Abstract
Biochar and compost application in soil has been proved as an environmental management and soil remediation strategy for upgrading soil quality and growth-promoting soil microorganisms. A detailed examination of the fluctuations and stoichiometric interactions between β-glucosidase (BG), N-acetylglucosaminidase (NAG), leucine aminopeptidase (LAP), and acid phosphatase (AP) after applying biochar and compost to the heavy metal soils was conducted in the paper. However, biochar stimulated the activity of AP and inhibited BG, NAG, and LAP. Compost and biochar-compost might strengthen BG, NAG, LAP, and AP activities. Redundancy analysis discovered that the crucial parameters that affected soil enzyme activity were TN, NO3−-N, and TP. However, the stoichiometric ratio of C/N, C/P, and N/P with the application of the biochar, compost, and biochar-compost could be changed. While alleviating the P-limitation in soils under Cu stress, biochar may also alleviate the C-limitation in soils under As, Cd, and Zn stress. Compost and biochar-compost application alleviated C-limitation in heavy-metal (Cu, As, Cd, and Zn) polluted soil. This work will help us further understand the stoichiometry and energy restrictions of biochar and compost on ecological functional enzymes.
Highlights
Compost increased hydrolase activities while biochar reduced activities of C and N cycles.
Compost decreased enzymatic stoichiometric ratios of C:N, C:P, and N:P.
Biochar-compost and compost alleviated C-limitation levels in heavy metal polluted soils.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Industrial production, fertilization, and chemical manufacturing are the main anthropogenic activities that cause heavy metal deposition into the soil environment (Zhao et al. 2022). The land animals and plant species could be affected by the risk of heavy metal, and even soil microorganism that enrolls in the nutrient cycle and metabolic process within the environment can be affected. Given the characteristics of toxicity, nondegradable and irreversible in heavy metals, it remains a constant strain on the farmland ecosystem until being restrained or removed (Zhao et al. 2021). Numerous studies have been conducted on biochar (Qiu et al. 2022; Sanchez-Hernandez et al. 2019) and compost (Tang et al. 2020) as effective soil remediation amendments to alter soil characteristics, alleviate heavy metal toxicity in soils, affect the structure of functional micro populations and enzyme activities (Tang et al. 2019; Liang et al. 2020), and improve C, N, and P levels in soils (Abujabhah et al. 2016; Zhang et al. 2021). Biochar can decrease the available content of heavy metal to improve the habitat preferences of microbes in soils through a variety of mechanisms, including physical adsorption, ion exchange, precipitation complexation, and cationic interaction, as a metal passivator. In soils that had been contaminated with heavy metals (Cd, Zn, and Pb), Houben et al. (2013) applied straw biochar. They discovered that as the amount of biochar added increased, the content of available heavy metals in the soil steadily reduced (Houben et al. 2013). Additionally, the application of biochar to soil would also impact the activities of the soil’s enzymes (Sandhu et al. 2019). Yang et al. (2016) discovered that the activities of urease and catalase were boosted by application of 1% and 5% biochar, but the activity of acid phosphatase was decreased by adding the 5% biochar. Urease and invertase activities in Cd and Cu contaminated soil could be improved by wheat straw biochar application (Jia et al. 2017). Compost can passivate soil heavy metal to reduce its toxicity in the soils by precipitation, adsorption, oxidation-reduction, complexation, and chelation with its abundance organic matter and microbes. According to Mariusz and Dorota (2016) and Lin et al. (2022), humus is abundant in compost, has the ability to combine with metal ions to create stable organometallic complexes, reducing the available content of Cd and Zn in soil. Besides, compost is a rich source of nutrients, which can supply the soil’s microbes with the building blocks and energy they need to grow and reproduce. For example, applying compost made from municipal solid waste boosts the activity of the enzymes β-glucosidase (Bhattacharyya et al. 2005) and acid phosphatase (Abujabhah et al. 2016; Arif et al. 2018) in heavy metal combined polluted soil.
It has been discovered that soil extracellular enzyme activity (EEA) gives fundamental information on the ecological enzyme stoichiometry limitations and the connections between soil enzyme activity and soil structure (Sinsabaugh et al. 2008; Sinsabaugh et al. 2009), which may potentially link microbial production with soil environmental and soil nutrient availability. Ecological stoichiometry refers to the proportion of soil functional enzymes engaged in C, N, and P cycling in ecosystems (Bowles et al. 2014), and has been widely used to assess the soil microbial nutrient requirements and limitations, as a method of evaluating the biogeochemical balance pattern between microbial and soil nutrients (Tapia-Torres et al. 2015; Bell et al. 2014; Cenini et al. 2016; Xu et al. 2017). Sinsabaugh et al. (2008) discovered that the stoichiometric ratio of soil enzyme C:N:P was about 1:1:1 globally, where it was slightly off due to regional variances in environmental conditions and biological factors (Doi et al. 2010; Williams et al. 2013). Therefore, research into the stoichiometric properties of soil mineralized enzymes for carbon, nitrogen, and phosphorus will help understand the impact of soil microbial mechanisms on soil C, N, and P cycles in heavy metal pollution.
It has been discovered that biochar (Wang et al. 2021) and compost (Zeng et al. 2018) contain a significant quantity of organic carbon, total nitrogen, and total phosphorus. Thus, we hypothesized that the addition of biochar and compost might alleviate the degree of C, N, and P limitation of soil microorganisms because higher resource concentration might minimize the potential for resource limitation. Compost (Tang et al. 2020) and biochar (Chen et al. 2018a) have the potential to fix heavy metals in soil. Therefore, we hypothesized that the toxicity of heavy metals to soil microorganisms can be alleviated by the interaction between biochar and compost, which could explain previous research’s variable direction of microbial resource limitation in soil remediation.
2 Material and method
2.1 Soil preparation and amendments characterization
Changde City (29°N, 111°E), Hunan, Central South China, was chosen as the location for soil sampling. Mining and agricultural operations in this area have polluted the soil with heavy metals such as Zn, Cd, Cu, and As. After a basic physical treatment, the soil samples were separated. Rice straw was used to make biochar in a tube furnace under hypoxic conditions (500 °C, 5 h) (Li et al. 2019). According to our prior research, compost was created from agricultural waste (e.g., vegetable leaves, rice straw) (Ren et al. 2018; Zeng et al. 2011). The primary physicochemical characteristics of soils and additives, and heavy metal properties after the addition of biochar and compost were depicted in Table 1 (Tang et al. 2020).
2.2 Scheme of experiment and sample acquisition
The majority of the investigations (Huang et al. 2017; Zeng et al. 2015) used 1% or 5% biochar addition. Yang et al. (2016) found that 5% straw biochar might raise soil pH, EC, and AP while lowering the available content of Cd, Cu, and Zn. Thus, to investigate biochar’s ability to remediate heavy metal-contaminated soils, we chose to utilize 5% of it. The four groups of experiments’ designs were as follows: Treatment A, 5 kg soil (control); treatment B, C and D added with 0.25 kg biochar (5%), 0.25 kg compost (5%), and 0.25 kg biochar (5%) + 0.25 kg compost (5%), respectively. All soil treatments were incubated for 80 days in a climate incubator at room temperature with a moisture content regulated to 0–70%, with subsamples being collected at 0, 30, 50 and 80 days.
2.3 Soil physicochemical property measurement
The EC, pH, nitrate-nitrogen (NO3−-N), and ammonia nitrogen (NH4+-N) were determined based on a previous study (Li et al. 2019). Dry combustion was used to analyze the OM content, and the content of TOC was equal to OM/1.724 content. Wet digestion, potassium dichromate oxidation, and the Kjeldahl technique were used to measure the amounts of total nitrogen (TN). NaHCO3 (pH 8.5)-colorimetric approach was used for extracting the AP. TP was digested by H2SO4-HClO4 and measured by a flow injection auto-analyzer (Huang et al. 2011).
2.4 The available contents of the heavy metal examination
The available content of heavy metals in soil was extracted using CaCl2 (0.01 mol/L) and measured using inductively coupled plasma mass spectrometry (ICP-MS) (Zhao et al. 2022).
2.5 Soil enzyme activity determination
Soil enzyme activities indicative of C-cycling (BG), N-cycling (NAG and LAP), and P-cycling (AP) were monitored. The release of p-nitrophenol (PNP) was used to determine the activities of BG and AP. The activities of NAG and LAP were measured by the method (Saiya-Cork et al. 2002).
2.6 The method of vector length and vector angle calculated
The vector length (VL) is determined by taking the square root of the sum of the squares of the C/N and C/P stoichiometry ratios, which indicates the limited degree of carbon. The longer the VL, the higher the C-limitation. By using the DEGREES functions after the ATAN2 functions of the C/N and C/P stoichiometry ratios vector angles (VA) were calculated, to reflect the N-limitation and P-limitation in soils. If the VA is greater than 45°, the N-limitation reduces with reducing VA. When the VA is less than 45°, the P-limitation increases with increasing VA. We set the ln(BG): ln(AP) ratio to be a, and the ln(BG): ln(NAG+LAP) ratio to be b. Thus, a stands for relative C-acquiring and P-acquiring enzyme activity, and b stands for relative C-acquiring and N-acquiring enzyme activity (Chen et al. 2019; Moorhead et al. 2016).
2.7 Statistical analysis
One-way analysis of variance (ANOVA) and correlation analysis were performed using IBM SPSS Statistics 22 (USA). Redundancy analysis (Canoco 5.0) was used to analyze the significance of environmental factors in explaining soil enzyme activities. Origin 2022 was used to create all of the figures with the exception of the redundancy analysis ordination biplot. The natural logarithm of soil enzyme activity was used to calculate the ecological stoichiometric ratio to represent the C:N:P acquisition ratio, in an attempt to optimize the normality distribution and homoscedasticity hypothesis for responding to the ratios of ln(BG): ln(NAG + LAP): ln(AP).
3 Results and discussion
3.1 The impact of biochar and compost on soil physicochemical characteristics
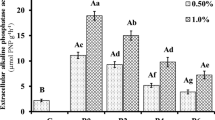
The content of pH, EC, NO3−-N, NH4+-N, OM, and AP in soil under the different treatments was displayed in Fig. 1. The addition of biochar and compost had essentially little effect on soil pH for the whole culture time, whereas biochar-compost considerably increased soil pH to 6.56 (Fig. 1a). The results were similar to those in previous research (Beesley et al. 2014; Liang et al. 2017). In comparison to control soil, EC with biochar addition observed no significant alterations (Fig. 1b). The EC of the soil was enhanced by 285% and 261%, respectively, using compost and biochar-compost. Our findings contradicted Igalavithana’s research, which claimed that biochar increased soil EC levels (Igalavithana et al. 2017). Biochar lowered NO3−-N levels, while compost, and biochar-compost raised NO3−-N levels, with compost having a stronger impact than biochar-compost (Fig. 1c). However, biochar, compost, and biochar-compost increased NH4+-N levels, although there was no statistical difference when compared to control soil (Fig. 1d). Prior research also found that biochar significantly lowered NO3−-N while somewhat increasing NH4+-N (Chen et al. 2013). It might be ascribed to the fact that the ammonia-oxidizing communities were available in compost, which produced and accumulated a large amount of NH4+-N (Ren et al. 2018). Soil OM (Fig. 1e) increased by 39%, 18%, and 66%, respectively, as biochar, compost, and biochar-compost were employed, compared to control soil (54.28 g·kg− 1), while soil AP (Fig. 1f) increased by 11%, 27%, and 31% respectively, and the content of AP in control soil was 32.78 g·kg− 1. Biochar addition and composted industrial sludge considerably enhanced the concentration of soil AP, according to Yang et al. (2016) and Arif et al. (2018). Phenolic, hydroxyl, and carboxyl functional groups on the surface of biochar bind to H+ in the soil, to raise the pH of the soil (Gul et al. 2015). The contents of NO3−-N and NH4+-N also have an impact on pH. Tang et al. (2020) demonstrated that an increase in NO3−-N content raises pH while an increase in NH4+-N content caused soil acidification. An elevation in pH could result from ammonia’s dissolution as well (Gil et al. 2008). Previous research has hypothesized that microbial assimilation of NO3− and SO42− through OM decomposition may be responsible for the decline in soil EC. Both biochar and compost have high levels of organic matter, and compost has a lot of humus, which is the primary organic carbon store in the carbon cycle (Gusiatin and Kulikowska 2016). Furthermore, studies have demonstrated that biochar adsorption of soil organic molecules can boost organic matter content by inducing polymerization of tiny organic molecules via surface catalytic activity. The detailed analysis has been depicted in our previous paper (Tang et al. 2020).
The effects of biochar and compost on soil physicochemical properties during the whole culture period. EC: Electrical conductivity; NH4+-N: Ammonium; NO3−-N: Nitrate-nitrogen; OM: Organic matter; AP: Available phosphorus. Treatment A: soil without any addition. Treatment B: soil with biochar addition. Treatment C: soil with compost addition. Treatment D: soil with biochar-compost addition. The results on day 0 and 30 were demonstrated in the previous study (Tang et al. 2020). The different letter on the bar represents significant difference at P < 0.05 level on each sampling occasion
TOC (Fig. 2a) increased by 33%–47%, 6%–29%, and 52%–83%, respectively, when biochar, compost, and biochar-compost treatments were used, compared to control soil, as biochar and compost had a strong capacity of soil C sequestration. Several other research (Abujabhah et al. 2016; Arif et al. 2018; Liang et al. 2017) found that biochar, compost, and their combination had positive impacts on TOC, which were consistent with what we discovered. Biochar, compost, and biochar-compost could have the potential to boost TN (Fig. 2b) and TP (Fig. 2c) levels. The impacts of soil remediation agents on TN were as follows: biochar-compost > compost > biochar, with the preferable remediation effect on day 80. The results were consistent with those found by Wang et al. (2022a, 2022b, 2022c). Similarly, the improvement of TP by soil remediation agents reached a maximum on day 50, with biochar-compost having the greatest, followed by biochar and compost. Thijs Vanden Nest’s investigation of the P availability of compost and biochar found that compost was superior to biochar in the P availability, which contradicted with our results (Vanden Nest et al. 2021). The ratio of soil C:N:P was demonstrated in Table 2 and the results revealed that C:N:P ratio has declined over time. The C:N:P ratio could be raised after applying biochar (Liao et al. 2022), and decreased by compost, and biochar-compost, compare to the control soil. The level of humification of OM is indicated by the soil C/N ratio, which means the higher the level of humification of OM, the higher the C/N ratio. The ratio of C/P in soil is typically considered as a sign of soil P mineralization or as a measure of the environment’s capacity to absorb and retain P. Higher C/P implies that soil microorganisms have a proclivity to assimilate available P in soil, and it is easy for microorganisms and plants to compete for the uptake of available P in soil, indicating that soil has a strong capacity for phosphate fixation. The soil N/P ratio is a powerful predictor of nutrient restriction as well as a diagnostic index of nitrogen sufficiency. A lower soil N/P ratio usually signifies that N is the principal limiting factor for plants, which indicates higher primary productivity. We found that compost and biochar-compost could lower the soil C/N ratio, and increase the soil C/P and N/P ratio, indicating that lower primary productivity after compost and biochar-compost were added to soils. However, better primary productivity and stronger ability to fix phosphorus, but with a lower level of soil OM humification were shown in the treated soil under biochar, indicating that N was the principal limiting factor in soil. The increase of soil C:N:P ratio induced by biochar might be due to the fact that: (i) its highly aromatic structure made a higher degree of biochemical and thermal stability (Ji et al. 2022; Xu et al. 2022); (ii) its abundance of carbon can increase the soil’s capacity to store carbon (Yang et al. 2022); (iii) the strong capacity of N adsorption, which may improve the soil nitrogen retention, and increase the amount of organic matter and nutrient availability (Kamali et al., 2022); (iv) the organic functional groups on the surface can increase soil’s capacity to absorb HPO42− and lessen phosphorus loss (Li et al. 2020). The reduction in soil C:N:P ratio that compost causes may be related to abundant microorganisms carried by compost, because there may be competition for C, N, and P absorption between the microbes and soil. However, the use of biochar can compensate for this deficiency of compost (Wu et al. 2017), because the addition of biochar delivered the recalcitrant carbon that can increase the C/N ratio (**do et al. 2012). Biochar had the greatest impact on the soil C:N:P ratio, followed by biochar-compost and compost.
The effects of biochar and compost on soil total organic C (a), total N (b), and total P (c) contents. Treatment A: soil without any addition. Treatment B: soil with biochar addition. Treatment C: soil with compost addition. Treatment D: soil with biochar-compost addition. The different letter on the bar represents significant difference at P < 0.05 level on each sampling occasion
3.2 The variations of biochar and compost to the heavy metal available content
According to Table 3, the passivation rate of Cu by biochar ranged from 12% to 26%, according to the findings. Compost and biochar-compost increased the content of soil available Cu, with the activation rates of 107%–276% and 47%–335% respectively. The availability of As content rose to various degrees after biochar, compost, and biochar-compost application, with activation rates of 82%–85%, 64%–70%, and 53%–58%, respectively. The increase of available Cu and As induced by compost might be due to the fact that the humic acids’ aromatic systems in compost contain functional groups that can form stable oxygen-radicals, which might increase the Cu and As content in the acidic soil (Clemente and Bernal 2006; Zeng et al. 2015), whereas strongly decrease the bioavailability of Pb (Zhang et al. 2017). Different remediation strategies could reduce the available Cd and Zn contents in soil. Biochar-compost had the most notable passivation impact on Cd, with a passivation rate ranging from 75% to 87%. Biochar and compost were used to passivate 39%–73% and 42%–70% of Cd in soil, respectively. For 30 days, biochar, compost, and biochar-compost reduced 39%–60%, 50%–77%, and 64%–86% of the available Zn in the soil, and were even able to fix Zn in the soil on days 50 and 80. By electrostatically interacting with heavy metals and chelating with them, biochar, particularly biochar with large surface area, functional groups, and high pH, can aid in the immobilization of metal cations. A significant portion of compost is made up of humus, which can combine with soil metal ions to form stable organometallic complexes and lessen metal mobility. The detailed analysis has been depicted in our previous study (Tang et al. 2020).
3.3 The alterations in soil enzymatic activities induced by biochar and compost utilized
To evaluate the dynamical effects of environmental amendments on enzymatic action, the activities of BG, NAG, LAP, and AP were assessed (Fig. 3). Soil enzyme activities varied between different treatments of soils polluted by heavy metals. Microorganisms use BG as a rate-limiting enzyme in the degradation of cellulose to glucose (Arif et al. 2018). Throughout the trial, biochar reduced BG enzyme activity by 5%–23% (Fig. 3a), compared to the control. Compost and biochar-compost were shown to be more effective in increasing BG enzyme activity, which increased by 17%–46% and 28%–52% increased, respectively. NAG is the enzyme that controls the degradation of glucosamine polymers and chitin. The amino terminal of leucine and other hydrophobic amino acid polypeptides can be hydrolyzed by LAP. Therefore, the nitrogen availability in soil is well described by NAG and LAP. Except for an 18% rise in NAG activity on day 30, biochar inhibited NAG (Fig. 3b) and LAP (Fig. 3c) enzyme activity. NAG and LAP enzyme activity was raised by 72%–110% and 31%–42%, by compost, and raised by 65%–90% and 12%–29%, by biochar-compost, respectively. AP regulates the hydrolysis of phosphate monomers and diesters to phosphate and influences the pace of soil organophosphorus mineralization (Bünemann et al. 2012). Biochar, compost, and biochar-compost treatments stimulated AP activity by 26%–37%, 109%–227%, and 170%–296%, respectively (Fig. 3d). It is worth noting that the activity of BG, NAG, LAP, and AP increased with the increase of the cultivation time. Under various remediation strategies, BG, NAG, and AP attained their maximum activity on day 80, while LAP reached the maximum value on day 50. Biochar had the minimum effect on AP activation and even inhibited BG, NAG, and LAP activity. Biochar-compost outperformed compost in terms of BG and AP activities, whereas compost activated NAG and LAP better.
The effects of biochar and compost on soil β-glucosidase activity (a), N-acetylglucosaminidase activity (b), leucine aminopeptidase activity (c), and acid phosphatase activity (d). Treatment A: soil without any addition. Treatment B: soil with biochar addition. Treatment C: soil with compost addition. Treatment D: soil with biochar compost addition
Previous studies have demonstrated that biochar addition may increase enzyme activity (Luo et al. 2017), inhibit enzyme activity (Foster et al. 2018), or have no effects on enzyme activity (Liao et al. 2022). Our study showed that biochar had no effects on BG, NAG, and LAP activity on day 0 and 30, but activities reduced on day 50 and 80. However, the activity of AP increased by addition of biochar. The direct adsorption of the enzyme is the principal factor affecting the decrease in enzyme activity caused by biochar. Foster et al. (2018) discovered that the decrease in BG activity may be due to adsorption of partial biochar by BG substrate because polar glucose substrate is easily adsorbable to negatively charge solid phase. C-cycling enzyme activity may be inhibited by small compounds produced by biochar such as phenols and polyphenols, or caused by the presence of microbes and carbon on the surface of biochar, which boosts effectiveness while lowering enzyme production. Lehmann et al. (2011) proposed that this was due to the coexistence of microbes and carbon on the surface of biochar, that is, biochar adsorption on -glucosidase resulted in decreased enzyme synthesis (Liao et al. 2016). Liao et al. (2022) found that the activity of the N-acquiring enzyme was considerably inhibited by biochar, but increased the P-acquiring enzyme activity (Zhang et al. 2019), and according to our findings, biochar application enhanced NAG activity within 30 days but inhibited it at 30–80 days (Fig. 3b). Furthermore, the biochar utilized in this study had a high pH of 9.10, which could raise soil pH and stimulate microbial metabolism, restrict microbial N use (Chen et al. 2017; Chen et al. 2018a, b), and drive enzyme synthesis, thus increasing the activity of the LAP (Pokharel et al. 2020). The priming effect provided by biochar is strongly associated with increased AP activity (Gul et al., 2015). Biochar-induced increases in soil TOC and pH may enhance the reaction kinetics of soil enzymes (Zimmerman et al. 2011). Compost is a soil amendment with a diverse flora and fauna community that differs from the original soil in terms of species composition (Kaurin et al. 2018). The increase in BG, NAG, LAP, and AP activity caused by compost application might be attributed to: (i) the reduction of unstable carbon pools or potentially hazardous components in contaminated soils (Garau et al. 2019); (ii) the compost induced that increased the soil pH (Alvarenga et al. 2008); (iii) the abundant organic matter in compost that provided an energy-rich substrate for soil enzyme; and (iv) the increase of substrate availability. The combination of heavy metals with organic matter in compost can also alleviate the heavy metals toxicity on soil microbial and enzyme activity. Moreover, the interaction between biochar and compost may be correlated with the increase in soil enzyme activity induced by biochar-compost.
3.4 Stoichiometry of soil enzyme activities by applying biochar and compost
The stoichiometry of the four enzymatic activities was analyzed in (Fig. 4). The average C:N:P acquisition ratios by BG: (NAG + LAP), BG: AP, and (NAG + LAP): AP were 2.18, 5.39, and 2.48 in control soil. The C:N:P acquisition ratios after biochar application were 2.02, 3.59, and 1.77. The C:N:P acquisition ratios after compost application were 1.86, 2.85, and 1.55, while after biochar-compost addition the C:N:P acquisition ratios were 2.12, 2.28, and 1.07. The ratio of the BG: (NAG + LAP), BG: AP, and (NAG + LAP): AP remediated by soil amendments was much lower than that of the control soil. However, in control soil, the average ratios of ln(BG): ln(NAG + LAP), ln(BG): ln(AP), and ln(NAG + LAP): ln(AP) were 1.32, 2.09, and 1.58, suggesting that the enzyme activity ratio of C:N:P was roughly 1.3:2.1:1.6. Biochar application had no influence on the ln(C/N) (Fig. 4a), while considerably lowered the ln(C/P) (Fig. 4b) and ln(N/P) (Fig. 4c) ratios, compared to the control soil. Compost and biochar-compost, on the other hand, decreased the ratios of ln(C/N), ln(C/P), and ln(N/P). The stoichiometric ratio of ecological enzymes became closer to 1 as culture time increased. On day 80, the ln(C/P) and ln(N/P) ratios did not significantly differ between compost and biochar-compost treatment. In general, biochar, compost, and biochar-compost improved the stoichiometric ratio of soil ecological enzymes substantially, and the stoichiometric ratio of soil ecological enzymes became closer to 1 as the culture days increased. According to Fig. 5, AP, NO3−-N, EC, TN, the soil C/N and N/P ratios were shown to be strongly associated with fluctuations and varied amendments with the logarithms of soil enzyme activity. Positive relationships between ln(BG) and EC (P < 0.001), TN (P < 0.001), NO3−-N (P = 0.001), AP (P = 0.014), soil N/P (P < 0.001), however, a negative correlation between ln(BG) and soil C/N (P = 0.003) were found. Moreover, ln(NAG) was positively linked with EC (P < 0.001), TN (P < 0.001), NO3−-N (P = 0.003), AP (P < 0.001), soil N/P (P < 0.001), and negatively with soil C/N (P = 0.000). It also indicated that ln(LAP) and EC (P < 0.001), TN (P < 0.001), NO3−-N (P = 0.002), AP (P = 0.014), soil N/P (P < 0.001) were positively correlated, but negatively correlated with C/N (P < 0.001). There were positive connection between ln(AP) and EC (P < 0.001), TN (P < 0.001), NO3−-N (P = 0.003), AP (P < 0.001), soil N/P (P = 0.009). Soil enzyme activity changed as a result of changes in environmental factors. Therefore, pH, EC, TOC, TN, TP, NO3−-N, NH4+-N, AP, soil C/N, soil C/P, and soil N/P were selected as soil physicochemical factors in this study, and the correlation between environmental factors and soil enzyme activity was assessed using redundancy analysis. The eigenvalues of Axis 1 and Axis 2 were 94.62% and 3.44%, respectively, in redundancy analysis, and the cumulative explanatory variables were 98.06% (Fig. 6). The primary parameters that affected soil enzyme activity were TN, NO3−-N, and TP. TN could account for more than 85% of the variability in soil enzyme activity, followed by NO3−-N (51.3%) and TP (22.4%). Alterations in soil physicochemical characteristics, as well as heavy metal concentration, induced by biochar or compost, could cause physiological and metabolic changes in soil functional enzymes.
The stoichiometry ratio of ln(BG):ln(NAG+LAP) (a), ln(BG):ln(LAP) (b), and ln(NAG+LAP):ln(AP) (c) under different remediation methods. Treatment A: soil without any addition. Treatment B: soil with biochar addition. Treatment C: soil with compost addition. Treatment D: soil with biochar-compost addition
Redundancy analysis (RDA) ordination biplot of soil enzyme activities and soil physicochemical factors under various soil additives addition. EC: electrical conductivity, NO3−-N: nitrate nitrogen, NH4+-N: ammonia nitrogen, TP: total phosphorus, TN: total nitrogen, soil C/N: soil carbon to nitrogen ratio; soil C/P: soil carbon to phosphorus ratio; soil N/P: soil nitrogen to phosphorus ratio. A, B, C, D indicate different remediation strategies as follows: Treatment A, soil without any addition; Treatment B, soil with biochar addition; Treatment C, soil with compost addition; Treatment D, soil with biochar-compost addition
The enzyme acquisition C:N:P ratio was not near 1:1:1 in this investigation, in contrast to the global and regional scales (Sinsabaugh et al. 2008; Sinsabaugh et al. 2009). This might be owing to the use of biochar and compost, which may prevent the C/N, C/P, and N/P from reaching the full potential. The ratio of soil N/P acquisition was reduced in control and experimental soil, but there were no significant variations across the days. Throughout all the treatments, the enzyme N/P in control soil was greater than in experimental soil, indicating that microbial metabolism in soils with biochar and compost preferred to engage more NAG and LAP than AP. Modified treatments had decreased soil N concentrations, which is another evidence in favor of this. When soil N becomes scarce, N-acquiring enzymes were further potentially synthesized by microorganisms to get the most out of their resource reserves through obtaining the most scarce resource (Bloom et al. 1985). This supports prior findings that N is the most important indicator in temperate soils (Wardle et al. 2004). As a result, the substantial microbial research in N-acquiring enzymes suggested N was severely constrained in soils studied. The pace of litter decomposition and carbon balance of an ecosystem could be regulated by N and P availability. Thus, the stoichiometric ratios of extracellular enzyme activity in soil may be critical parameters of the energy cycle and microbial metabolism. Our results indicated that if nutrient availability decreased or increased, with the addition of amendments and duration increase, the enzyme activity did not rise or decrease constantly. The average enzymatic C:N:P ratio indicated that the rates at which digestible substrates were supplied from the magnitudes of the carbon, nitrogen, and phosphorus sources were similar, thus close limitations on microbial production may be transferred directly between them (Sinsabaugh and Shah 2010). The C, N, and P acquisition rates of EEA can give an empirical basis for building the connection between extracellular enzyme activity and net primary productivity, along with reallocating supplies in the microbial diversity (Sinsabaugh et al. 1992). The stoichiometric ratio of the ecological enzyme C:N:P is a crucial index of energy and nutrient cycling in microorganisms (Hessen 1997; James et al. 1996). Biochar and compost additions primarily alerted soil enzymatic activity and stoichiometric ratios, which explained the temporal variation in soil enzyme activity. Biochar and compost altered the nutritional needs of the soil and ecological enzymes, causing changes in enzyme activity and stoichiometric ratios. Microbes must release comparable enzymes to fulfill their nutritional needs when soil N and P are scarce (Allison et al. 2007).
3.5 Analysis of the correlation between soil physicochemical characteristics and ecological enzyme C, N, and P stoichiometry
The link between soil physicochemical parameters and the logarithms of enzyme activity measurement was depicted in Fig. 7. Electrical conductivity was negatively correlated with enzymatic stoichiometry ratio of C/N activity (P < 0.001), C/P activity (P < 0.001), N/P activity (P < 0.001), respectively (Fig. 7a). A negative relationship between organic matter and C/P activity (P < 0.018), N/P activity (P = 0.006) was found (Fig. 7b). Available phosphorus was negatively associated with C/N activity (P = 0.003), C/P activity (P < 0.001), N/P activity (P < 0.001) (Fig. 7c). Additionally, it demonstrated a negative correlation between soil N/P and C/N activity (P < 0.001) and C/P activity (P < 0.039) (Fig. 7d). Enzymatic C/N and soil C/N had a positive connection (P = 0.004) (Fig. 7e).
The tested soil originated from paddy fields polluted by heavy metal, not natural ecosystems. Long-term flooding circumstances limit microbial activity associated to organic matter decomposition and C mineralization, which causes a high level of soil carbon storage. The addition of compost with a greater N and P content as well as slower N mineralization rate and microbial biomass cycling were other factors that contributed to the rise in N and P content in the tested soil. In this study, biochar, compost, and biochar-compost all had similar patterns of influence on soil nutrient stoichiometric ratios, lowering the stoichiometric ratios of C/N, C/P, and N/P. The increase of the AP activity with the P limitation increased and C/P acquisition ratio decreased globally. When the C/N acquisition ratio was high, the activities of BG, NAG, LAP, and AP were comparatively low, but when the N/P was high, the activities of BG, NAG, LAP, and AP were relatively high. The shift in soil C, N, and P ratios might be attributed to the addition of exogenous fertilizers such as biochar and compost, which significantly altered the stoichiometric balance of soil components. By modifying the activity of functional enzymes involved in limiting nutrient cycling, soil microorganisms can produce a more ecological stoichiometric ratio between nutrients and other nutrient components. Soil microorganisms maintain the balance of soil material cycle and energy flow by controlling the growth and metabolism of C, N, and P by changing their stoichiometric ratio and redistributing the percentage of functional enzymes involved in soil C, N, and P cycling (Mooshammer et al. 2014; Zhu et al. 2018). Microbes will also release additional enzymes to fulfill their nutritional requirements once soil nutrient availability is insufficient (Wallenius et al. 2011). To maintain the stability of their metrology, microbes would increase or decrease the activities of corresponding functional enzymes and absorb various nutritional components needed for their development from the soil to accomplish the dynamic balance of internal stability under varying C, N, and P ratios.
3.6 The assessments of biochar and compost on the resource limitation in heavy metal contaminated soils
Different responses to biochar and compost in soil contaminated with heavy metals were used to indicate the indexes of C, N, and P resource limitation (Table 4). Under different remediation strategies, neither the changes in vector length nor the vector angle increased or decreased continuously as the culture time increased. Biochar, compost, and biochar-compost can reduce the vector length or increase the vector angle, respectively. In comparison to the control soil, the vector length under biochar-compost addition was the lowest on day 80 while the vector angle was the largest on day 50. However, the vector lengths of biochar and compost addition were the lowest while the vector angles were the greatest on day 50. Compared to compost and biochar compost, biochar had the greatest vector length, indicating that the highest relative C limitation, in accordance with the greatest ratios of C vs. nutrient acquisition for both elements. Soils treated with biochar were less P-limited than those treated with compost and biochar-compost. Biochar-compost had the greatest vector angle, suggesting the highest relative P limitation. However, the lowest vector length had, the minimum C limitation in biochar-compost application soils had.
Heavy metals might have a great impact on the ln(C/N), ln(C/P), ln(N/P), vector length, and vector angle, as demonstrated by the Pearson correlations (Table 5). It showed that the vector length might be positively correlated with ln(C/N), and ln(C/P). Vector angle might be negatively correlated with the ln(C/N), ln(C/P), ln(N/P). On the one hand, biochar decreased the available content of Cu, Cd, and Zn whereas increased As content. On the other hand, there might be a negative correlation between Cu, As, and ln(C/N), ln(C/P), ln(N/P), and vector length, respectively. These indicated that the reduction of Cu content or the increase of As content induced by biochar could raise or decrease the ln(C/N), ln(C/P), ln(N/P), and vector length. And the vector angle might positively link to the Cu and As, showing that biochar could alleviate the soil P-limitation and enhance the C-limitation under Cu stress while enhancing the soil P-limitation and alleviating the C-limitation under As stress. There might be a positive relationship between Cd, Zn, and ln(C/P), ln(N/P), and vector length, respectively. It showed that the decrease of Cd and Zn content induced by biochar could increase the ln(C/P), ln(N/P), and vector length. Also, the vector angle might be correlated with Cd and Zn negatively, implying that biochar could alleviate the C-limitation under Cd and Zn stress. Similarly, compost and biochar-compost could alleviate the C-limitation under Cu, As, Cd, and Zn stress. The outcomes also demonstrated that applying compost and biochar greatly increased soil C content. Firstly, it was because, when added to soil, their high carbon concentration might directly raise the soil C content. Secondly, through surface catalytic activity, biochar could adsorb soil organic molecules and encourage the polymerization of tiny organic molecules to create soil organic matter (Liang et al. 2010). Finally, the use of biochar and compost influenced the variety, quantity, and activity of microorganisms involved in the C turnover process, increasing the amount of C in the soil and alleviating the soil C-limitation. In acidic soil, P can be adsorbed by metal oxides (Cui et al. 2011), while the application of biochar may adsorb some metal ions and keep them in the soil solution without precipitating, thus improving their available content. At the same time, the fixation of metal ions to P is reduced to improve the available phosphorus content (Rajkovich et al. 2012), thus alleviating the soil P-limitation (Parvage et al. 2013).
Biochar and compost, in addition to improving the soil nutrient availability, have also been proved to alter enzyme activities. We suggested that the stoichiometric patterns of biochar, compost, and soil extracellular enzyme activity can be linked to the soil nutrient cycle through biochar-compost-soil-microbe feedbacks. The soil stoichiometry of enzyme activity varies greatly based on the soil types, soil structures, and soil additives. Limitations on soil resources have a significant impact on microbial activity. Uncertainty remains regarding the relative significance and mechanisms of biochar and compost on modifications in microbial activity. Is it sufficient to know that compost and biochar can have varying effects on the stoichiometry of soil enzyme activity in this situation? We insisted that a more conclusive thorough investigation of the C, N, and P levels of the microbial biomass is required.
4 Conclusions
Biochar and compost can improve soil quality, and change the microhabitat of soil microbes. Under Cu stress, biochar might alleviate soil P-limitation while alleviate the C-limitation in soils caused by As, Cd, and Zn stress. Furthermore, the C-limitation in soils with Cu, As, Cd, and Zn toxicity may be alleviated by compost and biochar-compost. The stoichiometry of soil nutrients and microbial nutrition requirements are connected to the enzymatic capacity of hydrolases in heavy metal-contaminated soils. A rough control of soil nutrient accumulation and alleviation of heavy metal stress are provided by the potential of oxidases in refractory components of soil organic matter. These findings provide insight on the ecological stoichiometry and biogeochemical processes of heavy metal soils being remedied with biochar and compost.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon request.
Abbreviations
- EEA:
-
extracellular enzyme activity
- BG:
-
β-1,4-glucosidase
- AP:
-
acid phosphatase
- AKP:
-
alkaline phosphatase
- NAG:
-
N-acetylglucosaminidase
- LAP:
-
leucine aminopeptidase
- EC:
-
electrical conductivity
- OM:
-
organic matter
- TOC:
-
total organic carbon
- NO3 −-N:
-
nitrate nitrogen
- NH4 +-N:
-
ammonia nitrogen
- TN:
-
total nitrogen
- TP:
-
total phosphorus
- AP:
-
available phosphorus
- VL:
-
vector length
- VA:
-
vector angles
References
Abujabhah IS, Bound S, Doyle R, Bowman JP (2016) Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl Soil Ecol 98:243–253. https://doi.org/10.1016/j.apsoil.2015.10.021
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781. https://doi.org/10.1016/j.soilbio.2007.02.006
Alvarenga P, Goncalves AP, Fernandes RM, de Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406:43–56. https://doi.org/10.1016/j.scitotenv.2008.07.061
Arif MS, Riaz M, Shahzad SM, Yasmeen T, Ashraf M, Siddique M, Mubarik MS, Bragazza L, Buttler A (2018) Fresh and composted industrial sludge restore soil functions in surface soil of degraded agricultural land. Sci Total Environ 619:517–527. https://doi.org/10.1016/j.scitotenv.2017.11.143
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJ (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202. https://doi.org/10.1016/j.envpol.2013.11.026
Bell C, Carrillo Y, Boot CM, Rocca JD, Pendall E, Wallenstein MD (2014) Rhizosphere stoichiometry: are C : N : P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol 201:505–517. https://doi.org/10.1111/nph.12531
Bhattacharyya P, Chakrabarti K, Chakraborty A (2005) Microbial biomass and enzyme activities in submerged rice soil amended with municipal solid waste compost and decomposed cow manure. Chemosphere 60:310–318. https://doi.org/10.1016/j.chemosphere.2004.11.097
Bloom AJ, Chapin FS III, Mooney HA (1985) Resource limitation in plants-an economic analogy. Annu Rev Ecol Evol S 16:363–392. https://doi.org/10.1146/annurev.es.16.110185.002051
Bowles TM, Acosta-Martínez V, Calderón F, Jackson LE (2014) Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol Biochem 68:252–262. https://doi.org/10.1016/j.soilbio.2013.10.004
Bünemann EK, Oberson A, Liebisch F, Keller F, Annaheim KE, Huguenin-Elie O, Frossard E (2012) Rapid microbial phosphorus immobilization dominates gross phosphorus fluxes in a grassland soil with low inorganic phosphorus availability. Soil Biol Biochem 51:84–95. https://doi.org/10.1016/j.soilbio.2012.04.012
Cenini VL, Fornara DA, McMullan G, Ternan N, Carolan R, Crawley MJ, Clément JC, Lavorel S (2016) Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol Biochem 96:198–206. https://doi.org/10.1016/j.soilbio.2016.02.015
Chen D, Liu X, Bian R, Cheng K, Zhang X, Zheng J, Joseph S, Crowley D, Pan G, Li L (2018b) Effects of biochar on availability and plant uptake of heavy metals – a meta-analysis. J Environ Manage 222:76–85. https://doi.org/10.1016/j.jenvman.2018.05.004
Chen H, Zheng M, Mao Q, **ao K, Wang K, Li D (2019) Cropland conversion changes the status of microbial resource limitation in degraded karst soil. Geoderma 352:197–203. https://doi.org/10.1016/j.geoderma.2019.06.018
Chen J, Chen D, Xu Q, Fuhrmann JJ, Li L, Pan G, Li Y, Qin H, Liang C, Sun X (2018a) Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol Fertil Soils 55:185–197. https://doi.org/10.1007/s00374-018-1333-2
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33. https://doi.org/10.1016/j.scitotenv.2016.08.190
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44. https://doi.org/10.1016/j.apsoil.2013.05.003
Clemente R, Bernal MP (2006) Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 64(8):1264–1273. https://doi.org/10.1016/j.chemosphere.2005.12.058
Cui HJ, Wang MK, Fu ML, Ci E (2011) Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soil Sediment 11:1135–1141. https://doi.org/10.1007/s11368-011-0405-9
Doi H, Cherif M, Iwabuchi T, Katano I, Stegen JC, Striebel M (2010) Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 119:752–765. https://doi.org/10.1111/j.1600-0706.2009.18540.x
Foster E, Fogle E, Cotrufo M (2018) Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 8(10):158. https://doi.org/10.3390/agriculture8100158
Garau M, Garau G, Diquattro S, Roggero PP, Castaldi P (2019) Mobility, bioaccessibility and toxicity of potentially toxic elements in a contaminated soil treated with municipal solid waste compost. Ecotoxicol Environ Saf 186:109766. https://doi.org/10.1016/j.ecoenv.2019.109766
Gil MV, Carballo MT, Calvo LF (2008) Fertilization of maize with compost from cattle manure supplemented with additional mineral nutrients. Waste Manag 28:1432–1440. https://doi.org/10.1016/j.wasman.2007.05.009
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Gusiatin ZM, Kulikowska D (2016) Behaviors of heavy metals (cd, cu, Ni, Pb and Zn) in soil amended with composts. Environ Technol 37:2337–2347. https://doi.org/10.1080/09593330.2016.1150348
Hessen DO (1997) Stoichiometry in food webs: Lotka revisited. Oikos 79:195–200. https://doi.org/10.2307/3546108
Houben D, Evrard L, Sonnet P (2013) Beneficial effects of biochar application to contaminated soils on the bioavailability of cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 57:196–204. https://doi.org/10.1016/j.biombioe.2013.07.019
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553. https://doi.org/10.1016/j.chemosphere.2017.01.130
Huang Z, Clinton PW, Baisden WT, Davis MR (2011) Long-term nitrogen additions increased surface soil carbon concentration in a forest plantation despite elevated decomposition. Soil Biol Biochem 43:302–307. https://doi.org/10.1016/j.soilbio.2010.10.015
Igalavithana AD, Lee SE, Lee YH, Tsang DC, Rinklebe J, Kwon EE, Ok YS (2017) Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 174:593–603. https://doi.org/10.1016/j.chemosphere.2017.01.148
James JE, Dean RD, Neil AM, John HS (1996) Organism Size, Life History, and N:P Stoichiometry: Toward a unified view of cellular and ecosystem processes. Bioscience 46(9):674–684. https://doi.org/10.2307/1312897
Ji M, Wang X, Usman M, Liu F, Dan Y, Zhou L, Campanaro S, Luo G, Sang W (2022) Effects of different feedstocks-based biochar on soil remediation: a review. Environ Pollut 294:118655. https://doi.org/10.1016/j.envpol.2021.118655
Jia W, Wang B, Wang C, Sun H (2017) Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J Environ Chem Eng 5:2107–2114. https://doi.org/10.1016/j.jece.2017.04.015
**do K, Sánchez-Monedero MA, Hernández T, García C, Furukawa T, Matsumoto K, Sonoki T, Bastida F (2012) Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci Total Environ 416:476–481. https://doi.org/10.1016/j.scitotenv.2011.12.009
Kamali M, Sweygers N, Al-Salem S, Appels L, Aminabhavi TM, Dewil R (2022) Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J 428:131189. https://doi.org/10.1016/j.cej.2021.131189
Kaurin A, Cernilogar Z, Lestan D (2018) Revitalisation of metal-contaminated, EDTA-washed soil by addition of unpolluted soil, compost and biochar: effects on soil enzyme activity, microbial community composition and abundance. Chemosphere 193:726–736. https://doi.org/10.1016/j.chemosphere.2017.11.082
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota-a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Li H, Li Y, Xu Y, Lu X (2020) Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 244:125471. https://doi.org/10.1016/j.chemosphere.2019.125471
Li M, Ren L, Zhang J, Luo L, Qin P, Zhou Y, Huang C, Tang J, Huang H, Chen A (2019) Population characteristics and influential factors of nitrogen cycling functional genes in heavy metal contaminated soil remediated by biochar and compost. Sci Total Environ 651:2166–2174. https://doi.org/10.1016/j.scitotenv.2018.10.152
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizão FJ (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213. https://doi.org/10.1016/j.orggeochem.2009.09.007
Liang J, Tang S, Gong J, Zeng G, Tang W, Song B, Zhang P, Yang Z, Luo Y (2020) Responses of enzymatic activity and microbial communities to biochar/compost amendment in sulfamethoxazole polluted wetland soil. J Hazard Mater 385:121533. https://doi.org/10.1016/j.jhazmat.2019.121533
Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X, Wu H, Qian Y, Li X, Luo Y (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288. https://doi.org/10.1016/j.chemosphere.2017.04.081
Liao N, Li Q, Zhang W, Zhou G, Ma L, Min W, Ye J, Hou Z (2016) Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. Eur J Soil Biol 72:27–34. https://doi.org/10.1016/j.ejsobi.2015.12.008
Liao X, Kang H, Haidar G, Wang W, Malghani S (2022) The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: a meta-analysis. Geoderma 411:115692. https://doi.org/10.1016/j.geoderma.2021.115692
Lin X, Wang N, Li F, Yan B, Pan J, Jiang S, Peng H, Chen WG, Zhang J, Zhang L, Huang H, Luo L (2022) Evaluation of the synergistic effects of biochar and biogas residue on CO2 and CH4 emission, functional genes, and enzyme activity during straw composting. Bioresour Technol 360:127608. https://doi.org/10.1016/j.biortech.2022.12760
Luo L, Meng H, Gu JD (2017) Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J Environ Manage 197:539–549. https://doi.org/10.1016/j.jenvman.2017.04.023
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22. https://doi.org/10.3389/fmicb.2014.00022
Parvage MM, Ulén B, Eriksson J, Strock J, Kirchmann H (2013) Phosphorus availability in soils amended with wheat residue char. Biol Fertil Soils 49:245–250. https://doi.org/10.1007/s00374-012-0746-6
Pokharel P, Ma Z, Chang SX (2020) Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar 2:65–79. https://doi.org/10.1007/s42773-020-00039-1
Qiu M, Liu L, Ling Q, Cai Y, Yu S, Wang S, Fu D, Hu B, Wang X (2022) Biochar for the removal of contaminants from soil and water: a review. Biochar 4:19. https://doi.org/10.1007/s42773-020-00039-1
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284. https://doi.org/10.1007/s00374-011-0624-7
Ren L, Cai C, Zhang J, Yang Y, Wu G, Luo L, Huang H, Zhou Y, Qin P, Yu M (2018) Key environmental factors to variation of ammonia-oxidizing archaea community and potential ammonia oxidation rate during agricultural waste composting. Bioresour Technol 270:278–285. https://doi.org/10.1016/j.biortech.2018.09.042
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Sanchez-Hernandez JC, Rio JM, Attademo AM, Malcevschi A, Andrade Cares X (2019) Assessing biochar impact on earthworms: implications for soil quality promotion. J Hazard Mater 366:582–591. https://doi.org/10.1016/j.jhazmat.2018.12.032
Sandhu S, Sekaran U, Ozlu E, Hoilett NO, Kumar S (2019) Short-term impacts of biochar and manure application on soil labile carbon fractions, enzyme activity, and microbial community structure. Biochar 1:271–282. https://doi.org/10.1007/s42773-019-00025-2
Sinsabaugh RL, Antibus RK, Linkins AE, Mcclaugherty CA, Rayburn L, Repert D, Weiland T (1992) Wood decomposition over a first-order watershed: mass loss as a function of lignocellulase activity. Soil Biol Biochem 24:743–749. https://doi.org/10.1016/0038-0717(92)90248-V
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. https://doi.org/10.1038/nature08632
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Sinsabaugh RL, Shah JJF (2010) Integrating resource utilization and temperature in metabolic scaling of riverine bacterial production. Ecology 91(5):1455–1465. https://doi.org/10.1890/08-2192.1
Tang J, Zhang J, Ren L, Zhou Y, Gao J, Luo L, Yang Y, Peng Q, Huang H, Chen A (2019) Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Environ Manage 242:121–130. https://doi.org/10.1016/j.jenvman.2019.04.061
Tang J, Zhang L, Zhang J, Ren L, Zhou Y, Zheng Y, Luo L, Yang Y, Huang H, Chen A (2020) Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci Total Environ 701:134751. https://doi.org/10.1016/j.scitotenv.2019.134751
Tapia-Torres Y, Elser JJ, Souza V, García-Oliva F (2015) Ecoenzymatic stoichiometry at the extremes: how microbes cope in an ultra-oligotrophic desert soil. Soil Biol Biochem 87:34–42. https://doi.org/10.1016/j.soilbio.2015.04.007
Vanden Nest T, Amery F, Fryda L, Boogaerts C, Bilbao J, Vandecasteele B (2021) Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci Total Environ 750:141699. https://doi.org/10.1016/j.scitotenv.2020.141699
Wallenius K, Rita H, Mikkonen A, Lappi K, Lindström K, Hartikainen H, Raateland A, Niemi RM (2011) Effects of land use on the level, variation and spatial structure of soil enzyme activities and bacterial communities. Soil Biol Biochem 43(7):1464–1473. https://doi.org/10.1016/j.soilbio.2011.03.018
Wang M, Fu Y, Wang Y, Li Y, Shen J, Liu X, Wu J (2021) Pathways and mechanisms by which biochar application reduces nitrogen and phosphorus runoff losses from a rice agroecosystem. Sci Total Environ 797:149193. https://doi.org/10.1016/j.scitotenv.2021.149193
Wang N, Awasthi MK, Pan J, Jiang S, Wan F, Lin X, Yan B, Zhang J, Zhang L, Huang H, Li H (2022a) Effects of biochar and biogas residue amendments on N2O emission, enzyme activities and functional genes related with nitrification and denitrification during rice straw composting. Bioresour Technol 357:127359. https://doi.org/10.1016/j.biortech.2022.127359
Wang N, Ren L, Zhang J, Awasthi MK, Yan B, Zhang L, Wan F, Luo L, Huang H, Zhao K (2022b) Activities of functional enzymes involved in C, N, and P conversion and their stoichiometry during agricultural waste composting with biochar and biogas residue amendments. Bioresour Technol 345:126489. https://doi.org/10.1016/j.biortech.2021.126489
Wang N, Zhao K, Li F, Peng H, Lu Y, Zhang L, Pan J, Jiang S, Chen A, Yan B, Luo L, Huang H, Li H, Wu G, Zhang J (2022c) Characteristics of carbon, nitrogen, phosphorus and sulfur cycling genes, microbial community metabolism and key influencing factors during composting process supplemented with biochar and biogas residue. Bioresour Technol 366:128224. https://doi.org/10.1016/j.biortech.2022.128224
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305(5683):509–513. https://doi.org/10.1126/science.1098778
Williams MA, Jangid K, Shanmugam SG, Whitman WB (2013) Bacterial communities in soil mimic patterns of vegetative succession and ecosystem climax but are resilient to change between seasons. Soil Biol Biochem 57:749–757. https://doi.org/10.1016/j.soilbio.2012.08.023
Wu H, Lai C, Zeng G, Liang J, Chen J, Xu J, Dai J, Li X, Liu J, Chen M, Lu L, Hu L, Wan J (2017) The interactions of composting and biochar and their implications for soil amendment and pollution remediation: a review. Crit Rev Biotechnol 37(6):754–764. https://doi.org/10.1080/07388551.2016.1232696
Xu Q, Xu Q, Zhu H, Li H, Yin W, Feng K, Wang S, Wang X (2022) Does biochar application in heavy metal-contaminated soils affect soil micronutrient dynamics? Chemosphere 290:133349. https://doi.org/10.1016/j.chemosphere.2021.133349
Xu Z, Yu G, Zhang X, He N, Wang Q, Wang S, Wang R, Zhao N, Jia Y, Wang C (2017) Soil enzyme activity and stoichiometry in forest ecosystems along the north-south transect in eastern China (NSTEC). Soil Biol Biochem 104:152–163. https://doi.org/10.1016/j.soilbio.2016.10.020
Yang X, Liu J, McGrouther K, Huang H, Lu K, Guo X, He L, Lin X, Che L, Ye Z (2016) Effect of biochar on the extractability of heavy metals (cd, cu, Pb, and Zn) and enzyme activity in soil. Environ Sci Pollut R 23:974–984. https://doi.org/10.1007/s11356-015-4233-0
Yang Y, Sun K, Han L, Chen Y, Liu J, **ng B (2022) Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol Biochem 169:108657. https://doi.org/10.1016/j.soilbio.2022.108657
Zeng G, Wu H, Liang J, Guo S, Huang L, Xu P, Liu Y, Yuan Y, He X, He Y (2015) Efficiency of biochar and compost (or composting) combined amendments for reducing cd, cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. RSC Adv 5(44):34541–34548. https://doi.org/10.1039/c5ra04834f
Zeng G, Zhang J, Chen Y, Yu Z, Yu M, Li H, Liu Z, Chen M, Lu L, Hu C (2011) Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting. Bioresour Technol 102(19):9026–9032. https://doi.org/10.1016/j.biortech.2011.07.076
Zeng G, Zhang L, Dong H, Chen Y, Zhang J, Zhu Y, Yuan Y, **e Y, Fang W (2018) Pathway and mechanism of nitrogen transformation during composting: functional enzymes and genes under different concentrations of PVP-AgNPs. Bioresour Technol 253:112–120. https://doi.org/10.1016/j.biortech.2017.12.095
Zhang J, He Y, Fang Y, Zhao K, Wang N, Zhou Y, Luo L, Yang Y (2021) Characteristics and influencing factors of microbial Community in Heavy Metal Contaminated Soil under silicon fertilizer and biochar remediation. Adsorpt Sci Technol 2021:1–10. https://doi.org/10.1155/2021/9964562
Zhang L, **ang Y, **g Y, Zhang R (2019) Biochar amendment effects on the activities of soil carbon, nitrogen, and phosphorus hydrolytic enzymes: a meta-analysis. Environ Sci Pollut R 26:22990–23001. https://doi.org/10.1007/s11356-019-05604-1
Zhang X, Qin B, Deng J, Wells M (2017) Whole-cell bioreporters and risk assessment of environmental pollution: a proof-of-concept study using lead. Environ Pollut 229:902–910. https://doi.org/10.1016/j.envpol.2017.07.068
Zhao K, Yang Y, Peng H, Zhang L, Zhou Y, Zhang J, Du C, Liu J, Lin X, Wang N, Huang H, Luo L (2022) Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci Total Environ 822:153483. https://doi.org/10.1016/j.scitotenv.2022.153483
Zhao K, Yang Y, Zhang L, Zhang J, Zhou Y, Huang H, Luo S, Luo L (2021) Silicon-based additive on heavy metal remediation in soils: toxicological effects, remediation techniques, and perspectives. Environ Res 205:112244. https://doi.org/10.1016/j.envres.2021.112244
Zhu Z, Ge T, Luo Y, Liu S, Xu X, Tong C, Shibistova O, Guggenberger G, Wu J (2018) Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol Biochem 121:67–76. https://doi.org/10.1016/j.soilbio.2018.03.003
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43(6):1169–1179. https://doi.org/10.1016/j.soilbio.2011.02.005
Acknowledgements
Not applicable.
Funding
This research was financially supported by the National Key Research and Development Program of China (No. 2018YFD0500205), the Science and Technology project of Changsha (No. kh1801219), and the National Natural Science Foundation of China (51408219).
Author information
Authors and Affiliations
Contributions
KZ: Software, Formal analysis, Writing – original draft; NW: Data curation, Writing – original draft; SJ: Data curation, Writing – original draft; FL: Formal analysis, Writing – original draft; SL: Writing – review & editing; AC: Conceptualization, Methodology; YZ: Writing – review & editing; HL: Conceptualization, Methodology; XL: Formal analysis, Writing – review & editing; JZ: Supervision, Project administration, Writing – review & editing; LZ: Conceptualization, Methodology; HH: Conceptualization, Methodology; LL: Conceptualization, Methodology, Software, Writing – review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The corresponding author consents on behalf of all the authors that this is original work and has permission to be published.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, K., Wang, N., Jiang, S. et al. Potential implications of biochar and compost on the stoichiometry-based assessments of soil enzyme activity in heavy metal-polluted soils. carbon res 1, 29 (2022). https://doi.org/10.1007/s44246-022-00029-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-022-00029-x