1249–1258.
Trobacher CP . Ethylene and programmed cell death in plants. Botany 2009; 87: 757–769.
Ahlfors R, Lang S, Overmyer K, Jaspers P, Brosche M, Tauriainen A et al. Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein-protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. Plant Cell 2004; 16: 1925–1937.
Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y et al. Structure and function of sulfotransferases. Arch Biochem Biophys 2001; 390: 149–157.
Baek D, Pathange P, Chung JS, Jiang J, Gao L, Oikawa A et al. A stress-inducible sulphotransferase sulphonates salicylic acid and confers pathogen resistance in Arabidopsis. Plant Cell Environ 2010; 33: 1383–1392.
Acknowledgements
OVA was supported by Australian Research Council APD fellowship and grants (DP110102868, DP160103573 and DP130102918), and facilities provided by CE140100008. BJP was supported by Australian Research Council Centre of Excellence Program (CE140100008). We thank Dr Kai Xun Chan for critically reading the manuscript and for useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Chandel
Rights and permissions
About this article
Cite this article
Van Aken, O., Pogson, B. Convergence of mitochondrial and chloroplastic ANAC017/PAP-dependent retrograde signalling pathways and suppression of programmed cell death. Cell Death Differ 24, 955–960 (2017). https://doi.org/10.1038/cdd.2017.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2017.68
- Springer Nature Limited
This article is cited by
-
Genome-wide analysis of the NAC transcription factor family in broomcorn millet (Panicum miliaceum L.) and expression analysis under drought stress
BMC Genomics (2020)
-
Proteomics: a powerful tool to study plant responses to biotic stress
Plant Methods (2019)
-
Cell death in cancer in the era of precision medicine
Genes & Immunity (2019)
-
Combinatorial Interactions of Biotic and Abiotic Stresses in Plants and Their Molecular Mechanisms: Systems Biology Approach
Molecular Biotechnology (2018)