Abstract
Background
Identification of prognostic biomarkers for outcomes in gastrointestinal (GI) cancer with immunotherapy is important. This study investigated the relationship between the prognostic biomarker, pretreatment neutrophil-to-lymphocyte ratio (preNLR), and immunotherapeutic outcomes in patients with advanced GI cancer.
Methods
We searched the PubMed, Embase, and Cochrane Library databases for studies reporting predictive values for preNLR in patients with advanced GI cancer treated with immune checkpoint inhibitor (ICI). The primary outcomes considered were progression-free survival (PFS) and overall survival (OS). Hazard ratios (HRs) with 95% confidence intervals (CIs) for PFS and OS were pooled using a random effects model. We then validated the results observed in an in-house cohort of patients treated with ICIs for advanced GI cancers. Other prognostic factors for PFS and OS were explored using Cox proportional hazard analyses.
Results
Overall, 27 observational studies involving 3,610 patients with advanced GI cancer were included. Patients with higher preNLR were associated with poorer PFS (HR 1.70, 95% CI 1.50–1.92) and OS (HR 2.35, 95% CI 1.82–3.03) with ICI treatment. Subgroup analyses based on NLR cut-off value, research center, sample size, and ICI drugs used were consistent with the primary results. A retrospective analysis of the in-house patient cohort validated these results (PFS: HR 3.173, 95% CI 2.314–4.351; OS: HR 3.004, 95% CI 1.837–4.912). Multivariable Cox regression analysis of 174 patients showed that higher preNLR and negative programmed death ligand-1 (PD-L1) expression were independently, significantly, and unfavorably associated with PFS and OS.
Conclusion
PreNLR might be an effective prognostic biomarker for patients with advanced GI cancer treated with ICIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal (GI) tract cancers develo** in the stomach, liver, esophagus, pancreas, and colorectum represent over a quarter of all cancers and more than a third of all cancer-related deaths globally [1]. In recent decades, chemotherapy has been the cornerstone for managing advanced GI cancers; however, only minimal improvements in survival have been achieved for these patients [2]. More recently, immunotherapy has revolutionized cancer treatment by extending survival in various cancer types, including GI malignancies [3, 4]. Unfortunately, the favorable benefits of immune checkpoint inhibitor (ICI) treatment do not apply to all GI cancer patients, even to those with a positive programmed death ligand-1 (PD-L1) expression [5,6,7]. This renders the identification of prognostic biomarkers a clinical necessity for screening and predicting which cancer patients are likely to respond to immunotherapy, thereby improving therapeutic efficacy.
The neutrophil-to-lymphocyte ratio (NLR) is a marker of systemic inflammation, and it is defined as the neutrophil count divided by the lymphocyte count. Recently, it has been shown to be related to outcomes in various cancers [8,9,10]. Therefore, the NLR may be a potential prognostic factor for cancer patients, especially those treated with immunotherapy, as ICI therapies stimulate T lymphocyte activation in the immune system. Several retrospective studies have reported the prognostic value of pretreatment NLR (preNLR) in patients with inoperable GI cancers undergoing ICI treatment [11,12,13]. However, these studies have yielded conflicting results. In addition, they are limited by small sample sizes and the inclusion of specific cancer types only. These deficiencies have inevitably introduced bias and led to over- or under-estimation of the prognostic value of preNLR. Therefore, we pooled the available studies to explore the prognostic value of preNLR in ICI-treated patients with advanced GI cancers, and we also performed a retrospective, validating analysis using clinical data obtained from our center to verify the pooled results.
Methods
Pooled analysis
Data sources and selection criteria
Following a previously established protocol (PROSPERO: CRD42022383439) and the PRISMA guidelines [14], we searched the PubMed, Embase, and Cochrane Library databases systematically (from their inception to 31 August 2022). Table S1 presents the detailed search strategies. The inclusion criteria for the studies were as follows: (I) studies conducted in populations with advanced GI cancer (including gastric cancer, esophageal cancer, intestinal cancer, pancreatic cancer, hepatocellular carcinoma, cholangiocarcinoma); (II) treatments that included one or more ICI drugs (targeting programmed death-1 [PD-1], PD-L1, cytotoxic T lymphocyte-associated antigen-4 [CTLA-4], etc.); and (III) studies that included the prognostic value for preNLR, detailed data of overall survival (OS) and/or progression-free survival (PFS), and the related hazard ratio (HR) with 95% confidence intervals (CIs). Studies were excluded if they were published only as conference abstracts, posters, or as presentations of ongoing trials. Eligibility screening of the studies based on the above criteria was conducted by two authors (Y.Y. and S.R.).
Study outcomes, data extraction, and quality assessment
PFS and OS were the primary outcomes. Two authors (Y.Y. and S.R.) applied a predesigned table to extract data from the main text, including study characteristics, patient demographics and characteristics, reported NLR cut-off values, and efficacy outcomes. The methodological quality of the studies included was appraised using the Newcastle-Ottawa Scale (NOS) [15]. Summary risk of bias was stratified according to the NOS score (low, NOS score ≥ 7; moderate, 4 ≤ NOS score ≤ 6; high, NOS score ≤ 3). Any disagreements that occurred in the study selection, data extraction, and quality assessment were settled by discussion with the corresponding author (Q.X.).
Statistical analyses
HRs with 95% CIs for PFS and OS were calculated by employing a random-effects model. The I2 test was used to evaluate heterogeneity among studies, with I2 > 50% indicating considerable heterogeneity. Subgroup analyses were performed based on cancer type, cut-off value of NLR, research center, sample size, and ICI treatment. A sensitivity analysis was conducted to test the robustness of the results by excluding each study from the pool in sequence. Publication bias was assessed by employing funnel plots, Begg’s, and Egger’s tests. All data were analyzed using STATA software (version 13.0).
Retrospective analysis of in-house patients
Participants
Information for all patients diagnosed with advanced GI cancer and treated with at least one ICI drug for more than one cycle at a university hospital in Shanghai, China, between 2018 and 2022, was obtained from the hospital information system (HIS). Clinical information obtained from the medical records included gender, age, cancer type, the status of PD-L1 expression, remote metastasis, clinical interventions, line of ICI treatment, ICI agent and combinations, and concomitant medication.
Assessment
The primary end point of the present study was to assess the prognostic significance of different preNLR levels in relation to PFS and OS. PFS and OS were defined as the time from treatment initiation until radiographic disease progression or death, and death from any cause, respectively. Depending on the drug regimen, Response Evaluation Criteria in Solid Tumors (RECIST) was used to assess treatment efficacy. The response was stratified into four classes: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR plus PR was defined as an objective response, while CR, PR, plus SD was defined as disease control. The preNLR was calculated as the neutrophil count divided by the lymphocyte count at the first ICI treatment. The receiver operating characteristic (ROC) curve for predicting 6-month PFS by NLR was depicted to determine the cut-off value of the NLR. The point at the upper left corner of the ROC curve, namely, the highest point on the vertical axis and the point furthest to the left on the horizontal axis, was identified as the optimal cut-off value. The patients were divided into low and high NLR groups based on their cut-off values.
Statistical analyses
Continuous variables were reported as mean ± standard deviation, and these were compared using the independent t-test. Categorical variables were expressed as numbers and percentages, and these were compared using the chi-square test. The survival curves for PFS and OS were estimated using the Kaplan–Meier method. Prognostic factors for OS and PFS were performed by univariate and multivariate Cox proportional hazard analyses. SPSS software (version 22.0) was used for the above statistical analyses, and P < 0.05 was considered statistically significant.
Results
Pooled analysis
Characteristics and quality of studies
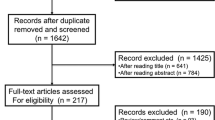
The flow diagram for the study selection is depicted in Fig. 1. The initial search identified 342 studies, of which 42 full-text articles were obtained to be further assessed for eligibility. Finally, 27 studies fulfilled the inclusion criteria. Their characteristics are listed in Table S2. Most were retrospective studies conducted in single centers in Asian countries. Regarding cancer type, 12 studies involved patients with gastric cancer, 11 involved patients with hepatocellular carcinoma, and the remaining four studies involved patients with esophageal cancer, gastric and colorectal cancers, and pancreatic cancer. A total of 3,610 advanced GI cancer patients were included, with 2,289, 722, 453, and 146 patients receiving PD-1 inhibitors, unclassified ICIs, PD-L1 inhibitors combined with bevacizumab, and PD-1/L1 inhibitors, respectively. The NOS scores for the included studies ranged from 4 to 9, as presented in Table S3.
Relationship between preNLR and immunotherapy outcome
Having pooled data from 21 studies with a total of 2,739 patients, we found that a higher NLR was related to a poorer PFS (HR 1.70, 95% CI 1.50–1.92,I2 = 21.4%; Fig. 2A, Fig. S1). Subgroup analyses, stratified by factors that included NLR cut-off value, research center, sample size, and drug given for the immunotherapy, consistently showed trends similar to that of the primary result (Fig. S2). Data on NLR and OS were extracted for 3,562 patients in 26 studies who were treated with immunotherapy. The pooled results revealed a significant association between an elevated NLR and a shorter OS in patients with GI cancers who were receiving immunotherapy (HR 2.35, 95% CI 1.82–3.03), with high heterogeneity (I2 = 89.3%; Fig. 2B,Fig.S3). To detect the potential source of heterogeneity among studies reporting OS data, subgroup analyses were performed as previously described (Fig. S4). The high heterogeneity was primarily derived from studies involving hepatocellular carcinoma, using a cut-off value ≥ 3. These were performed in a single center, with a sample size ≥ 100, and receiving treatment with PD-1 inhibitors.
The results of the sensitivity analyses, including PFS and OS, were consistent with those of the primary analyses (Table S4). A visual inspection of the funnel plot showed relative symmetry in studies reporting an association between NLR and PFS (Fig. S5A). Regarding the OS outcome, the funnel plot and Egger’s test showed significant publication bias (Fig. S5B, Table S5). To mitigate this publication bias, we used the trim-and-fill method and then found the results were consistent with the primary outcomes (P for interaction > 0.05; Fig. S5C–S5D, Table S5).
Retrospective analysis
Baseline characteristics
A total of 205 patients with advanced GI cancer who were receiving ICI agents were enrolled in the in-house patient cohort, of whom 174 were included in the analyses (6 were excluded due to autoimmune and chronic infectious diseases, 11 developed acute infection before the first dose of ICI therapy, and 14 had missing data). The patients’ clinical features are provided in Table 1. Among these patients, 116 (66.7%) were male, 114 (65.5%) had gastric cancer, 76 (43.7%) had positive PD-L1 expression, and 159 (91.4%) had metastasis. Nearly two-thirds (64.4%) of the patients received immunotherapy combined with chemotherapy, and most received PD-1 inhibitors. Regarding concomitant medication, half of the patients used glucocorticoids (50.0%) or proton pump inhibitors (PPIs, 47.7%) during their immunotherapy. Based on the ROC analysis, the optimal cut-off value for NLR was determined as 2.98 (AUC = 0.724, P < 0.001; Fig. S6). The patients were divided into a low NLR group (NLR < 2.98, n = 95) and a high NLR group (NLR ≥ 2.98, n = 79; Table 1).
Efficacy and safety based on low and high NLR groups
The optimal treatment efficacy was evaluated in 163 patients: 1 (0.6%) patient had CR, 37 (22.7%) patients had PR, 83 (50.9%) patients had SD, and 42 (25.8%) patients had PD. The objective response rate (ORR) was 23.3%, and the disease control rate (DCR) was 74.2%. A significant difference in DCR was observed between the low and high NLR groups (83.3% vs. 63.0%, P = 0.003; Table 2). As shown in Fig. 3, the PFS in the high NLR group was 4.4 months, while the PFS in the low NLR group was 9.1 months (HR 3.173, 95% CI 2.314–4.351, P < 0.001). Similarly, the OS was 6.3 months in the high NLR group and 13.5 months in the low NLR group (HR 3.004, 95% CI 1.837–4.912, P < 0.001). The curves for both groups were well separated over time.
Safety data were obtained from 103 patients, of whom over 90% experienced adverse events (Table 2). Of these, myelosuppression (46, 44.7%) was the most common adverse event, followed by cutaneous reactions (40, 38.8%), and gastrointestinal reactions (35, 34.0%). Adverse events, regardless of whether total or individual, occurred with comparable frequencies in the low and high NLR groups, with the exception of myelosuppression (56.1% in low NLR vs. 30.4% in high NLR, P = 0.009).
Univariate and multivariate analyses of preNLR
As shown in Table S6, 14 potential variables were initially included in the univariate Cox regression analysis. Six of them (age, metastatic sites, line of immunotherapy, NLR, use of PPI, and myelosuppression) and four of them (age, metastatic sites, PD-L1 expression, and NLR) were identified as statistically associated with the risk of disease progression and death, respectively (P < 0.1 for each variable). Since PD-L1 expression is recognized as an important predictor of the efficacy of immunotherapy, we also included it in the multivariate Cox regression analysis of PFS. After the multivariate Cox regression analysis, the results showed that preNLR ≥ 2.98 increased the risk of disease progression independently and significantly (HR 3.638, 95% CI 2.144–6.173, P < 0.001). This was similar for immunotherapy ≥ 2 (HR 3.213, 95% CI 1.608–6.418, P = 0.001), for PD-L1 negative (HR 1.672, 95% CI 1.007–2.775, P = 0.047), and for the concomitant use of PPI (HR 2.568, 95% CI 1.488–4.433, P = 0.001; Table 3). In addition, preNLR ≥ 2.98 (HR 2.600, 95% CI 1.481–4.566, P = 0.001), PD-L1 negative (HR 1.754, 95% CI 1.006–3.058, P = 0.047), and metastatic sites ≥ 3 (HR 1.966, 95% CI 1.109–3.485, P = 0.021) were independently associated with worse OS.
Discussion
To the best of our knowledge, the present study reports the most comprehensive assessment of the association between preNLR and outcomes during immunotherapy across multiple gastrointestinal cancers by pooling eligible studies to give a relatively large sample size and by analyzing a retrospective GI cancer cohort in the Chinese population. We consistently observed an increased risk of disease progression and death with elevated preNLR among patients with advanced GI across the different analyses. Further analyses of the factors influencing clinical outcomes, as taken from retrospective data, suggest that preNLR and PD-L1 expression are associated significantly and independently with PFS and OS in patients with GI cancers.
Previously published meta-analyses have documented the prognostic value of neutrophils, particularly NLR, and their link to poor outcomes from immunotherapy for various cancers, such as advanced non-small cell lung cancer [8], renal cell cancer [9], and head and neck squamous cell carcinoma [10]. However, whether preNLR has a predictive role in patients treated with ICI for advanced GI cancer remains unknown. Jiang et al. conducted a meta-analysis with 36 studies, involving a total of 8,614 patients with gastric cancer, and demonstrated that high preNLR predicts a poor prognosis for systemic therapy, including chemotherapy, targeted therapy, and immunotherapy [16]. Recently, several real-world studies have been performed to explore the association between NLR value and prognosis for various GI cancers being treated with immunotherapy [11,12,13]. Notably, these studies have yielded confounding results, with different preNLR cut-off values. In addition, they were limited by small sample sizes, which inevitably reduced their statistical power. Given these limitations, the random effects model was used regardless of the presence of heterogeneity in the present study. Furthermore, all GI cancer types were included to provide a comprehensive estimate of the preNLR value in relation to immunotherapeutic outcomes. These results were further verified using real-world data from our center.
Consistent with previous studies that focused on other cancers, high preNLR was associated with short PFS and OS in GI cancer. An epidemiological study indicated that almost a quarter of all cancer cases are accompanied by acute or chronic inflammation [17]. Thus, the relationship between cancer and inflammation has been extensively discussed. Over a long period of exploration, the role of systemic inflammation in the development and progression of cancer has gradually been acknowledged [18]. Neutrophils are a systemic inflammatory index, and it is hypothesized that they suppress the anti-tumor immune response by secreting chemokines and cytokines [19]. On the other hand, the increased infiltration of lymphocytes around solid tumor tissues has been shown to be related to superior therapeutic efficiency and better patient outcomes [20]. Therefore, a lower NLR value represents relative lymphocyte dominance, while it also indicates the unique properties of a favorable inflammatory microenvironment for a subsequent immunotherapeutic reaction.
Together with the baseline NLR, when tumors were labeled as PD-L1 positive, it was noted as a significantly favorable factor for a better prognosis in multivariate analyses. As an immune checkpoint, PD-L1 is a reasonable biomarker for predicting the treatment response to anti-PD-1 or anti-PD-L1 therapies. Regarding other characteristics, patients receiving immunotherapy as a first-line treatment were independently associated with improved PFS but not with improved OS. In general, patients with initial immunotherapy had a longer PFS and OS than those with previous antitumor treatments [6, 21]. There was no clear discrepancy in relation to OS in our study, probably due to insufficient follow-up time. We also found that concomitant ICI–PPI therapy appeared to significantly shorten the PFS. Studies analyzing the microbiome of patients undergoing ICI treatment have found that patients responding to immunotherapy have a more diverse gut microbiome, which, in turn, has been shown to correlate with the tumor microenvironment [22, 23]. Theoretically, a decrease in the diversity of bacterial species may have adverse effects on the outcomes of patients using ICIs. Studies have proven that PPIs affect the gut microbiome, possibly through the direct inhibition of stomach acid, which normally provides the main defense system against bacterial influx from food and oral bacterial flora [24]. In addition, patients who had fewer organ metastases (< 3) demonstrated a favorable trend in OS. This finding was consistent with that of a previous study [11].
While every effort was made, we acknowledge that the study had several limitations. First, our findings were based on exploratory retrospective data collected from different sources, including a pooled analysis and a cohort study, so there were differences between patients across the analyses. However, it is common to achieve stronger confidence by using independent datasets for cross-validation [25, 26]. Second, in the pooled analysis, the studies included displayed heterogeneity in cancer subtypes, NLR cut-off values, ICI agents, etc. Sensitivity and subgroup analyses were therefore carried out to control for the effects of possible confounders. Third, some intrinsic and extrinsic factors, such as previous treatments and the gut microbiomes of patients, were not reported in previous studies, so they could not be considered in our analysis. Furthermore, our own cohort study was based on a relatively small sample size of patients from a single center, which may have caused selection bias. Future prospective studies with larger sample sizes, refined cancer types, and more detailed clinical information are required to further investigate the impact of preNLR on immunotherapy outcomes among GI cancer patients.
Conclusion
Our comprehensive study suggested that a higher preNLR was significantly and independently associated with poorer PFS and OS in advanced GI cancer patients undergoing ICI treatment. Hence, NLR should be regarded as an effective prognostic predictor, and as a valuable aid in clinical ICI treatment strategy decisions. Importantly, given the limitations mentioned above, more prospective cohort studies are needed to confirm our results.
Availability of data and materials
The data that support the findings of this study are available on reasonable request to the corresponding authors.
References
Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335-349.e15.
Ham IH, Lee D, Hur H. Cancer-associated fibroblast-induced resistance to chemotherapy and radiotherapy in gastrointestinal cancers. Cancers. 2021;13(5):1172.
Abdul-Latif M, Townsend K, Dearman C, et al. Immunotherapy in gastrointestinal cancer: the current scenario and future perspectives. Cancer Treat Rev. 2020;88:102030.
Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29(3):1559–74.
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80.
Fuchs CS, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer. 2022;25(1):197–206.
** J, Yang L, Liu D, et al. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: a meta-analysis. BMJ Open. 2020;10(6):e035031.
Yanagisawa T, Mori K, Katayama S, et al. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2022;14(9):709–25.
Takenaka Y, Oya R, Takemoto N, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck. 2022;44(5):1237–45.
Pan YT, Si HY, Deng GC, et al. A composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-PD-1 antibodies. Front Oncol. 2021;11:798415.
Toshida K, Itoh S, Tomiyama T, et al. Comparison of the prognostic effect of sarcopenia on atezolizumab plus bevacizumab and lenvatinib therapy in hepatocellular carcinoma patients. JGH Open. 2022;6(7):477–86.
Gao Y, Zhang Z, Li Y, et al. Pretreatment neutrophil-to-lymphocyte ratio as a prognostic biomarker in unresectable or metastatic esophageal cancer patients with anti-PD-1 therapy. Front Oncol. 2022;12:834564.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother. 2018;67(5):713–27.
Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–80.
Hung RJ, Ulrich CM, Goode EL, et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J Natl Cancer Inst. 2015;107(11):djv246.
Pęczek P, Gajda M, Rutkowski K, et al. Cancer-associated inflammation: pathophysiology and clinical significance. J Cancer Res Clin Oncol. 2023;149(6):2657–72.
Jacquelot N, Tellier J, Nutt SI, et al. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology. 2021;10(1):1900508.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–31.
Yan YD, Zhao Y, Zhang C, et al. Toxicity spectrum of immunotherapy in advanced lung cancer: a safety analysis from clinical trials and a pharmacovigilance system[J]. EclinicalMedicine. 2022;50:101535.
**g Y, Chen X, Li K, et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J Immunother Cancer. 2022;10(1):e003779.
Acknowledgements
Not applicable.
Funding
This work was supported by project grants from the National Natural Science Foundation of China (82271883, 82073195), Hospital-pharma Integration Project on Innovative Achievement Translation (SHDC2022CRD043).
Author information
Authors and Affiliations
Contributions
QX, MHY: guarantors of the entire manuscript. QX, YDY: study conception and design, critical revision of the manuscript for important intellectual content. SYR, YFX, HXP, and XW: data acquisition, analysis, and interpretation. XMW, XQW, TYZ, and GYW: verification of the underlying data. All the authors have read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was implemented according to the Declaration of Helsinki, and was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (KY2021-097-B).
Consent for publication
All the authors have approved the final version of this manuscript, and agreed the publication.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Material 1.
Association of pretreatment neutrophil-to-lymphocyte ratio with outcome in patients with advanced gastrointestinal cancer receiving immunotherapy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, Y., Rong, S., **, Y. et al. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in advanced gastrointestinal cancer immunotherapy: evidence from published studies and a Chinese single center cohort. CCB 3, 9 (2024). https://doi.org/10.1007/s44272-024-00014-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44272-024-00014-y