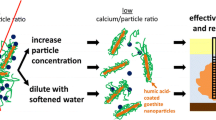
Abstract
Iron oxide nanomaterials play important roles in biogeochemical processes. This study investigates the effects of representative natural carbonaceous materials (humic acid [HA] and extracellular polymeric substances [EPS]) and cations on the heteroaggregation and sedimentation of engineered and natural iron oxide nanomaterials with montmorillonite and sulfate- and amine-modified polystyrene (PS) nanoparticles (NPs) (S- and N-PS NPs, respectively) in water, assessing their environmental behavior and differences in colloidal stability parameters. In addition, a novel extended Derjaguin–Landau–Verwey–Overbeek theory (XDLVO) was developed to describe the mechanism of colloidal behavior that concurrently considers gravitational and magnetic attraction forces. In CaCl2 solution and most natural water samples, negatively charged S-PS NPs promoted heteroaggregation with goethite and iron oxide (Fe3O4) NPs more than positively charged N-PS NPs with increased nanoplastic particle concentration. In seawater, the introduction of S- and N-PS NPs increased the maximum net energy (barrier) (ΦMAX) of heteroaggregation and sedimentation with goethite and Fe3O4 NPs, facilitating dispersal and suspension of the system. The X-ray photoelectron spectroscopy (XPS) and molecular dynamics simulation results suggested that Ca2+ forms bridging interactions between Fe3O4 and S-PS NPs to promote aggregation, while competitive adsorption occurs between the N atoms of N-PS NPs and Ca2+ on the surface of Fe3O4 NPs. The study findings will help to improve the understanding of interfacial processes affecting ions at nanomaterial/water interfaces and assessments of the geochemical behavior and ecological risks of nanoplastics.
Highlights
· XDLVO theory provides a reasonable explanation for the colloidal behavior of particles.
· Cation bridging occurs between S-PS and iron oxide NPs.
· Competitive adsorption occurs between the N atoms of N-PS NPs and Ca2+.
· HA and EPS have different effects on heteroaggregation and sedimentation.
· The introduvction of S- and N-PS NPs facilitates dispersal and suspension of the heteroaggregation system in seawater.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plastic pollution, which widely exists in aqueous environments, is recognized as a global environmental problem that poses a serious threat to the survival of aquatic organisms (Alimi et al. 2018; Jeong et al. 2018; Pinheiro et al. 2023; Wang et al. 2021b). Plastic fragments in the environment can be broken down into microplastics through solar light radiation, mechanical abrasion, wave action, and biodegradation, ultimately forming nanoplastics (Ding et al. 2020; Liu et al. 2019; Zhu et al. 2020). As engineered carbonaceous nanomaterials, incidentally produced nanoplastics exhibit a diversity of compositions and morphologies and a heterogeneity that is typically absent from engineered nanomaterials. This heterogeneity affects their environmental fate and potential impact on biological communities and human health (Gigault et al. 2021; Liebgott et al. 2023). For example, nanoplastics are reportedly toxic to organisms such as bivalves (Corbicula fluminea) (Li et al. 2020b), green algae (Scenedesmus obliqus) (Besseling et al. 2014), zooplankton (Daphnia magna) (Barreto et al. 2023; Besseling et al. 2014), marine rotifers (Brachionus koreanus) (Jeong et al. 2018), zebrafish larvae (Liu et al. 2021b; Wang et al. 2023), bacteria, and fungi (Shruti et al. 2023; Wu et al. 2022b). In fact, the availability and toxicity of nanoplastics to aquatic organisms depend on their stability in the environment (Tiwari et al. 2020). Various stability and aggregation studies of nanoplastics under varying environmental conditions have found that pH (Shams et al. 2020), ionic strength (Chen et al. 2018; Mao et al. 2020), dissolved organic matter (Liu et al. 2020; Singh et al. 2019), and suspended solids play important roles in controlling their fate in aquatic environments.
Notably, low-density nanoplastics (1.03–1.07 g/cm3) (Li et al. 2018) or by provoking destabilization via the promotion of bridging mechanisms (Schaumann et al. 2015). However, the composition and properties of EPS from different sources differ (Yang et al. 2021), and the effects of EPS and HA on the colloidal behavior of nanomaterials have not been well studied (Quigg et al. 2013). Recent research has focused on the influence of EPS from activated sludge in sewage treatment plants and aquatic algae on the stability of nanomaterials such as Ag (Fernando et al. 2020), TiO2 (Lin et al. 2016), CeO2 (Song et al. 2020), nanoplastics (Grassi et al. 2020; Mao et al. 2020; Summers et al. 2018), ZnO (Huang et al. 2023; Xu and Jiang 2015), quantum dots (Zhang et al. 2012), and carbon nanotubes (Adeleye and Keller 2014), but less attention has been paid to the effect of EPS produced by bacteria, especially harmful bacteria, on nanomaterial stability. In actuality, harmful bacteria might have a more direct and important impact on the environment and human health than algae (Graham and Cady 2014). Among such harmful bacteria, Pseudomonas putida plays a key role in the maintenance of environmental quality. It can survive in a variety of polluted environments and participate in activities such as element circulation, pollutant biodegradation, etc. (Timmis 2002). In recent years, some rare clinical opportunistic pathogens have been identified as P. putida, causing urethral infections, skin infections, osteomyelitis, etc. (Meireles et al. 2013; Yang et al. 1996). Therefore, determining the influence of EPS produced by P. putida on nanomaterial stability is of great significance for understanding the behavior of nanomaterials and bacteria in aqueous environments and their combined environmental risks (Dimkpa et al. 2011; Wu et al. 2020).
Compared to artificial nanomaterials, natural nanomaterials are present in large volumes and contain geological material impurities (e.g., clay and SiO2), resulting in quite different colloidal stabilities in aqueous solution (Brar Satinder et al. 2015; Jeevanandam et al. 2018; Malakar et al. 2021). However, differences in the colloidal stability of heteroaggregates of natural and artificial nanomaterials with nanoplastics in aqueous solution have not been considered to date. Herein, fine superparamagnetic artificial magnetite NPs (Fe3O4 NPs) and nonmagnetic bulk natural goethite (containing varying amounts of MgO, SiO2, CaO, and Al2O3) were selected as model materials to probe the differences in their heteroaggregation and sedimentation with polystyrene (PS) NPs in water. Several colloidal stability parameters impacted by sulfate- and amine-modified PS NPs (S- and N-PS NPs, respectively) and representative NOM fractions (HA and EPS) under laboratory conditions were examined to assess their effects on the colloidal behavior of the heteroaggregates. Apart from well-controlled solution chemistry studies, we also compared the effects of positively and negatively charged nanoplastics on their heteroaggregation and sedimentation behaviors in different natural water samples. In addition, the interactions by which these factors affected the heteroaggregation and sedimentation of nanomaterials were quantitatively investigated using a newly developed XDLVO theory that concurrently considered various non-DLVO interactions. Finally, the interaction mechanisms involved in the heteroaggregation of nanoplastics with goethite and Fe3O4 NPs in the presence or absence of Ca2+ were described at the molecular level by MD simulations. The study findings provide insight for understanding the interfacial processes affecting ions at nanomaterial/water interfaces and assessing the geochemical behavior and ecological risks of nanoplastics with engineered and natural nanomaterials.
2 Materials and methods
2.1 Materials and chemicals
Goethite (30%–63% Fe), montmorillonite clay (682,659), S-PS NPs (L1528), and N-PS NPs (L9904) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Leonardite HA (1S104H) was obtained from the International Humic Substances Society (Denver, CO, USA). FeCl2·4H2O, FeCl3·6H2O, CaCl2, HCl, and NaOH were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The stock solutions of goethite (500 mg/L), Fe3O4 NP (20 mg/mL), HA, seawater, lake water, and river water were prepared as previously described (Wu et al. 2022a). The PS NP stock solution was prepared by diluting the PS suspension with deionized water to a final concentration of 1000 mg/L, followed by sonication for 20 min. The montmorillonite stock solution was prepared at a concentration of 1000 mg/L by dissolving the powder in ultrapure water. The Chlorella vulgaris EPS (EPS1) and P. putida strain MnB1 EPS (EPS2) extraction methods are included in Sect. 1.1 of the Supplementary Information.
2.2 Characterization of HA or EPS and heteroaggregates of goethite and Fe3O4 NPs with PS NPs
The heteroaggregates of goethite and Fe3O4 NPs with PS NPs were visualized using transmission electron microscopy (TEM) and photographed. The TEM images were recorded on an H7500 transmission electron micrograph (Hitachi, Tokyo, Japan) operated at 120 kV. The FTIR spectrum of EPS was obtained using a Magna-IR 750 FTIR spectrometer (Nicolet, Madison, WI, USA) with KBr powder as the background, which was recorded from 400 to 4000 cm−1 at a resolution of 4 cm−1 and averaged over 200 scans. The surface compositions of EPS and heteroaggregates of goethite and Fe3O4 NPs with PS NPs were determined by X-ray photoelectron spectroscopy using an ESCALAB 250** (Thermo Scientific, Loughborough, UK). X-ray diffraction (XRD) analysis that determined the crystal structures of heteroaggregates of goethite and Fe3O4 NPs with PS NPs was performed with an X'Pert Alpha 1 (Malvern Panalytical Ltd., Malvern, UK) using Cu K-α1 and λ = 1.5406 Å. Scanning electron microscopy (SEM) using a JSM-7800F (JEOL, Tokyo, Japan) equipped with energy dispersive spectroscopy (EDS) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were used to observe goethite and Fe3O4 NPs with PS NPs aggregates in CaCl2 solution in the presence and absence of HA or EPS. A superconducting quantum interference device (Quantum Design, San Diego, CA, USA) was used to analyze the effect of PS NPs/montmorillonite on the magnetic properties of Fe3O4 NPs.
2.3 Heteroaggregation and sedimentation of goethite and Fe3O4 NPs with PS NPs under various conditions
All batch aggregation and sedimentation experiments were conducted at room temperature (25 °C ± 1 °C) with 20 mg/L goethite and Fe3O4 NP solutions. The final volume of the mixture was 30 mL, and all stock solutions were simultaneously vibrated and sonicated for 10 min prior to use.
Determination of the critical coagulation concentration (CCC) for heteroaggregates (completed within approximately 30 min) was based on the following steps: Aliquots of suspended goethite and Fe3O4 NPs were mixed with different concentrations of PS NPs, HA/EPS, and CaCl2 solutions. Changes in the hydrodynamic diameter (Dh) value of heteroaggregates within 20–30 min were measured by time-resolved dynamic light scattering (TR-DLS) using a Nano-ZS90 Zetasizer (Malvern Panalytical, Malvern, UK). Details of the aggregation studies are included in Sect. 1.2 of the Supplementary Information.
Aggregation and sedimentation of heteroaggregates (after shaking for 24 h) are based on the following steps: Aliquots of suspended goethite and Fe3O4 NPs were added to different concentrations of electrolyte solution (CaCl2) in the presence or absence of varying concentrations of HA, PS NPs, or montmorillonite. Aliquots of suspended goethite and Fe3O4 NPs in the presence of varying concentrations of PS NPs were mixed with different natural water samples. The mixtures were sealed and shaken at 150 rpm for 24 h. Subsequently, aliquots of the mixture were transferred immediately into a cuvette (for Dh and ζ potential detection) and a quartz cell. Sedimentation of heteroaggregates of goethite and Fe3O4 NP with PS NPs or montmorillonite suspensions in the cell was monitored by ultraviolet–visible spectroscopy using an 8453 UV–Vis spectrometer (Agilent Technologies, Santa Clara, CA, USA). Measurements were obtained from the time-resolved optical density (OD = Ce [absorbance at different time intervals]/C0 [initial absorbance]) of heteroaggregates of goethite and Fe3O4 NP suspensions at 441 and 381 nm over 120 min time periods, respectively (Wu et al. 2022a; Wu and Bi 2019).
Settling curves were fitted using the least squares method when quantifying settling parameters with an exponential model (Chappell et al. 2009; Lin et al. 2012; Long et al. 2012; Ma et al. 2015): y = OD Plateau + OD1 exp (Rt), where t is the settling time (min), OD1 is the optical density of the first point > t = 0, R is the settling rate (OD/min), and ODPlateau is the offset value or the value at which the settling curve reached a plateau.
2.4 Extended Derjaguin − Landau − Verwey − Overbeek (XDLVO) theory
In our previous study (Wu et al. 2022a), we proposed integrating osmotic repulsion (OSM), elastic-steric repulsion (ELAS), gravitational energy (VG) (Birdi 2015; Chen et al. 2010; Kim et al. 2005; Prieve and Ruckenstein 1974), and magnetic attraction (VM) interaction energies into the classic DLVO theory to quantitatively analyze various interactions affecting the colloidal behavior of Fe3O4 NPs. However, OSM and ELAS interaction energies, leading to the sorption and/or coating of HA onto NPs, have been proven to be negligible compared to other interaction energies (Wu et al. 2022a). Thus, VG and VM interaction energies were integrated into the classic DLVO theory to quantitatively analyze various interactions that affected the colloidal behavior of heteroaggregates of goethite and Fe3O4 NPs with PS NPs or montmorillonite. The Dh and ζ potential information was used in combination with XDLVO theory to calculate the aggregation and sedimentation interaction energies of NPs (see Sect. 1.3 of the Supplementary Information), and the XDLVO interaction energy profiles were calculated.
The total XDLVO interaction energy (ETOT-XDLVO) for the aggregation of NPs is given by
The total XDLVO interaction energy (ETOT-XDLVO) for the sedimentation of NPs is given by
where VLW, VEDL, VM, and VG are the van der Waals forces, electric double layer (EDL) force, magnetic attraction interaction energy, and gravitational energy, respectively.
2.5 MD simulations
To ascertain the bonding mode of cations on PS NP and goethite/Fe3O4 NP surfaces at the molecular level, MD simulations (see Sect. 1.4 of the Supplementary Information) were incorporated to determine the fundamental difference in the behavior of Ca2+ ions over PS NPs and goethite (111) /Fe3O4 NP (311) planes.
3 Results and discussion
3.1 Heteroaggregation and sedimentation of goethite and Fe3O4 NPs with S- and N-PS NPs
Figs. 3 and Table S15 present the attachment efficiency (α) and CCC (CaCl2) values of natural goethite and artificial Fe3O4 NPs in S- and N-PS NP suspensions. As the S-PS NP concentration increased from 1 to 20 mg/L, the CCC (CaCl2) value of the mixed suspension of goethite and S-PS NPs increased slightly, but that of the mixed suspension of Fe3O4 and S-PS NPs basically remained unchanged. This was mainly because negatively charged S-PS NPs were adsorbed onto the goethite surface (Figs. 3c), reducing its surface potential and thus reducing EDL repulsion. In addition, the adsorption of Fe3O4 NPs on the surface increased the magnetic attraction interaction between particles (Figs. 3g, h). Figs. 3e, f also showed that the maximum aggregation rate of particles significantly increased with increasing S-PS NP concentration. By contrast, as the N-PS NP concentration increased from 1 to 20 mg/L, the CCC (CaCl2) values of goethite and Fe3O4 NPs suspensions mixed with N-PS NPs significantly increased by 17.33 and 7.71 times, respectively. This was mainly due to the positively charged N-PS NPs adsorbed on the goethite surface (Figs. 3c), which increased its surface potential and thus increased EDL repulsion, whereas the adsorption of positively charged N-PS NPs on the Fe3O4 NP surface (Figs. 3d) weakened the magnetic attraction interaction between particles (Figs. 3g, h). Figs. 3e, f also shows that the maximum aggregation rate of particles significantly decreased with increasing N-PS NP concentration. As a result, negatively charged S-PS NPs seemed to promote the aggregation of goethite and Fe3O4 NPs in CaCl2 solution more than positively charged N-PS NPs with increasing nanoplastic particle concentration. Similarly, the CCC values of carboxylated PS NPs and hematite NPs in NaCl and CaCl2 solutions were significantly lower than those of amino-functionalized PS NPs and hematite NPs in NaCl and CaCl2 solutions. This was mainly because Fe3O4 NPs were more likely to heteroaggregate with negatively charged COOH-PS NPs (Yu et al. 2021).
The sedimentation experimental data in deionized water (Fig. 1a, b) revealed that the dispersion stability of goethite and PS suspensions increased with increasing concentrations of S- and N-PS NPs. However, the dispersion stability of Fe3O4 and N-PS NP suspensions only increased with increasing N-PS NP concentration. The sedimentation of Fe3O4 and S-PS NP suspensions gradually increased with increasing S-PS NP concentration, which was supported by the change in Dh value (Fig. 1c). In addition, contrary to the influence of negatively charged S-PS NPs, the ζ potential of goethite and Fe3O4 NPs gradually increased with the addition of positively charged N-PS NPs due to a strong EDL repulsive force generated between particles (Fig. 1c), which was mainly due to the adsorption of N-PS NPs on their surface (Fig. 2).
Settling data showing the optical density (Ce/C0) of 20 mg/L (a) goethite and (b) Fe3O4 NPs dispersions in varying concentrations of HA, S-PS NPs, and N-PS NPs. c Dh and ζ potentials of goethite and Fe3O4 NPs in varying concentrations of HA, S-PS NPs, and N-PS NPs. d The maximum net energy (barrier) (ΦMAX) for aggregation and sedimentation of goethite and Fe3O4 NPs in different concentrations of HA, S-PS NPs, and N-PS NPs. (e) Settling data showing the optical density (Ce/C0) of 20 mg/L goethite and Fe3O4 NP dispersions in varying concentrations of N-PS NPs
XDLVO theory was employed to analyze the aggregation and sedimentation behaviors of goethite and Fe3O4 NPs in deionized water (Fig. 1d and Figs. 4, Tables S5–S7), revealing that (1) aggregation and sedimentation ΦMAX of goethite in S-PS NP suspension, mainly due to EDL repulsion (Fig. 1c, d), rapidly increased with increasing S-PS NP concentration, becoming greater than that of goethite in N-PS NP suspension (Fig. 1d). This finding confirmed that S-PS NPs could promote the dispersion and suspension of their heteroaggregation system with goethite more than the same concentration of N-PS NPs (Fig. 1a), which seemed to be contrary to the above observation that S-PS NPs promoted heteroaggregation with goethite and Fe3O4 NPs more than N-PS NPs in 5 mM CaCl2 solution. This may be due to the fact that the same concentration of Ca2+ might form a stronger bridging effect between goethite and S-PS NPs than between goethite and N-PS NPs. (2) S-PS NPs promoted the suspension and dispersion of their heteroaggregation system with Fe3O4 NPs more than N-PS NPs only in low concentrations of S- and N-PS NP solutions (0–1 mg/L) (Fig. 1b, c), although the opposite was true in high concentrations of N- and S-PS NP solutions (5–20 mg/L). This was because Fe3O4 NPs had weak magnetic attraction interactions (Figs. 4c) and larger aggregation and sedimentation ΦMAX in high concentrations of N-PS NP solution compared with those in the same concentration of S-PS NP solution (Fig. 1d), thus exhibiting smaller Dh values (< 1100 nm) (Fig. 1c) and gravity interaction (Figs. 4d). Similarly, when compared with the same concentrations of montmorillonite (see Sect. 1.5 of the Supplementary Information), S-PS NPs and N-PS NPs (Fig. 1a, b), HA could effectively improve the dispersion and suspension of goethite (because the adsorption of HA on the particle surface provided strong EDL repulsion) and Fe3O4 NPs (because of their weak magnetic attraction in HA solution) (Figs. 4c, d). Additionally, N-PS NPs could promote the dispersion of their heteroaggregation system with goethite and Fe3O4 NPs more than the same concentration of montmorillonite (Fig. 1c). (3) heteroaggregation and sedimentation of N-PS and Fe3O4 NPs were facilitated in a 1 mg/L N-PS NP solution compared to N-PS NPs and goethite (Fig. 1c and Figs. 4e) because magnetic attraction promoted the aggregation of Fe3O4 NPs, increasing the gravity effect (Figs. 4c, d). However, with increasing N-PS NP concentration (5–20 mg/L), the ΦMAX gap between goethite and Fe3O4 NPs heteroaggregation and sedimentation systems with N-PS NPs (Fig. 1d) continued to decrease because stronger EDL repulsion (Figs. 4c, d) led to smaller Dh values (Fig. 1c) and gravity interaction. Thus, the heteroaggregation system of Fe3O4 and N-PS NPs had poorer settleability compared with that of goethite and N-PS NPs. Furthermore, the sedimentation experimental data in deionized water (Fig. 1a, b) demonstrated that the dispersion stability of goethite suspension increased with increasing concentration of S- and N-PS NP solutions. However, the dispersion stability of Fe3O4 and N-PS NP suspensions only increased with increasing N-PS NP concentration. With increasing S-PS NP concentration, the sedimentation of their heteroaggregation system with Fe3O4 NPs gradually increased, supported by the change in Dh value (Fig. 1c). In addition, unlike the influence of negatively charged HA, montmorillonite, and S-PS NPs, the addition of positively charged N-PS NPs gradually increased the ζ potential of goethite and Fe3O4 NPs in the N-PS NPs heteroaggregation system because a strong EDL repulsive force was generated between particles (Fig. 1c), which was mainly due to the adsorption of N-PS NPs on their surface (Fig. 2).
3.2 Heteroaggregation and sedimentation of goethite and Fe3O4 NPs with S- and N-PS NPs in the presence of HA or EPS
Figure 3 and Table S16 present the attachment efficiency (α) and CCC (CaCl2) values of heteroaggregates of natural goethite and artificial Fe3O4 NPs with S- and N-PS NPs in HA and EPS suspensions. In the presence of 5 mg/L HA and EPS2, the CCC (CaCl2) values of the heteroaggregation system of goethite in 1 and 20 mg/L S-PS NP solutions were approximately 4–7.5 and 1.67–1.25 times lower, respectively, than those in their absence. This was mainly due to negatively charged HA or EPS adsorbing onto the goethite and S-PS NP heteroaggregate surfaces and reducing their surface potential (Figs. 5a, b), thereby reducing EDL repulsion (Figs. 6d). However, the CCC (CaCl2) values of goethite and S-PS NP solutions in the presence of 5 mg/L EPS1 remained unchanged in 1 mg/L S-PS NP solution or were slightly increased by 2.63 times in 20 mg/L S-PS NP solution compared to that in its absence. Figs. 5a, b also demonstrates that the maximum aggregation rate of the heteroaggregation system of goethite and S-PS NPs in 5 mg/L HA solution was greater than that in 5 mg/L EPS solution, indicating that Ca2+ might be more likely to form Ca2+ bridging interactions in the presence of HA than that in the presence of EPS. Meanwhile, in the presence of 5 mg/L HA and EPS, the CCC (CaCl2) values of the Fe3O4 and S-PS NPs heteroaggregation systems in 1 and 20 mg/L S-PS NP solutions were approximately 1.11–7.50 times higher than those in their absence. Figs. 5c, d also shows that the adsorption of negatively charged HA or EPS onto the surface of Fe3O4 NPs reduced the surface potential, and the maximum aggregation rate of particles was in the order of EPS2 > HA > EPS1. This might imply that Ca2+ was more likely to form Ca2+ bridging interactions in the presence of HA or EPS2 than that in the presence of EPS1 in the heteroaggregation system of Fe3O4 and S-PS NPs. The XDLVO theoretical analysis indicated (Figs. 6c and Table S8) that the addition of HA and EPS reduced the ΦMAX of the heteroaggregation of goethite and S-PS NPs in 1 and 20 mg/L S-PS NP solutions mainly due to the reduction in EDL repulsion (Figs. 6d), but it increased the ΦMAX of the heteroaggregation of Fe3O4 and S-PS NPs in the solutions mainly due to the reduction in magnetic attraction (Figs. 6d). This might explain the phenomenon that the CCC (CaCl2) values of goethite heteroaggregated with S-PS NPs tended to decrease while those of Fe3O4 NPs heteroaggregated with S-PS NPs tended to increase after the addition of HA and EPS. In addition, the ΦMAX of the heteroaggregation of goethite and Fe3O4 NPs with 20 mg/L S-PS NPs in deionized water (without CaCl2) was significantly higher than that in 5 mM CaCl2 solution (Figs. 6c), mainly due to the obvious reduction in EDL repulsion between particles due to the addition of CaCl2 (Figs. 6d).
Settling data showing the optical density (Ce/C0) of (a) 20 mg/L goethite and (b) 20 mg/L Fe3O4 NPs dispersed in S-PS NP suspensions in the presence or absence of HA, EPS1, and EPS2. c Dh and ζ potentials of goethite and Fe3O4 NPs dispersed in 20 mg/L S-PS NP suspensions in the presence or absence of 5 mg/L HA, EPS1, and EPS2. d The ΦMAX for the heteroaggregation of goethite and Fe3O4 NPs together with 20 mg/L S-PS NP suspensions in the presence or absence of 5 mg/L HA, EPS1, and EPS2
The sedimentation experimental data in deionized water (Fig. 3a, b) demonstrated that the dispersion stability of the goethite and 20 mg/L S-PS NPs heteroaggregation system was gradually enhanced in the order of EPS2 < EPS1 < HA. However, the dispersion stability of the Fe3O4 NPs and 20 mg/L S-PS NPs heteroaggregation system gradually increased in the order of EPS2 < HA < EPS1, which was supported by the change in Dh value (Fig. 3c). Additionally, the adsorption of negatively charged HA and EPS onto the particle surface substantially reduced the ζ potential. XDLVO theory was used to analyze the aggregation and sedimentation behaviors of the goethite and Fe3O4 NPs with S-PS NPs heteroaggregation systems in deionized water. The analysis revealed (Fig. 3d, Figs. 7 and Table S9–S10) that (1) aggregation and sedimentation ΦMAX of the goethite and Fe3O4 NPs with S-PS NPs heteroaggregation systems, which were dominated by EDL repulsion (Figs. 7c, d), increased in the order of EPS2 < EPS1 < HA and EPS2 < HA < EPS1, respectively, in deionized water (Fig. 3d), which explained their aggregation and sedimentation behaviors (Fig. 3a–c). (2) The addition of HA and EPS significantly improved the heteroaggregation and sedimentation ΦMAX of the Fe3O4 NPs with S-PS NPs heteroaggregation system compared with that without their addition (Fig. 3d). This was mainly due to increased EDL repulsion and decreased magnetic attraction (Figs. 7c, d), which was consistent with the results observed in the presence of 5 mM CaCl2. However, the addition of EPS2 reduced the aggregation and sedimentation ΦMAX of the goethite and S-PS NPs heteroaggregation system (Tables S9–S10 and Fig. 3d), mainly due to the reduction in EDL repulsion, which was in opposition to the results observed with the addition of HA and EPS1.
3.3 Heteroaggregation and sedimentation of goethite and Fe3O4 NPs with S- and N-PS NPs in natural water samples
The sedimentation curves (Figs. 8), ζ potential, and Dh (Fig. 4a) of 1 and 20 mg/L S- and N-PS NPs in seawater, river water, and lake water are presented in Sect. 1.6 of the Supplementary Information.
a Dh and ζ potentials of goethite and Fe3O4 NPs together with N- or S-PS NPs in seawater, river water, and lake water. b The ΦMAX for the sedimentation and heteroaggregation of goethite and Fe3O4 NPs together with N- or S-PS NPs in seawater, river water, and lake water. c The ΦMAX for the sedimentation and heteroaggregation of N- or S-PS NPs in seawater, river water, and lake water
The sedimentation curves (Figs. 8) and Dh values (Fig. 4) of goethite and Fe3O4 NPs heteroaggregated with 1 and 20 mg/L PS NPs in seawater, river water, and lake water indicated that, in most cases, goethite and Fe3O4 NPs in the same concentration as the S-PS NPs heteroaggregation system seemed to suspend more easily than in the N-PS NPs heteroaggregation system, and the ζ potential of the goethite and Fe3O4 NPs with S-PS NPs heteroaggregation system in natural water samples was more negative than that of the goethite and Fe3O4 NPs with N-PS NPs heteroaggregation system (Fig. 4a). This was mainly due to the fact that goethite and Fe3O4 NPs are usually negatively charged due to the adsorption of organic matter in natural water bodies. The heteroaggregation and sedimentation ΦMAX of negatively charged S-PS NPs heteroaggregated with goethite and Fe3O4 NPs in natural water samples were mostly higher than those of the same concentration of positively charged N-PS NPs heteroaggregated with goethite and Fe3O4 NPs (Fig. 4b), which were more conducive to particle dispersion and suspension. This was because the dispersion and suspension of goethite are mainly caused by increased EDL repulsion, while those of Fe3O4 NPs are mainly caused by decreased magnetic attraction and gravity interaction (Figs. 9g, h). In seawater, the heteroaggregation and sedimentation ΦMAX of goethite and Fe3O4 NPs increased with increasing concentrations of N- and S-PS NPs (Fig. 4b), thereby facilitating their dispersal (Fig. 4a) and suspension (Figs. 8a, b and Tables S13–S14). Notably, the EDL repulsion of heteroaggregated goethite and Fe3O4 NPs with PS NPs disappeared in seawater (Table S13), and the aggregation ΦMAX between particles was dominated by the van der Waals effect. In river water, the heteroaggregation ΦMAX of Fe3O4 NPs changed slightly with increasing concentrations of S- and N-PS NPs (Fig. 4b), mainly due to the small change in EDL repulsion (Figs. 9g), resulting in a non-obvious change in Dh value (Fig. 4a). However, the sedimentation ΦMAX exhibited significant increases of 355.42–4023.56 KT, mainly due to the decrease in gravity interaction (Figs. 9h), leading to the Fe3O4 NPs heteroaggregation system being easily suspended with increasing concentrations of S- and N-PS NPs (Figs. 8d). With increasing S-PS NP concentration, the heteroaggregation and sedimentation ΦMAX of goethite (Figs. 9g and Table S13) slightly increased by 16.72–185.67 KT. However, ΦMAX decreased by 393.19–1476.30 KT with increasing N-PS NP concentration mainly due to the reduction in EDL repulsion (Figs. 9g) and increase in gravity interaction (Figs. 9h and 4b). This better explains the phenomenon that goethite and S-PS NP heteroaggregates were more easily dispersed (Fig. 4a) and suspended (Figs. 8c) when the S-PS NP concentration increased, whereas goethite and N-PS NP heteroaggregates more easily aggregated and settled when the N-PS NP concentration increased. In lake water, the heteroaggregation and sedimentation ΦMAX of goethite increased by 19.58 and 52.05 KT with increasing S-PS NP concentration, respectively, whereas the heteroaggregation and sedimentation ΦMAX of Fe3O4 NPs decreased by 1314.33 and 1678.53 KT, respectively. Therefore, the goethite and S-PS NPs heteroaggregation system tended to suspend (Figs. 8e), while the Fe3O4 NPs and S-PS NPs heteroaggregation systems tended to aggregate (Fig. 4a). However, with increasing N-PS NP concentration, the opposite trend was found in their aggregation and sedimentation ΦMAX, dispersion, and suspension characteristics with goethite and Fe3O4 NPs (Figs. 10).
The 3D fluorescence spectrum data (Figs. 13) revealed that the organic matter in seawater was mainly composed of HA-like and fulvic acid-like substances, while river water contained microbial metabolites and aromatic proteins in addition to HA-like and fulvic acid-like substances. Lake water was dominated by soluble microbial product-like substances. Figure 4a and S8g demonstrate that the Dh value of goethite and S-PS NP heteroaggregates in lake water was less than that in river water and seawater, facilitating their suspension in lake water compared to that in river water and seawater. On the one hand, the heteroaggregation and sedimentation ΦMAX of goethite and S-PS NPs in lake water were greater than those in river water and seawater (Fig. 4b). On the other hand, the maximum aggregation rate of goethite and S-PS NPs in river water and seawater containing HA (which might more easily form Ca2+ bridging) was greater than that in lake water rich in EPS (Figs. 5a, b). However, the Dh value of Fe3O4 and S-PS NP heteroaggregates in lake water was greater than that in river water and seawater. Additionally, the ΦMAX of the Fe3O4 and S-PS NP heteroaggregates in lake water was smaller than that in river water and seawater (Fig. 4b). However, there might be more bacterial EPS in lake water than in river water or seawater, which may be more likely to form Ca2+ bridging; thus, the maximum aggregation rate of Fe3O4 and S-PS NPs in lake water was greater than that in river water and seawater (Figs. 5c, d). Interestingly, the sedimentation ΦMAX of Fe3O4 and S-PS NPs in seawater was considerably smaller than that in river water and lake water (Fig. 4b), which might explain the phenomenon that Fe3O4 and S-PS NP heteroaggregates settled more easily in seawater (Figs. 8h) than in river and lake water. The Dh values of Fe3O4 NP heteroaggregates with 1 and 20 mg/L N-PS NPs in lake water were greater than those in seawater and river water (Fig. 4b); thus, they were more prone to sedimentation in lake water (Figs. 8j) than in seawater and river water. This was mainly because their heteroaggregation and sedimentation ΦMAX in lake water were considerably smaller than those in seawater and river water due to greater magnetic attraction (Figs. 9g) and gravity interaction (Figs. 9h) in lake water, respectively. However, the Dh value of goethite and 1 mg/L N-PS NPs heteroaggregates in seawater was larger than that in lake and river water (Fig. 4b); thus, they were more prone to sedimentation in seawater than in lake and river water (Figs. 8j). This was mainly because the heteroaggregation and sedimentation ΦMAX of goethite and 1 mg/L N-PS NP in seawater were considerably smaller than those in lake and river water due to greater EDL repulsion during heteroaggregation in river and lake water (Figs. 9g) and gravity interaction being largely offset by EDL repulsion during settlement (Figs. 9h). The Dh value of goethite and 20 mg/L N-PS NP heteroaggregates in lake water was greater than that in seawater and river water (Fig. 4b); thus, they settled more easily in lake water than in seawater and river water (Figs. 8i). This was mainly because the heteroaggregation and sedimentation ΦMAX of goethite and 20 mg/L N-PS NPs in lake water were considerably smaller than those in seawater and river water due to the smaller EDL repulsion during heteroaggregation (Figs. 9g) and larger gravity interaction during sedimentation in lake water (Figs. 9h). In addition, another study indicated that although no heteroaggregation of negatively charged CeO2 and Fe2O3 NPs was observed in ultrapure water due to electrostatic repulsion, specific adsorption of divalent cations caused cation bridging and electrostatic shielding in lake water, thereby promoting the heteroaggregation of CeO2 and Fe2O3 NPs (Oriekhova and Stoll 2019). The heteroaggregation rate of 5 mg/L α-Fe2O3 and amidine-modified PS plastic NPs in Rhône river water depended on the nanoplastics concentration and charge neutralization process. As the plastic concentration increased, the aggregation rate and heteroaggregate Dh value rapidly increased (0–5 mg/L) and then rapidly decreased (5–40 mg/L) (Oriekhova and Stoll 2018). The research findings herein were in good agreement with previously reported results.
3.4 XRD, FTIR, SEM, and XPS analyses and MD simulations
3.4.1 Bonding modes for the adsorption of S- and N-PS NPs on goethite and Fe3O4 NPs in the absence of Ca2+
Descriptions of the XRD, FTIR, SEM, and XPS analyses are included in Sect. 1.7 of the Supplementary Information. The MD simulation results indicated that Fe3O4 NPs could bind to the grooves formed by –SO4 in S-PS NPs and –NH3 in N-PS NPs (Fig. 5a, b). Specifically, the Fe atoms of Fe3O4 NPs could form polar interactions with the O atoms of the S–O (3.2–3.6 Å) and S = O (3.3–3.5 Å) bonds in S-PS NPs, while the O atoms of Fe3O4 NPs could form polar interactions with the O atoms of the S–O bond in S-PS NPs (3.4 Å) (Fig. 5a). Similarly, the O atoms of Fe3O4 NPs could form polar interactions with the N atoms of N-PS NPs (2.7–3.5 Å), and the Fe atoms of Fe3O4 NPs could form polar interactions with the O atoms of the C = O bond in N-PS NPs (2.9–3.4 Å) (Fig. 5b). Moreover, the –SO4 in S-PS NPs and –NH3 in N-PS NPs could also bind to the surface of goethite (Fig. 5c, d). In particular, the O atoms of the S–O and S = O bonds in S-PS NPs could form polar interactions with the Fe atoms (2.8–3.6 Å) and H atoms (2.6–2.9 Å) of goethite (Fig. 5c). Additionally, the N atoms of –NH3 in N-PS NPs could form polar interactions with the O atoms of goethite (2.7–3.4 Å), while the O atoms of the C = O bond in N-PS NPs could form polar interactions with the Fe atoms of goethite (3.4 Å) (Fig. 5d). Table 1 shows that the binding energy of Fe3O4 NPs bound to S-PS NPs was greater than that of Fe3O4 NPs bound to N-PS NPs, indicating that SPS NPs were more likely to form heteroaggregates with Fe3O4 NPs. This finding supported the observation in Sect. 3.1 that S-PS NPs promoted the aggregation and sedimentation of their heteroaggregation system with Fe3O4 NPs more than N-PS NPs in high concentrations of N- and S-PS NP solutions (5–20 mg/L) (Fig. 1b, c). Conversely, the binding energy of S-PS NPs bound to goethite was less than that of N-PS NPs bound to goethite, explaining why S-PS NPs in deionized water promoted the dispersion and suspension of their heteroaggregation system with goethite more than N-PS NPs.
3.4.2 Bonding modes for the adsorption of S- and N-PS NPs on goethite and Fe3O4 NPs in the presence of Ca2+
The MD simulation results demonstrated that the presence of Ca2+ had different effects on the aggregation of S- and N-PS NPs with Fe3O4 NPs and goethite. First, Ca2+ formed polar interactions with the O atoms of the S–O and S = O bonds in S-PS NPs, and it also formed a polar bridging effect (1.7–3.9 Å) between the O atoms of the S–O bonds in S-PS and Fe3O4 NPs, thus promoting their heteroaggregation (Fig. 6a). However, this Ca2+ bridging effect did not occur when Fe3O4 NPs were adsorbed on N-PS NPs, as both the N atoms of the NH3 groups in N-PS NPs and Ca2+ could form polar interactions with the O atoms on the surface of Fe3O4 NPs, resulting in a competitive adsorption binding phenomenon (Fig. 6b). Second, Ca2+ formed a polar bridging effect between the O atoms of the S–O and S = O bonds in S-PS NPs and the O atoms of goethite (1.7–3.9 Å), promoting their heteroaggregation (Fig. 6c). However, this Ca2+ bridging effect did not occur when N-PS NPs were adsorbed on goethite, as both the N atoms of the NH3 groups in N-PS NPs and Ca2+ could form polar interactions with the O atoms on the surface of goethite, leading to a competitive adsorption binding phenomenon (Fig. 6d). Furthermore, the binding energies of the two types of PS with goethite and Fe3O4 NPs in the presence of Ca2+ (Table 1) revealed that the binding energy of Fe3O4 NPs bound to S-PS NPs was greater than that of Fe3O4 NPs bound to N-PS NPs. This indicated that Fe3O4 NPs were more likely to bind to S-PS NPs than the same expansion unit of N-PS NPs, leading to the formation of heteroaggregates. This finding might explain why the CCC value of the heteroaggregation system formed by Fe3O4 and S-PS NPs (Table S15) was significantly lower than that of the heteroaggregation system formed by Fe3O4 NPs and the same concentration of N-PS NPs. The results indicated that the binding energy of S-PS NPs bound to goethite was smaller than that of N-PS NPs bound to goethite, suggesting that N-PS NPs were more easily adsorbed than S-PS NPs on goethite to form heteroaggregates. This finding was consistent with the CCC values reported in Table S15. Furthermore, Table 1 shows that the total binding energies of S-PS NPs bound to natural goethite and artificial Fe3O4 NPs in the absence of Ca2+ were significantly less than those in its presence. This observation was likely due to the Ca2+ bridging effect between Fe3O4 and S-PS NPs. Similarly, the total binding energy of N-PS NPs and natural goethite in the absence of Ca2+ was significantly less than that in its presence. Meanwhile, the total binding energy of N-PS and Fe3O4 NPs in the absence of Ca2+ was greater than that in its presence. This result was attributed to the competitive adsorption between Ca2+ and N-PS NPs on the surface of natural goethite and Fe3O4 NPs in the presence of Ca2+. Specifically, the reduction in the amount of attraction interaction energy, which includes van der Waals forces, hydrogen bonding, and desolvation, induced Fe3O4 and N-PS NP aggregation. This reduction was significantly smaller than that involved when natural goethite and N-PS NP aggregation was induced.
4 Conclusions
This study demonstrated that the heteroaggregation and sedimentation of natural iron oxide nanomaterials with PS differ in aqueous environments and are considerably affected by ion, HA, and EPS concentrations. The main mechanism of stabilization or destabilization arising from these factors was suitably explained by the developed XDLVO theory, in which multiple non-DLVO interaction forces were introduced, including gravitational and magnetic attraction forces. Observations derived from this study indicated that (1) heteroaggregates formed by natural goethite and S-PS NPs were more easily dispersed and suspended than those formed by goethite and clay particles, thus prolonging their suspension residence time in water. However, heteroaggregates formed by Fe3O4 and S-PS NPs more easily aggregated and settled than those formed by Fe3O4 NPs and nanoclay particles, which was conducive to the entry of NPs into sediment. (2) As the S-PS NP concentration increased, the CCC (CaCl2) value of the mixed suspension of goethite and S-PS NPs slightly increased, but the CCC (CaCl2) value of the mixed suspension of Fe3O4 and S-PS NPs basically remained unchanged. However, as the N-PS NP concentration increased, the CCC (CaCl2) value of the goethite and Fe3O4 NP suspension mixed with N-PS NPs significantly increased. This was mainly due to PS NPs adsorbing on the goethite and Fe3O4 NP surfaces, resulting in changes in EDL repulsion and magnetic attraction interaction. (3) In CaCl2 solution and most natural water samples, goethite and Fe3O4 NPs in the same concentration as the S-PS NPs heteroaggregation system seemed to suspend more easily than those in the N-PS NPs heteroaggregation system because negatively charged S-PS NPs were more easily adsorbed on the surface of Fe3O4 NPs than positively charged N-PS NPs. In seawater, the heteroaggregation and sedimentation ΦMAX with goethite and Fe3O4 NPs increased with increasing concentrations of positively charged PS NPs, facilitating their dispersal and suspension. This was not exactly the same behavior as in lake and river water, which might be due to different aggregation rates resulting from different organic matter compositions or changes in heteroaggregation and sedimentation ΦMAX between particles with increasing PS NP concentration in different natural water samples. The XPS, SEM–EDS, and MD simulation results suggested that Ca2+ could form bridges between Fe3O4 and S-PS NPs to promote aggregation, while competitive adsorption occurred between the N atoms of N-PS NPs and Ca2+ on the surface of Fe3O4 NPs. These results will be helpful in understanding the effect of natural carbonaceous materials on the heteroaggregation and sedimentation behaviors of engineered and natural iron oxide nanomaterials with nanoplastic particles and their environmental fate and ecological risks in aquatic environments. In addition, the DLVO model should be improved to concurrently introduce more non-DLVO interactions, including hydration forces, Lewis acid–base interactions, and hydrophobic and hydrogen bonding interactions.
5 Availabilityof data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeleye AS, Keller AA (2014) Long-term colloidal stability and metal leaching of single wall carbon nanotubes: Effect of temperature and extracellular polymeric substances. Water Res 49:236–250
Adeleye AS, Pokhrel S, Mädler L, Keller AA (2018) Influence of nanoparticle do** on the colloidal stability and toxicity of copper oxide nanoparticles in synthetic and natural waters. Water Res 132:12–22
Ali I, Tan X, Li J, Peng C, Naz I, Duan Z, Ruan Y (2022) Interaction of microplastics and nanoplastics with natural organic matter (NOM) and the impact of NOM on the sorption behavior of anthropogenic contaminants – A critical review. J Clean Prod 376:134314
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ Sci Technol 52(4):1704–1724
Argun BR, Statt A (2023) Influence of shape on heteroaggregation of model microplastics: a simulation study. Soft Matter 19(42):8081–8090
Barreto A, Silva ARR, Capitão A, Sousa ÉML, Calisto V, Maria VL (2023) Nanoplastics increase the toxicity of a pharmaceutical, at environmentally relevant concentrations – A mixture design with Daphnia magna. Environ Toxicol Pharmacol 103:104258
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environmental Science & Technology 48(20):12336–12343
Birdi KS (2015) Handbook of Surface and Colloid Chemistry, 4th edn. CRC Press, Boca Raton
Brar Satinder K, Zhang Tian C, Verma M, Surampalli Rao Y, Tyagi Rajeshwar D (2015) Nanomaterials in the Environment. American Society of Civil Engineers, Reston, Virginia
Cerbelaud M, Bennani Y, Peyratout C (2022) Heteroaggregation between particles modified by polyelectrolyte multilayers. Colloids Surf, A 650:129572
Chappell MA, George AJ, Dontsova KM, Porter BE, Price CL, Zhou P, Morikawa E, Kennedy AJ, Steevens JA (2009) Surfactive stabilization of multi-walled carbon nanotube dispersions with dissolved humic substances. Environ Pollut 157(4):1081–1087
Chen G, Hong Y, Walker SL (2010) Colloidal and Bacterial Deposition: Role of Gravity. Langmuir 26(1):314–319
Chen K, ** R, Luo C, Song G, Hu Y, Cheng H (2018) Synthesis of polydopamine-functionalized magnetic graphene and carbon nanotubes hybrid nanocomposites as an adsorbent for the fast determination of 16 priority polycyclic aromatic hydrocarbons in aqueous samples. J Sep Sci. 41(8):1847–1855
Chen Y, Tang H, Cheng Y, Huang T, **ng B (2023) Interaction between microplastics and humic acid and its effect on their properties as revealed by molecular dynamics simulations. J Hazard Mater 455:131636
Cheng S, Ye Z, Wang X, Lian C, Shang Y, Liu H (2023) The effects of adsorbed benzo(a)pyrene on dynamic behavior of polystyrene nanoplastics through phospholipid membrane: A molecular simulation study. Colloids Surf, B 224:113211
Chrysikopoulos CV, Sotirelis NP, Kallithrakas-Kontos NG (2017) Cotransport of Graphene Oxide Nanoparticles and Kaolinite Colloids in Porous Media. Transp Porous Media 119(1):181–204
Chrysikopoulos CV, Syngouna VI (2012) Attachment of bacteriophages MS2 and ΦX174 onto kaolinite and montmorillonite: Extended-DLVO interactions. Colloids Surf, B 92:74–83
Delattre C, Pierre G, Laroche C, Michaud P (2016) Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol Adv 34(7):1159–1179
Dimkpa CO, Calder A, Britt DW, McLean JE, Anderson AJ (2011) Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions. Environ Pollut 159(7):1749–1756
Ding L, Mao R, Ma S, Guo X, Zhu L (2020) High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res 174:115634
Enyoh CE, Ovuoraye PE, Qingyue W, Wang W (2023) Examining the impact of nanoplastics and PFAS exposure on immune functions through inhibition of secretory immunoglobin A in human breast milk. J Hazard Mater 459:132103
Feng H, Liu Y, Xu Y, Li S, Liu X, Dai Y, Zhao J, Yue T (2022) Benzo[a]pyrene and heavy metal ion adsorption on nanoplastics regulated by humic acid: Cooperation/competition mechanisms revealed by molecular dynamics simulations. J Hazard Mater 424:127431
Fernando I, Lu D, Zhou Y (2020) Interactive influence of extracellular polymeric substances (EPS) and electrolytes on the colloidal stability of silver nanoparticles. Environ Sci Nano 7(1):186–197
Gigault J, El Hadri H, Nguyen B, Grassl B, Rowenczyk L, Tufenkji N, Feng S, Wiesner M (2021) Nanoplastics are neither microplastics nor engineered nanoparticles. Nat Nanotechnol 16(5):501–507
Graham MV, Cady NC (2014) Nano and Microscale Topographies for the Prevention of Bacterial Surface Fouling. Coatings 4(1):37–59
Grassi G, Gabellieri E, Cioni P, Paccagnini E, Faleri C, Lupetti P, Corsi I, Morelli E (2020) Interplay between extracellular polymeric substances (EPS) from a marine diatom and model nanoplastic through eco-corona formation. Sci Total Environ 725:138457
Gutierrez T, Teske A, Ziervogel K, Passow U, Quigg A (2018) Editorial: Microbial Exopolymers: Sources, Chemico-Physiological Properties, and Ecosystem Effects in the Marine Environment. Front Microbiol 9:1822
Hollóczki O (2021) Evidence for protein misfolding in the presence of nanoplastics. Int J Quantum Chem 121(3):e26372
Hollóczki O, Gehrke S (2020) Can Nanoplastics Alter Cell Membranes? ChemPhysChem 21(1):9–12
Huang R, Han Z, Ma C, Liu H, Huangfu X (2023) Stability and mobility of zinc oxide nanoparticles in aquatic environment: Influence of extracellular polymeric substances from cyanobacteria and microalgae. J Environ Chem Eng 11(1):109069
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074
Jeong CB, Kang HM, Lee YH, Kim MS, Lee JS, Seo JS, Wang M, Lee JS (2018) Nanoplastic Ingestion Enhances Toxicity of Persistent Organic Pollutants (POPs) in the Monogonont Rotifer Brachionus koreanus via Multixenobiotic Resistance (MXR) Disruption. Environ Sci Technol 52(19):11411–11418
Kim BJ, Chiu JJ, Yi GR, Pine DJ, Kramer EJ (2005) Nanoparticle-Induced Phase Transitions in Diblock-Copolymer Films. Adv Mater 17(21):2618–2622
Li C, Ma Y, Liu X, Huang R, Su R, Qi W, Che J, He Z (2021a) Synergistic effect of polystyrene nanoplastics and contaminants on the promotion of insulin fibrillation. Ecotoxicol Environ Saf 214:112115
Li M, He L, Zhang M, Liu X, Tong M, Kim H (2019a) Cotransport and Deposition of Iron Oxides with Different-Sized Plastic Particles in Saturated Quartz Sand. Environ Sci Technol 53(7):3547–3557
Li X, He E, **a B, Liu Y, Zhang P, Cao X, Zhao L, Xu X, Qiu H (2021b) Protein corona-induced aggregation of differently sized nanoplastics: impacts of protein type and concentration. Environ Sci Nano 8(6):1560–1570
Li X, He E, **a B, Van Gestel CAM, Peijnenburg WJGM, Cao X, Qiu H (2020a) Impact of CeO2 nanoparticles on the aggregation kinetics and stability of polystyrene nanoplastics: Importance of surface functionalization and solution chemistry. Water Res 186:116324
Li Y, Wang X, Fu W, **a X, Liu C, Min J, Zhang W, Crittenden JC (2019b) Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling. Water Res 161:486–495
Li Z, Feng C, Wu Y, Guo X (2020b) Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. J Hazard Mater 392:122418
Liebgott C, Chaib I, Doyen P, Robert H, Eutamene H, Duflos G, Reynaud S, Grassl B, Mercier-Bonin M (2023) Fate and impact of nanoplastics in the human digestive environment after oral exposure: A common challenge for toxicology and chemistry. TrAC, Trends Anal Chem 166:117175
Lin D, Drew Story S, Walker SL, Huang Q, Cai P (2016) Influence of extracellular polymeric substances on the aggregation kinetics of TiO2 nanoparticles. Water Res 104:381–388
Lin D, Ji J, Long Z, Yang K, Wu F (2012) The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res 46(14):4477–4487
Liu J, Zhang T, Tian L, Liu X, Qi Z, Ma Y, Ji R, Chen W (2019) Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ Sci Technol 53(10):5805–5815
Liu S, **e L, Liu G, Zhong H, Zeng H (2021a) Understanding the hetero-aggregation mechanism among sulfide and oxide mineral particles driven by bifunctional surfactants: Intensification flotation of oxide minerals. Miner Eng 169:106928
Liu X, Li J, Huang Y, Wang X, Zhang X, Wang X (2017) Adsorption, Aggregation, and Deposition Behaviors of Carbon Dots on Minerals. Environ Sci Technol 51(11):6156–6164
Liu Y, Huang Z, Zhou J, Tang J, Yang C, Chen C, Huang W, Dang Z (2020) Influence of environmental and biological macromolecules on aggregation kinetics of nanoplastics in aquatic systems. Water Res 186:116316
Liu Y, Wang Y, Ling X, Yan Z, Wu D, Liu J, Lu G (2021b) Effects of Nanoplastics and Butyl Methoxydibenzoylmethane on Early Zebrafish Embryos Identified by Single-Cell RNA Sequencing. Environ Sci Technol 55(3):1885–1896
Long Z, Ji J, Yang K, Lin D, Wu F (2012) Systematic and Quantitative Investigation of the Mechanism of Carbon Nanotubes’ Toxicity toward Algae. Environ Sci Technol 46(15):8458–8466
Ma S, Zhou K, Yang K, Lin D (2015) Heteroagglomeration of Oxide Nanoparticles with Algal Cells: Effects of Particle Type, Ionic Strength and pH. Environ Sci Technol 49(2):932–939
Malakar A, Kanel SR, Ray C, Snow DD, Nadagouda MN (2021) Nanomaterials in the environment, human exposure pathway, and health effects: A review. Sci Total Environ 759:143470
Mao Y, Li H, Huangfu X, Liu Y, He Q (2020) Nanoplastics display strong stability in aqueous environments: Insights from aggregation behaviour and theoretical calculations. Environ Pollut 258:113760
Meireles C, Costa G, Guinote I, Albuquerque T, Botelho A, Cordeiro C, Freire P (2013) Pseudomonas putida are environmental reservoirs of antimicrobial resistance to β-lactamic antibiotics. World J Microbiol Biotechnol 29(7):1317–1325
Nie X, **ng X, **e R, Wang J, Yang S, Wan Q, Zeng EY (2023) Impact of iron/aluminum (hydr)oxide and clay minerals on heteroaggregation and transport of nanoplastics in aquatic environment. J Hazard Mater 446:130649
Oliveira YM, Vernin NS, Maia Bila D, Marques M, Tavares FW (2022) Pollution caused by nanoplastics: adverse effects and mechanisms of interaction via molecular simulation. PeerJ 10:e13618
Oriekhova O, Stoll S (2018) Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ Sci Nano 5(3):792–799
Oriekhova O, Stoll S (2019) Heteroaggregation of CeO2 nanoparticles in presence of alginate and iron (III) oxide. Sci Total Environ 648:1171–1178
Phenrat T, Saleh N, Sirk K, Tilton R, Lowry G (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41(1):284–290
Pinheiro HT, MacDonald C, Santos RG, Ali R, Bobat A, Cresswell BJ, Francini-Filho R, Freitas R, Galbraith GF, Musembi P, Phelps TA, Quimbayo JP, Quiros TEAL, Shepherd B, Stefanoudis PV, Talma S, Teixeira JB, Woodall LC, Rocha LA (2023) Plastic pollution on the world’s coral reefs. Nature 619(7969):311–316
Prieve DC, Ruckenstein E (1974) Effect of London forces upon the rate of deposition of Brownian particles. AIChE J 20(6):1178–1187
Quigg A, Chin W, Chen C, Zhang S, Jiang Y, Miao A, Schwehr KA, Xu C, Santschi PH (2013) Direct and Indirect Toxic Effects of Engineered Nanoparticles on Algae: Role of Natural Organic Matter. ACS Sustainable Chemistry & Engineering 1(7):686–702
Rama P, Gallego-Urrea JA, Abbas Z (2023) Interfacial interactions of humic acids with polystyrene nano-plastics in aqueous/ionic environments: a molecular dynamics exploration. Environ Sci Nano 10(5):1385–1393
Schaumann GE, Philippe A, Bundschuh M, Metreveli G, Klitzke S, Rakcheev D, Grün A, Kumahor SK, Kühn M, Baumann T, Lang F, Manz W, Schulz R, Vogel H-J (2015) Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci Total Environ 535:3–19
Shams M, Alam I, Chowdhury I (2020) Aggregation and stability of nanoscale plastics in aquatic environment. Water Res 171:115401
Shang S, Liu Y, Liu M, Bai Y, Wang X, Wu B, Chen J, Dong J, Liu Y (2022) Studying the adsorption mechanisms of nanoplastics on covalent organic frameworks via molecular dynamics simulations. J Hazard Mater 421:126796
Shruti VC, Kutralam-Muniasamy G, Pérez-Guevara F (2023) Do microbial decomposers find micro- and nanoplastics to be harmful stressors in the aquatic environment? A systematic review of in vitro toxicological research. Sci Total Environ 903:166561
Singh N, Tiwari E, Khandelwal N, Darbha GK (2019) Understanding the stability of nanoplastics in aqueous environments: effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ Sci Nano 6(10):2968–2976
Song J, Xu Y, Liu C, He Q, Huang R, Jiang S, Ma J, Wu Z, Huangfu X (2020) Interpreting the role of NO3−, SO42−, and extracellular polymeric substances on aggregation kinetics of CeO2 nanoparticles: Measurement and modeling. Ecotoxicol Environ Saf 194:110456
Summers S, Henry T, Gutierrez T (2018) Agglomeration of nano- and microplastic particles in seawater by autochthonous and de novo-produced sources of exopolymeric substances. Mar Pollut Bull 130:258–267
Syngouna VI, Chrysikopoulos CV (2019) Bacteriophage MS2 and titanium dioxide heteroaggregation: Effects of ambient light and the presence of quartz sand. Colloids Surf, B 180:281–288
Timmis KN (2002) Pseudomonas putida: a cosmopolitan opportunist par excellence. Environ Microbiol 4(12):779–781
Tiwari E, Singh N, Khandelwal N, Monikh FA, Darbha GK (2020) Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J Hazard Mater 397:122769
Toh W, Ang EYM, Ng TY, Lin R, Liu Z (2022) Antifouling Bilayer Graphene Slit Membrane for Desalination of Nanoplastic-Infested Seawater: A Molecular Dynamics Simulation Study. ACS Appl Mater Interfaces 14(38):43965–43974
Trefalt G, Cao T, Sugimoto T, Borkovec M (2020) Heteroaggregation between Charged and Neutral Particles. Langmuir 36(19):5303–5311
Verdugo P, Alldredge AL, Azam F, Kirchman DL, Passow U, Santschi PH (2004) The oceanic gel phase: a bridge in the DOM–POM continuum. Mar Chem 92(1):67–85
Wang H, Han X, Chen Y, Guo W, Zheng W, Cai N, Guo Q, Zhao X, Wu F (2021a) Effects of F−, Cl−, Br−, NO3−, and SO42− on the colloidal stability of Fe3O4 nanoparticles in the aqueous phase. Sci Total Environ 757:143962
Wang J, Zhao X, Wu A, Tang Z, Niu L, Wu F, Wang F, Zhao T, Fu Z (2021b) Aggregation and stability of sulfate-modified polystyrene nanoplastics in synthetic and natural waters. Environ Pollut 268:114240
Wang Y, Wang J, Cong J, Zhang H, Gong Z, Sun H, Wang L, Duan Z (2023) Nanoplastics induce neuroexcitatory symptoms in zebrafish (Danio rerio) larvae through a manner contrary to Parkinsonian’s way in proteomics. Sci Total Environ 905:166898
Wu A, Zhao X, Yang C, Wang J, Wang X, Liang W, Zhou L, Teng M, Niu L, Tang Z, Hou G, Wu F (2022) A comparative study on aggregation and sedimentation of natural goethite and artificial Fe3O4 nanoparticles in synthetic and natural waters based on extended Derjaguin–Landau–Verwey–Overbeek (XDLVO) theory and molecular dynamics simulations. J Hazard Mater. 435:128876
Wu J, Jiang R, Liu Q, Ouyang G (2021) Impact of different modes of adsorption of natural organic matter on the environmental fate of nanoplastics. Chemosphere 263:127967
Wu J, Ye Q, Wu P, Xu S, Liu Y, Ahmed Z, Rehman S, Zhu N (2022b) Heteroaggregation of nanoplastics with oppositely charged minerals in aquatic environment: Experimental and theoretical calculation study. Chem Eng J 428:131191
Wu M, Bi E (2019) Stability of Artificial Nano-Hydroxyapatite in the Presence of Natural Colloids: Influence of Steric Forces and Chargeability. J Environ Qual 48(4):1100–1108
Wu S, Liu Y, Zhang H, Lei L (2020) Nano-graphene oxide with antisense vicR RNA reduced exopolysaccharide synthesis and biofilm aggregation for Streptococcus mutans. Dent Mater J 39(2):278–286
Xu H, Jiang H (2015) Effects of cyanobacterial extracellular polymeric substances on the stability of ZnO nanoparticles in eutrophic shallow lakes. Environ Pollut 197:231–239
Yang CH, Young T, Peng MY, Weng MC (1996) Clinical spectrum of Pseudomonas putida infection. J Formos Med Assoc. 95(10):754–761
Yang Y, Zheng S, Li R, Chen X, Wang K, Sun B, Zhang Y, Zhu L (2021) New insights into the facilitated dissolution and sulfidation of silver nanoparticles under simulated sunlight irradiation in aquatic environments by extracellular polymeric substances. Environ Sci Nano 8(3):748–757
Yong X, Du K (2023) Effects of Shape on Interaction Dynamics of Tetrahedral Nanoplastics and the Cell Membrane. J Phys Chem B 127(7):1652–1663
Yu SJ, Li QC, ShanHaoLiLiu WYZNPJF (2021) Heteroaggregation of different surface-modified polystyrene nanoparticles with model natural colloids. Sci Total Environ 784:147190
Zhang M, Xu L (2022) Transport of micro- and nanoplastics in the environment: Trojan-Horse effect for organic contaminants. Crit Rev Environ Sci Technol 52(5):810–846
Zhang S, Jiang Y, Chen C, Spurgin J, Schwehr KA, Quigg A, Chin W-C, Santschi PH (2012) Aggregation, Dissolution, and Stability of Quantum Dots in Marine Environments: Importance of Extracellular Polymeric Substances. Environ Sci Technol 46(16):8764–8772
Zhao J, Dai Y, Wang Z, Ren W, Wei Y, Cao X, **ng B (2018) Toxicity of GO to Freshwater Algae in the Presence of Al2O3 Particles with Different Morphologies: Importance of Heteroaggregation. Environ Sci Technol 52(22):13448–13456
Zhu H, Fan X, Zou H, Guo R, Fu S (2023) Effects of size and surface charge on the sedimentation of nanoplastics in freshwater. Chemosphere 336:139194
Zhu K, Jia H, Sun Y, Dai Y, Zhang C, Guo X, Wang T, Zhu L (2020) Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res 173:115564
Acknowledgements
Acknowledgments of Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers [41925031], [41991315] and [41521003]).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Aiming Wu and Chunyan Yang. The first draft of the manuscript was written by Aiming Wu and Chunyan Yang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Fengchang Wu is the Editor-in-chief of Carbon Research and was not involved in the editorial review, or the decision to publish, this article. All authors declare that there are no competing interests.
Additional information
Communicated by Baoshan **ng.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, A., Yang, C., Zhao, X. et al. Heteroaggregation and sedimentation of natural goethite and artificial Fe3O4 nanoparticles with polystyrene nanoplastics in water. Carbon Res. 3, 38 (2024). https://doi.org/10.1007/s44246-024-00107-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-024-00107-2