Abstract
Background
Conduct disorder (CD) involves a group of behavioral and emotional problems that usually begins during childhood or adolescence. Structural brain alterations have been observed in CD, including the amygdala, insula, ventrolateral and medial prefrontal cortex, anterior cingulate cortex, and fusiform gyrus. The current study developed a multivariate generalized linear model (GLM) to differentiate adolescents with CD from typically develo** (TD) adolescents in terms of grey matter volume (GMV).
Methods
The whole‐brain structural MRI data were collected from 96 adolescents with CD (mean age = \(16.188\pm 1.259\) years; mean IQ = \(104.292\pm 8.107\); 63 males) and 90 TD individuals (mean age = \(15.956\pm 1.506\) years; mean IQ = \(106.622\pm 9.076\); 59 males) matched on age, IQ, and sex. Region-wise GMV was extracted following whole-brain parcellation into 68 cortical and 14 subcortical regions for each participant. A multivariate GLM was developed to predict the GMV of the pre-hypothesized regions-of-interest (ROIs) based on CD diagnosis, with intracranial volume, age, sex, and IQ serving as the covariate.
Results
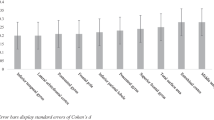
A diagnosis of CD was a significant predictor for GMV in the right pars orbitalis, right insula, right superior temporal gyrus, left fusiform gyrus, and left amygdala (F(1, 180) = 5.460–10.317, p < 0.05, partial eta squared = 0.029–0.054). The CD participants had smaller GMV in these regions than the TD participants (MCD–MTD = [− 614.898] mm3–[− 53.461] mm3).
Conclusions
Altered GMV within specific regions may serve as a biomarker for the development of CD in adolescents. Clinical work can potentially target these biomarkers to treat adolescents with CD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conduct disorder (CD) is a serious behavioral and emotional disorder that emerges in childhood and adolescence. It is characterized by a repetitive and persistent pattern of behavior in which the basic rights of others and/or major age-approximate social norms are violated [1]. CD is often seen as the precursor to adult antisocial personality disorder, thus has adverse long-term outcomes in both mental and physical domains of health [2]. CD is associated with an exceptionally high societal and economic burden, accounting for ~ 1% of all years lived with disability and surpassing autism spectrum disorders and attention-deficit/hyperactivity disorder (ADHD) in this measure of global health burden [3]. One significant critique of the diagnostic criteria for CD is that they solely rely on behavioral symptoms, providing little insight into the cognitive and emotional mechanisms that underlie these symptoms [4].
Recent functional neuroimaging studies have revealed that CD is associated with neurocognitive impairments in emotion processing [5], threat response [6, 7] and reinforcement-based decision-making [8,9,10]. The ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), insula, fusiform gyrus, temporal gyrus, and amygdala are particularly implicated as critical regions for the pathophysiology of CD. In addition, research has shown CD has structural abnormalities in the overlap** brain regions, suggesting that neural activity deficits may have a structural basis as well [8].
Previously, structural magnetic resonance imaging (sMRI) has been used to investigate the anatomical brain changes underlying CD, reflected by various morphometry parameters, including grey matter volume (GMV; i.e., cortical and subcortical volume [CV/SCV]). While a clear and consistent picture of the structural brain alterations seen in patients with CD remains lacking, two meta-analyses (each based on over 10 studies) of youth with CD, oppositional defiant disorder (ODD), or conduct problems concluded these conditions were associated with reductions in volume in cortical (ventrolateral, medial prefrontal, middle temporal, superior temporal, and anterior insular cortices) and subcortical (amygdala, caudate and putamen) regions [11, 12] though it should be noted that there have been several reports of both increased GMV in participants with CD relative to the controls in many of these regions since these meta-analyses were published [13,14,15] and a lack of group differences in GMV [16,17,18,19]. Within the pathology of CD, recent longitudinal studies suggested that higher CD-related symptomatology was associated with accelerated age-related cortical thinning [20, 21]. Specifically, Albaugh et al. [20] investigated a large community-based sample of 1039 adolescents. It revealed that a higher level of conduct problems (CP) was associated with reduced cortical thickness in late adolescence in the left rostral anterior cingulate, bilateral insula, and left inferior parietal cortices and this association was absent in early adolescence.
Moreover, there is a growing concern within the field of neuroimaging regarding replicability. For instance, MuGuire et al. [22] identified moderate-to-low reproducibility in sMRI when estimating gray matter thickness in several regions. Recent inquiries have also cast doubt on the replicability of structural brain-behavior associations [23, 24]. In developmental neuroimaging studies, maintaining consistency in repeated imaging has become increasingly challenging. This challenge arises from issues such as the difficulty in recruiting sufficiently large sample sizes within a limited age range and higher levels of in-scanner motion [25].
The current study primarily aimed to explore the structural brain alterations associated with CD using multivariate generalized linear modeling (GLM). We used the diagnosis of CD to predict the volumes of pre-hypothesized regions-of-interest (ROIs) taken from the meta-analysis by Rogers and De Brito [11]. The secondary aim of the current study was to evaluate if volume reduction in these ROIs reported by Rogers and De Brito [11] could be replicated. The ROIs we adopted were the right pars opercularis, right pars orbitalis, right pars triangularis, left superior frontal gyrus, right caudal anterior cingulate gyrus, bilateral insula, right superior temporal gyrus, left fusiform gyrus, and left amygdala (see Table 2, [11]). The diagnosis of CD was used as the predictor of the model, and intracranial volume, age, sex, and IQ served as the covariates. We hypothesized a significant association between the diagnosis of CD and reduced volume of the pre-hypothesized brain regions.
2 Methods
2.1 Participants
We included data from 186 young individuals aged ≥ 14 years (with IQ > 84; [26, 27]) from a residential care program and the surrounding community (mean age = \(16.075\pm 1.385\) years; mean IQ = \(105.419\pm 8.645\); 122 males). Youth recruited from the residential care program had been referred for behavioral and mental health problems. Participants from the community were recruited through flyers or social media. There were two groups of participants: participants with CD (N = 96; mean age = \(16.188\pm 1.259\) years; mean IQ = \(104.292\pm 8.107\); 63 males) and typically develo** (TD) adolescents (N = 90; mean age = \(15.956\pm 1.506\) years; mean IQ = \(106.622\pm 9.076\); 59 males); see Table 1.
In order to adhere to common clinical practice, diagnoses were determined via detailed interviews with child and adolescent psychiatrists and the participants and parents. The Boys Town National Research Hospital (BTNRH) institutional review board approved this study. A doctoral-level researcher or a member of the clinical research team obtained written informed consent and assent. In all cases, the youth had the right to decline participation at any time before or during the study. With respect to community participants, informed consent was obtained from the youths’ parents/legal guardians at the beginning of the on-site screening. After that, informed assent was obtained from the youth themselves. This procedure differed slightly for youth recruited from the Boys Town campus. Consent was typically obtained from parents during or shortly after the child’s arrival at Boys Town. Assent was obtained from the youth in a separate session, 5–10 days after parental consent had been obtained.
Exclusion criteria included IQ < 75 assessed with the Wechsler Abbreviated Scale of Intelligence (WASI two-subtest form; Wechsler, 2011), pregnancy, non-psychiatric medical conditions that require the use of medication that may have psychotropic effects (e.g., beta blockers or steroids), current psychosis, pervasive developmental disorders, Tourette’s disorder, neurological disorders, presence of metallic objects in the body (e.g., metal plates, pacemakers, etc.), and claustrophobia. Current psychiatric conditions (other than psychotic disorders or pervasive developmental disorders) were not exclusionary. The use of psychotropic medications for psychiatric indications (e.g., stimulants, selective serotonin reuptake inhibitors) was not exclusory. However, participants on stimulant medication were asked to withhold medication on the morning of the scan.
2.2 Data collection
2.2.1 Neuroanatomical data
High-resolution structural MRI (T1-weighted) data for each participant was collected using the same 3-Tesla Siemens MRI scanner located at BTNRH. Each participant was instructed to rest, relax, and try their best to minimize head movement during the entire scan. Whole‐brain anatomical data for each participant were acquired using a 3D magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence, which consisted of 176 axial slices (slice thickness = 1 mm, voxel resolution = 0.9 × 0.9 × 1 mm3, repetition time = 2200 ms; echo time = 2.48 ms; matrix size = 256 × 208; field of view (FOV) = 230 mm, and flip angle = 8°).
2.2.2 Measures
Measures included the conduct problems subscale from the Strength and Difficulties Questionnaire (SDQ-CP: [28]), the Reactive/Proactive Rating Scale (RPRS; [29]) to provide information on the severity of aggression in this population, and the Conners ADHD scale [30] to provide information on the severity of ADHD symptomatology in this population. The SDQ-CP shows moderate test–retest reliability [31] and good concurrent validity [32]. Previous research has found evidence supporting the reliability and validity of subscales of the RPRS [33, 34]. The Conners ADHD also had excellent test–retest reliability of 0.89 [35].
2.3 Image preprocessing
The recon‐all pipeline from the FreeSurfer toolbox (Version 6.0) was used to process the anatomical brain images [36, 37] and estimate GMV (i.e., CV and SCV). Structural images were processed following the basic image preprocessing steps, including head motion‐correction, brain extraction, automated transformation to the standard MNI template space, volumetric segmentation into cortical and subcortical matter, intensity correction, and parcellation of the cerebral cortex into gyral and sulcal matter [38]. For more technical details about the preprocessing steps, interested readers can access previous publications [36, 37, 39]. After preprocessing, we conducted quality control (QC) on each scan using the ENIGMA cortical surface segmentation protocol (for full details and scripts, please see www.enigma.ini.usc.edu). This QC process involved several steps, including outlier detection, visual inspection of surface segmentations for individual scans, and examination of external views of segmentations. Subjects exhibiting structural outliers underwent further scrutiny to address segmentation issues. MATLAB scripts were employed to assess both internal and external surfaces, ensuring the completeness and accuracy of reconstructions, including the presence of all lobes and correct labeling. Subjects were categorized as "pass" (no issues), "moderate" (some regions with potential issues), or "fail" (significant issues). The study initially recruited 1070 participants, with structural MRI data available from 616 participants who successfully passed the QC inspection. Of these 616, a sample of 378 participants (181 were healthy and 197 were diagnosed with CD) were aged ≥ 14 years and had IQ above 84. The final set of participants in healthy (N = 90) and CD (N = 96) categories were further selected so that the groups matched on age, IQ, and sex—this included the exclusion of (a) participants with disorders (other than CD); (b) participants with highest IQ; (c) youngest controls; (d) participants with lowest IQ; and (e) oldest participants, respectively. This process occurred before the analysis of the sMRI data.
2.4 Data extraction and preparation
The whole brain was parcellated into 68 cortical regions (i.e., 34 regions for each hemisphere) based on Desikan’s atlas [38]. CV data from 68 cortical regions, SCV data from 14 subcortical regions (i.e., 7 regions for each hemisphere), and intracranial volume (ICV; a measure of head size) data were evaluated using the recon‐all, mri_surf2surf, mris_anatomical_stats, and aparcstats2table pipelines developed by FreeSurfer.
2.5 Data analysis
2.5.1 Clinical data
Group differences in age, IQ, and scores in SDQ-CP, RPRS, and Connor’s ADHD scale were examined via independent samples t tests. Group difference in sex was examined via chi-squared tests.
2.5.2 Multivariate generalized linear model
The volumes of the right pars opercularis, right pars orbitalis, right pars triangularis, left superior frontal gyrus, right caudal anterior cingulate gyrus, bilateral insula, right superior temporal gyrus, left fusiform gyrus, and left amygdala were used as the dependent variables of the multivariate GLM. The diagnosis of CD was entered as the predictor of the model. ICV, age, sex, and IQ served as the covariates.
2.6 Follow-up analyses
2.6.1 Potential confounds
A number of participants with CD were also diagnosed with major depression disorder (MDD; N = 16), generalized anxiety disorder (GAD; N = 26), posttraumatic stress disorder (PTSD; N = 16), and had prescribed medications (e.g., antipsychotic medications, selective serotonin reuptake inhibitors [SSRIs], and/or stimulants; N = 37). Our multivariate GLM analysis was therefore repeated four times, once excluding the MDD participants, once excluding the GAD participants, once excluding the PTSD participants, and once excluding the prescribed participants.
2.6.2 Whole-brain grey matter volume
To examine if the CD participants and TD participants had different whole-brain GMV, a GLM analysis was conducted on the whole-brain GMV using the diagnosis of CD as the predictor and ICV, age, sex, and IQ as the covariates.
2.6.3 Aging effects
In order to address the effects of aging, we formed a new variable by splitting the current CD participants according to the median age and ran a multivariate GLM analysis on the volumes of the pre-hypothesized ROIs by using this new variable as the predictor, and ICV, sex, and IQ as the covariates.
3 Results
3.1 Clinical data
As expected, there were no group differences in age, sex, or IQ (p’s > 0.05); see Table 1. As expected, participants with CD scored significantly higher than TD participants on the SDQ-CP, the RPRS, and Connor’s ADHD scale; see Table 1.
3.2 Multivariate generalized linear model
There was a significant difference in GMV of the pre-hypothesized ROIs based on if the diagnosis of CD was made (F(10, 171) = 2.079, p = 0.029, \({\eta }_{p}^{2}\) = 0.108, Wilks’ Lambda = 0.892). The diagnosis of CD was a significant predictor for GMV in the right pars orbitalis, right superior temporal gyrus, right insula, left fusiform gyrus, and left amygdala (F(10, 171) = 5.460–10.317, p < 0.05, \({\eta }_{p}^{2}\) = 0.029–0.054; Fig. 1 & Table 2). Within each of these regions, the CD participants had smaller GMV than the TD participants (MCD–MTD = [− 614.898] mm3–[− 53.461] mm3).
3.3 Follow-up analyses
3.3.1 Potential confounds
Given the significant associations of CD with other diagnoses and prescribed medications (see Table 1), our multivariate GLM analysis was repeated four times, once excluding the MDD participants (N = 16), once excluding the GAD participants (N = 26), once excluding the PTSD participants (N = 16), and once excluding the prescribed participants (N = 37). The four analyses largely replicated the results of our main analysis except that (a) the right pars orbitalis was now trending towards significance when MDD was removed (p = 0.057); and (b) the right caudal anterior cingulate gyrus was significant when GAD was removed (F(10, 145) = 4.940, p = 0.028, \({\eta }_{p}^{2}\) = 0.03; for full details, see online Supplementary Table S1–S4).
3.3.2 Whole-brain grey matter volume
To examine if the CD participants and TD participants had different whole-brain GMV, a GLM analysis was conducted on the whole-brain GMV using the same predictor and covariates. The results indicated the CD participants had significantly smaller whole-brain GMV than the TD participants (Mean whole-brain GMV for CD = 590,036 mm3, Mean whole-brain GMV for TD = 607,538 mm3, F(1, 180) = 12.412, p < 0.05, \({\eta }_{p}^{2}\) = 0.065).
3.3.3 Aging effects
In order to evaluate the aging effects, we divided the current CD participants according to the median age and ran the multivariate GLM analysis on the volumes of the pre-hypothesized ROIs of using this new variable as the predictor, and ICV, sex, and IQ as the covariates. The results indicated there was no significant difference in GMV of the pre-hypothesized ROIs based on if the CD participants were older than the median age or not (F(10, 82) = 0.775, p = 0.652, \({\eta }_{p}^{2}\) = 0.086, Wilks’ Lambda = 0.914). For full details, see online Supplementary Table S5.
4 Discussion
The goal of the current study was to determine the extent to which diagnosis of CD was predicted by atypical volumes within regions identified as atypical in patients with CD in previous meta-analytic work [11]. By implementing multivariate GLM, we demonstrated that diagnosis of CD was predicted by significant differences in volumes of left amygdala, right insula, right pars orbitalis, right superior temporal gyrus, and left fusiform gyrus. In all cases, the participants with CD had smaller GMV than the TD participants. Our results demonstrated the findings of Rogers and De Brito [11] as largely replicable.
The reduction of GMV in the amygdala was consistently demonstrated by earlier CD work [40,41,42,43,44,45]. Moreover, Rogers and De Brito [11] noted in their review that grey matter reduction in the left amygdala in youths with CP was the most reliable finding that they observed. The current finding of reduced amygdala volume in the CD sample further supports this conclusion. The amygdala is involved in functional processes that have been found to be disrupted in patients with CD including aversive conditioning, decision-making, empathy, and emotional and threat processing [46,47,48,49,50,51]. Our finding is thus consistent with previous functional MRI data indicating atypical amygdala responding in tasks probing those processes in CD [52,53,54,55].
The insula is strongly implicated in empathy and risky decision-making [56]. The role of the insula in empathy has been supported by numerous neuroimaging studies reporting activation in response to others in pain [57]. Its role in risky decisions has been evidenced by robust insular activation in functional MRI studies during gambling tasks, in which the participants had to decide between options associated with uncertain outcomes [58, 59]. Rogers and De Brito [11] found that youths with CP exhibited reduced GMV in the bilateral anterior insula, while the volume of the right insula was reduced in the current CD sample. Reduced volume in the insula has been reported in several CD studies [40, 42, 45] and we replicated these findings. This result aligned with the functional MRI studies reporting altered insula response in youths with CD while watching others with emotional suffering [60, 61] and during decision-making [62]. It suggested that structural abnormality within the insula might partly underlie impaired empathy and poor decision-making in youths with CD.
The superior temporal gyrus has been involved in the perception of facial emotions and social cognition [63,64,65]. Previous functional MRI studies reported changed activity in the superior temporal gyrus during the processing of emotional faces versus neutral faces in male CD adolescents [54]. In addition, it was reported that the lifetime CD symptoms in female adolescents were negatively associated with activity in the superior temporal gyrus during face processing [66]. The current finding of decreased volume in the superior temporal gyrus in CD aligned with the findings of functional alternation in this region. The current structural finding was consistent with the meta-analysis conducted by Rogers and De Brito [11], although increased volume in the superior temporal gyrus was also reported [13, 67].
CD participants in the current study had smaller right pars orbitalis compared with the TD youth. The pars orbitalis has been shown to be involved in behavioral and motor inhibition and deductive reasoning [68]. The abnormalities within this region may disrupt the internal, intentional cognitive processes of individuals with CD, thereby impeding their ability to anticipate future outcomes of their actions and adhere to socially acceptable behavior.
Further, we observed decreased volume of the left fusiform gyrus in participants with CD. Few previous studies on CD have identified abnormalities in the structural or functional aspects of the visual system. Our discovery in the fusiform gyrus corresponded with the results of a previous study by Jiang et al. [69], which reported a decrease in cortical thickness in the same area in individuals with CD.
Several caveats should be considered with respect to the current results. First, many participants with CD had other psychiatric diagnoses (i.e., MDD, PTSD, and GAD) and/or were prescribed several psychiatric medications (i.e., antipsychotic medications, SSRIs, or stimulants). Ameliorating this concern, the follow-up analyses largely replicated the results of our main analysis. This suggests other psychiatric diagnoses or prescribed medications did not significantly affect the current results. Second, a follow-up GLM analysis revealed that the CD participants exhibited reduced whole-brain GMV compared to the TD participants. However, the primary research findings included ICV as one of the covariates. As such, our primary research findings could not be attributed to head size/total GMV differences between groups. Third, according to a follow-up multivariate GLM analysis, we observed no significant difference in GMV of the pre-hypothesized ROIs based on if the CD participants were older than the median age or not; i.e., within this sample, older CD participants did not show reduced GMV relative to younger CD participants—in contrast to some previous studies suggesting that higher CD-related symptomatology was associated with accelerated age-related cortical alteration. Fourth, diagnostic status was determined following clinical practice and included an interview by a board-certified psychiatrist rather than the implementation of a structured or semi-structured diagnostic interview. While the diagnosis method we used could raise concerns regarding the CD diagnoses, it is important to note that these diagnoses were supported by the SDQ-CP scores.
In conclusion, we found that volumes of the left amygdala, right insula, right pars orbitalis, right superior temporal gyrus, and left fusiform gyrus were reduced in the CD participants relative to the TD participants. These regions may serve as biomarkers for the development of CD in adolescents. Clinical work can potentially target those biomarkers for future therapeutic interventions as well as predict patients’ disease trajectories.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to IRB restrictions.
References
APA. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Blair RJ, Leibenluft E, Pine DS. Conduct disorder and callous-unemotional traits in youth. N Engl J Med. 2014;371(23):2207–16. https://doi.org/10.1056/NEJMra1315612.
Erskine HE, Ferrari AJ, Polanczyk GV, et al. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry. 2014;55(4):328–36. https://doi.org/10.1111/jcpp.12186.
Blair RJR, Mitchell D, Blair K. The psychopath. Emotion and the brain: Blackwell, 2005.
Alegria AA, Radua J, Rubia K. Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry. 2016;173(11):1119–30. https://doi.org/10.1176/appi.ajp.2016.15081089.
Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57(1):7–15. https://doi.org/10.1016/j.biopsych.2004.10.008.
Hwang S, Nolan ZT, White SF, Williams WC, Sinclair S, Blair RJ. Dual neurocircuitry dysfunctions in disruptive behavior disorders: emotional responding and response inhibition. Psychol Med. 2016;46(7):1485–96.
Fairchild G, Hawes DJ, Frick PJ, et al. Conduct disorder. Nat Rev Dis Primers. 2019;5(1):43. https://doi.org/10.1038/s41572-019-0095-y.
Blair RJR, Veroude K, Buitelaar JK. Neuro-cognitive system dysfunction and symptom sets: a review of fMRI studies in youth with conduct problems. Neurosci Biobehav Rev. 2018;91:69–90. https://doi.org/10.1016/j.neubiorev.2016.10.022.
Zhang R, Aloi J, Bajaj S, et al. Dysfunction in differential reward-punishment responsiveness in conduct disorder relates to severity of callous-unemotional traits but not irritability. Psychol Med. 2021. https://doi.org/10.1017/s0033291721003500.
Rogers JC, De Brito SA. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiat. 2016;73(1):64–72. https://doi.org/10.1001/jamapsychiatry.2015.2423.
Noordermeer SD, Luman M, Oosterlaan J. A Systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol Rev. 2016;26(1):44–72. https://doi.org/10.1007/s11065-015-9315-8.
Gao Y, Jiang Y, Ming Q, et al. Gray matter changes in the orbitofrontal-paralimbic cortex in male youths with non-comorbid conduct disorder. Front Psychol. 2020. https://doi.org/10.3389/fpsyg.2020.00843.
Gao Y, Jiang Y, Ming Q, et al. Neuroanatomical changes associated with conduct disorder in boys: influence of childhood maltreatment. Eur Child Adolesc Psychiatry. 2021. https://doi.org/10.1007/s00787-020-01697-z.
Zhang J, Liu W, Zhang J, et al. Distinguishing adolescents with conduct disorder from typically develo** youngsters based on pattern classification of brain structural MRI. Front Hum Neurosci. 2018;12:152. https://doi.org/10.3389/fnhum.2018.00152.
Hummer TA, Wang Y, Kronenberger WG, Dunn DW, Mathews VP. The relationship of brain structure to age and executive functioning in adolescent disruptive behavior disorder. Psychiatry Res. 2015;231(3):210–7. https://doi.org/10.1016/j.pscychresns.2014.11.009.
Michalska KJ, Decety J, Zeffiro TA, Lahey BB. Association of regional gray matter volumes in the brain with disruptive behavior disorders in male and female children. Neuroimage Clin. 2015;7:252–7. https://doi.org/10.1016/j.nicl.2014.12.012.
Vetter NC, Backhausen LL, Buse J, Roessner V, Smolka MN. Altered brain morphology in boys with attention deficit hyperactivity disorder with and without comorbid conduct disorder/oppositional defiant disorder. Hum Brain Mapp. 2020;41(4):973–83. https://doi.org/10.1002/hbm.24853.
Olvera RL, Glahn DC, O’Donnell L, et al. Cortical volume alterations in conduct disordered adolescents with and without bipolar disorder. J Clin Med. 2014;3(2):416–31. https://doi.org/10.3390/jcm3020416.
Albaugh MD, Hudziak JJ, Spechler PA, et al. Conduct problems are associated with accelerated thinning of emotion-related cortical regions in a community-based sample of adolescents. Psychiatry Res Neuroimaging. 2023;330: 111614. https://doi.org/10.1016/j.pscychresns.2023.111614.
Jirsaraie RJ, Kaczkurkin AN, Rush S, et al. Accelerated cortical thinning within structural brain networks is associated with irritability in youth. Neuropsychopharmacology. 2019;44(13):2254–62. https://doi.org/10.1038/s41386-019-0508-3.
McGuire SA, Wijtenburg SA, Sherman PM, et al. Reproducibility of quantitative structural and physiological MRI measurements. Brain Behav. 2017;7(9): e00759. https://doi.org/10.1002/brb3.759.
Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–60. https://doi.org/10.1038/s41586-022-04492-9.
Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, Genon S, Alzheimer’s Disease Neuroimaging Initiative. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife. 2019. https://doi.org/10.7554/eLife.43464.
Klapwijk ET, van den Bos W, Tamnes CK, Raschle NM, Mills KL. Opportunities for increased reproducibility and replicability of developmental neuroimaging. Dev Cogn Neurosci. 2021;47: 100902. https://doi.org/10.1016/j.dcn.2020.100902.
Melby L, Indredavik MS, Løhaugen G, Brubakk AM, Skranes J, Vik T. Is there an association between full IQ score and mental health problems in young adults? A study with a convenience sample. BMC Psychol. 2020;8(1):7. https://doi.org/10.1186/s40359-020-0372-2.
Diekema DS. Adolescent brain development and medical decision-making. Pediatrics. 2020;146(Suppl 1):S18–24. https://doi.org/10.1542/peds.2020-0818F.
Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–6. https://doi.org/10.1111/j.1469-7610.1997.tb01545.x.
Dodge KA, Coie JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146–58. https://doi.org/10.1037//0022-3514.53.6.1146.
Conners CK. Clinical use of rating scales in diagnosis and treatment of attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 1999;46(5):857–70. https://doi.org/10.1016/s0031-3955(05)70159-0.
Yao S, Zhang C, Zhu X, **g X, McWhinnie CM, Abela JR. Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J Adolesc Health. 2009;45(1):55–62. https://doi.org/10.1016/j.jadohealth.2008.11.006.
Muris P, Meesters C, van den Berg F. The strengths and difficulties questionnaire (SDQ)–further evidence for its reliability and validity in a community sample of Dutch children and adolescents. Eur Child Adolesc Psychiatry. 2003;12(1):1–8. https://doi.org/10.1007/s00787-003-0298-2.
Dodge KA, Lochman JE, Harnish JD, Bates JE, Pettit GS. Reactive and proactive aggression in school children and psychiatrically impaired chronically assaultive youth. J Abnorm Psychol. 1997;106(1):37–51. https://doi.org/10.1037//0021-843x.106.1.37.
Evans SC, Fite PJ, Hendrickson ML, Rubens SL, Mages AK. The role of reactive aggression in the link between hyperactive-impulsive behaviors and peer rejection in adolescents. Child Psychiatry Hum Dev. 2015;46(6):903–12. https://doi.org/10.1007/s10578-014-0530-y.
Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self-ratings of ADHD symptomas in auts II: reliability, validity, and diagnostic sensitivity. J Atten Disord. 1999;3(3):153–8. https://doi.org/10.1177/108705479900300304.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. https://doi.org/10.1006/nimg.1998.0395.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. https://doi.org/10.1006/nimg.1998.0396.
Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. https://doi.org/10.1093/cercor/bhg087.
Fairchild G, Passamonti L, Hurford G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011;168(6):624–33. https://doi.org/10.1176/appi.ajp.2010.10081184.
Huebner T, Vloet TD, Marx I, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(5):540–7. https://doi.org/10.1097/CHI.0b013e3181676545.
Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–42. https://doi.org/10.1016/j.neuroimage.2007.04.043.
Wallace GL, White SF, Robustelli B, et al. Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. J Am Acad Child Adolesc Psychiatry. 2014;53(4):456-65.e1. https://doi.org/10.1016/j.jaac.2013.12.008.
Stevens MC, Haney-Caron E. Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. J Psychiatry Neurosci. 2012;37(6):389–98. https://doi.org/10.1503/jpn.110148.
Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54(1):86–95. https://doi.org/10.1111/j.1469-7610.2012.02617.x.
Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–92. https://doi.org/10.1038/nature14188.
Gupta R, Koscik TR, Bechara A, Tranel D. The amygdala and decision-making. Neuropsychologia. 2011;49(4):760–6. https://doi.org/10.1016/j.neuropsychologia.2010.09.029.
Wang Z, Li Y, Childress AR, Detre JA. Brain entropy map** using fMRI. PLoS ONE. 2014;9(3): e89948. https://doi.org/10.1371/journal.pone.0089948.
Fernando AB, Murray JE, Milton AL. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci. 2013;7:190. https://doi.org/10.3389/fnbeh.2013.00190.
Fox AS, Oler JA, Tromp DP, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38(5):319–29. https://doi.org/10.1016/j.tins.2015.03.002.
Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–93. https://doi.org/10.1093/brain/122.5.883.
Fehlbaum LV, Raschle NM, Menks WM, et al. Altered neuronal responses during an affective stroop task in adolescents with conduct disorder. Front Psychol. 2018;9:1961. https://doi.org/10.3389/fpsyg.2018.01961.
Aggensteiner PM, Holz NE, Böttinger BW, et al. The effects of callous-unemotional traits and aggression subtypes on amygdala activity in response to negative faces. Psychol Med. 2022;52(3):476–84. https://doi.org/10.1017/S0033291720002111.
Passamonti L, Fairchild G, Goodyer IM, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67(7):729–38. https://doi.org/10.1001/archgenpsychiatry.2010.75.
Blair RJR, Zhang R. Recent neuro-imaging findings with respect to conduct disorder, callous-unemotional traits and psychopathy. Curr Opin Psychiatry. 2020;33(1):45–50. https://doi.org/10.1097/YCO.0000000000000559.
Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34(4):300–6. https://doi.org/10.1097/WNP.0000000000000377.
Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35(3):903–11. https://doi.org/10.1016/j.neubiorev.2010.10.009.
Clark L, Studer B, Bruss J, Tranel D, Bechara A. Damage to insula abolishes cognitive distortions during simulated gambling. Proc Natl Acad Sci U S A. 2014;111(16):6098–103. https://doi.org/10.1073/pnas.1322295111.
Von Siebenthal Z, Boucher O, Rouleau I, Lassonde M, Lepore F, Nguyen DK. Decision-making impairments following insular and medial temporal lobe resection for drug-resistant epilepsy. Soc Cogn Affect Neurosci. 2017;12(1):128–37. https://doi.org/10.1093/scan/nsw152.
Michalska KJ, Zeffiro TA, Decety J. Brain response to viewing others being harmed in children with conduct disorder symptoms. J Child Psychol Psychiatry. 2016;57(4):510–9. https://doi.org/10.1111/jcpp.12474.
Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80(2):203–11. https://doi.org/10.1016/j.biopsycho.2008.09.004.
Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166(1):83–94. https://doi.org/10.1176/appi.ajp.2008.08020212.
Bigler ED, Mortensen S, Neeley ES, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31(2):217–38. https://doi.org/10.1080/87565640701190841.
Radua J, Phillips ML, Russell T, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49(1):939–46. https://doi.org/10.1016/j.neuroimage.2009.08.030.
Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–58. https://doi.org/10.1002/hbm.20547.
Fairchild G, Hagan CC, Passamonti L, Walsh ND, Goodyer IM, Calder AJ. Atypical neural responses during face processing in female adolescents with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(6):677-87.e5. https://doi.org/10.1016/j.jaac.2014.02.009.
De Brito SA, Mechelli A, Wilke M, et al. Size matters: Increased grey matter in boys with conduct problems and callous–unemotional traits. Brain. 2009;132(4):843–52. https://doi.org/10.1093/brain/awp011.
Wildgruber D, Hertrich I, Riecker A, et al. Distinct frontal regions subserve evaluation of linguistic and emotional aspects of speech intonation. Cereb Cortex. 2004;14(12):1384–9. https://doi.org/10.1093/cercor/bhh099.
Jiang Y, Guo X, Zhang J, et al. Abnormalities of cortical structures in adolescent-onset conduct disorder. Psychol Med. 2015;45(16):3467–79. https://doi.org/10.1017/S0033291715001361.
Acknowledgements
We would like to thank Ron Copsey, Kim VanHorn, Michael Wright, Mark Timm, and Rhonda Tuel for their contributions to data collection. We would like to thank all participants and their families for their participation.
Funding
This research was in part supported by the National Institute of Mental Health under award number K22-MH109558. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Ru Zhang contributed to data collection, analyzed the data, and wrote the initial draft. R. James R. Blair designed the study, revised various versions of the draft, and obtained funding. Karina Blair contributed to the data analysis and writing. Matthew Dobbertin, Jaimie Elowsky, and Johannah Bashford-Largo collected the data and contributed to the writing. Ahria J. Dominguez and Melissa Hatch contributed to data analysis and writing. Sahil Bajaj analyzed the data, contributed to writing, and supervised all aspects of the study. All the authors participated in critical reading and manuscript revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors assert that all procedures followed were in accordance with the ethical standards of the Boys Town National Research Hospital (BTNRH) on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. The study was approved by the IRB at BTNRH (IRB protocol # 20–04-X).
Competing interests
All authors report no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, R., Blair, R.J.R., Blair, K.S. et al. Reduced grey matter volume in adolescents with conduct disorder: a region-of-interest analysis using multivariate generalized linear modeling. Discov Ment Health 3, 25 (2023). https://doi.org/10.1007/s44192-023-00052-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44192-023-00052-3