Abstract
The paper presents mathematical models and their implementation in C # language describing the phase transformations occurring in the process of austempered ductile iron (ADI) manufacture. The research includes two main stages: austenitization and isothermal holding at the bainitic range. The influence of free energy of austenite and ferrite on transformations was taken into account. Parameters of models were identified based on inverse analysis and experimental research. As part of the research, verification and validation of the developed models was carried out based on the results of experimental research. The tool developed and implemented enables the analysis of phase transformations occurring during heat treatment with isothermal holding of ductile iron with Ni addition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
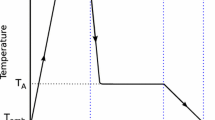
In the process of ADI manufacture by heat treatment of ductile iron, two most important stages can be distinguished, i.e. austenitizing and isothermal holding in the temperature range of bainitic transformation (Fig. 1).
Austenitizing should be carried out in such a way as to ensure the formation of homogeneous austenite under selected conditions of the transformation time and temperature. Obtaining the required austenitic microstructure, homogeneous in terms of carbon content, is fundamental importance as a starting point for the formation of ausferritic microstructure [1,2,3]. For example, too short austenitizing time may be the reason for incomplete transformation of pearlite into austenite, leaving an unprocessed microstructure that adversely affects the ADI parameters [1,2,3,4,5].
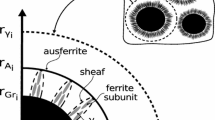
In case of isothermal holding of austenitized material at a given temperature, it is necessary to specify the time that will produce the matrix microstructure called ausferrite, which is a mixture of austenite and acicular ferrite. In ductile iron, the isothermal transformation in the bainitic range occurs in a mode completely different than in steel. The fundamental difference is the presence of graphite in cast iron, which results in a different microstructure. Moreover, it is assumed that in most steels, as a result of fluctuations in carbon concentration, ferrite and carbides are formed simultaneously. In case of ductile iron, the transformation during isothermal holding occurs much more slowly than in steel and can be presented as a multi-stage reaction including [6,7,8,9,10]:
-
Stage I—nucleation and growth of ferrite grains accompanied by carbon diffusion into the surrounding austenite
-
Stage II—end of the growth of ferrite plates, austenite saturation with carbon to a certain (maximum) concentration. With progressing enrichment of austenite in carbon (it may contain up to 2.2% C), the austenite becomes more and more stable. High-carbon austenite has a reduced temperature MS (below 20 °C), which increases its content in the cast iron matrix
-
Stage III—decomposition of carbon-saturated austenite into a carbide phase and the α solution:\(\gamma {\prime}({x}_{\gamma \alpha })\to \alpha ({x}_{\alpha \gamma })+{{\text{Fe}}}_{3}{\text{C}}\)
Too short isothermal holding results in low saturation of austenite with carbon. As a consequence, during cooling to ambient temperature or formation of mechanical stress (e.g. during machining), martensite may appear in the microstructure of ADI matrix.
On the other hand, too long isothermal holding can contribute to the decomposition of high-carbon austenite (Stage III). Carbon-saturated austenite \(\gamma {\prime}({x}_{\gamma \alpha })\) is transformed into “bainitic ferrite” and ε(Fe2,4C) carbides.
Considering the above, it is very important in the ADI manufacturing process to select technological parameters of this process (\({t}_{\gamma }\left({T}_{\gamma }\right), {t}_{i}\left({T}_{i}\right)\)) in such a way as to obtain homogeneous austenite and make the end of isothermal holding correspond to Stage II, which allows obtaining stable austenite, and consequently ausferrite [4, 7, 11, 12].
2 Modelling of phase transformations
As part of the research, two models covering the austenitizing and ausferritizing phase, schematically presented in Fig. 2, were proposed.
Model I describes the first stage of the transformation of pearlite + ferrite → austenite (Fig. 2a). It allows predicting the time \({t}_{\gamma }\) for a given austenitizing temperature \({T}_{\gamma }\), ensuring the formation of homogeneous austenite (Fig. 2b).
Model II relates to changes occurring during isothermal holding in the range of bainitic transformation (Fig. 2a) and allows determining the time \({t}_{i}\) for a given temperature of isothermal holding \({T}_{i}\), ensuring the formation of stable austenite, calculation of the austenite volume fraction and calculation of a fragment of the TTT diagram.
2.1 Model of pearlite + ferrite- > austenite transformation
To describe phase transformations occurring during pearlite + ferrite → austenite transformation, the solution of Fick's second law based on the finite difference method was used. The starting microstructure is composed of graphite spheroids surrounded by a ferrite layer and pearlite colonies (Fig. 3a), as schematically shown in Fig. 3b.
The model concept was based on the consumption of ferrite by austenite formed on the graphite/ferrite phase boundary and austenite formed during pearlitic transformation. The developed model was divided into three stages including austenitization and homogenization of carbon:
-
Stage I—during this stage, two transformations take place simultaneously, i.e. ferrite → austenite transformation and pearlite → austenite transformation (Fig. 4a).
-
Stage II—begins after the completion of pearlite → austenite transformation and only the transformation of ferrite into austenite takes place (Fig. 4b).
-
Stage III—begins after the completion of pearlite + ferrite → austenite transformation and involves homogenization of carbon in austenite (Fig. 4c).
The transformation process is controlled by carbon diffusion in austenite and ferrite. The aim of the model is to determine changes in carbon content in austenite and ferrite as a function of time and distance. Preliminary studies and assumptions concerning model I are presented in [13, 14].
2.2 Model of austenite- > ferrite transformation
The model of phase transformations during isothermal holding of ductile iron to obtain ausferritic microstructure is an adaptation of the Bhadeshia model developed for silicon steels [15,16,17,18] and it has been expanded with a thermodynamic calculation module.
The time of complete transformation was calculated taking into account the incubation period based on Eqs. (1)–(11):
where \({Q}^{\prime}, {C}_{4}, z, p\)—experimental constants: \({Q}^{\prime}=243{,}200 \, \mathrm{J}/{\text{mol}}\), \({C}_{4}=-135\), \(z=20\), \(p=5\) [19], \({T}_{i}\) ausferritization temperature, °C, \(R\) gas constant, \({\text{J}}/(\mathrm{mol K})\), \(\Delta {G}_{{\text{m}}}^{0}\) maximum free energy of nucleation, \({\text{J}}/{\text{mol}}\), \(u\) unit volume of bainitic ferrite, \(\upmu {{\text{m}}}^{3}\), \({u}_{t}\) length of ferrite needles, \(\upmu \mathrm{m}\), \({u}_{w}=0.001077T-0.2681\) width of ferrite needles, \(\upmu {{\text{m}}}^{2}\), \(r\) experimental constant: \(r=2540 {\text{J}}/{\text{mol}}\) [15], \({K}_{1}\) function of austenite grain size (the number of potential ferrite nucleation sites), \(1/({{\text{mm}}}^{3}{\text{s}})\), \(\overline{L }\) mean grain size of austenite, \(\mu \mathrm{m}\), \({K}_{1}^{\prime}, {K}_{2}\)—experimental constants: \({K}_{1}^{\prime}/u=0.57919{\text{E}}-6 {{\text{mm}}}^{2}{\text{s}}\), \({K}_{2}=0.20980{\text{E}}5 {\text{J}}/{\text{mol}}\), [17], \(\theta =({x}_{{T}_{0}^{\prime}}-\overline{x })/({x}_{{T}_{0}^{\prime}}-{x}_{\alpha })\)—maximum volume fraction of bainite, \({x}_{{T}_{0}}^{\prime}\) carbon content at the \({T}_{0}^{\prime}\) boundary, mole [17], \(\overline{x }=-1.7+0.0028\times {T}_{\gamma }+0.11{\text{Mn}}-0.057{\text{Si}}-0.058{\text{Ni}}- 0.12\mathrm{ Mo}\)—average carbon content in alloy [20], \({x}_{\alpha }\)—carbon content in ferrite, mole, \(\xi =v/\theta \)—relative volume fraction of bainite, \(v\) actual volume fraction of bainite, \(\beta ={\lambda }_{1}\left(1-{\lambda }_{2}\overline{x }\right)\)—autocatalysis factor, \({\lambda }_{1}, {\lambda }_{2}\)—experimental constants: \({\lambda }_{1}=147.5\), \({\lambda }_{2}=30.327\), \({G}_{N}\)—universal nucleation function, J/mol [17].
The driving force of transformation \(\Delta {G}_{{\text{m}}}^{0}\) was calculated using thermodynamic models developed by the CALPHAD (CALculations of PHAse Diagrams) method and parallel tangential structures (Fig. 5) as well as free energy calculations.
Changes in free energy during nucleation of ferrite from austenite with the composition \(\overline{x }\), construction of parallel curves—determination of maximum nucleation energy of ferrite with the composition (\({x}_{m}^{\alpha }\)) [21]
Free energies of austenite and ferrite were calculated from [22]. The thermodynamic calculation module was presented and described in detail in [23].
2.2.1 Identification of model parameters based on the experimental studies
The model comprises a number of experimental constants that have been grouped in vector x (12) and determined with an inverse solution algorithm:
Before identification of experimental constants, a local sensitivity analysis based on the difference quotients was carried out. The purpose of the analysis was to determine the significance of individual coefficients in the model. The results of the analysis showed that the impact of the coefficients \({\lambda }_{1}\), \({\lambda }_{2}\) and r was insignificant, and therefore the values of these coefficients were selected based on the literature data [15, 17].
The values of the coefficients x were determined by searching for the minimum of objective function:
where \({t}^{{\text{model}}}, {t}^{{\text{measurement}}}\)—vectors of output parameters (times of the beginning of transformation) obtained for the experimental test by model calculations and measurements, respectively, T—vector of test parameters (conditions) in inverse solution (temperature).
The applied solution does not allow us to check if the obtained results are optimal in a global sense, but it examines whether the adopted solution is better than the solutions found previously. Since the optimization method used is of a non-deterministic character, the analysis has been run many times.
An experimental TTT diagram (Fig. 6) made for ductile iron with the chemical composition given in Table 1 was used for model identification and verification.
TTT diagram [24]
The most important issue in the conducted research was the temperature range of bainitic transformation, i.e. about 220–500 °C. The transformation start line was used to identify model parameters, while the transformation end line was used to verify model calculations.
Coefficients selected by inverse analysis are presented in Table 2. Table 2 also contains values of coefficients based on literature data.
Since the method used is of a non-deterministic character, optimization was run many times and similar results were obtained. A broader analysis of the selection of model coefficients is a rather complex issue and may constitute the next stage of research.
2.2.2 Model verification
The models developed were verified by comparing the results of computer simulations with the results of own experimental research and research published in the technical literature.
Figure 7 shows the results of experimental studies published by Darwish et al. [6] regarding changes in carbon content as a function of austenitizing time at temperatures of 850, 900, 950 °C for cast iron with pearlitic-ferritic matrix microstructure. To compare the results obtained by Darwish et al. [6] with the results obtained by computer simulation of the austenitization process, the points determined by the symADI program were plotted on the diagram. The points shows the time needed for the transformation and homogenization of austenite at temperatures of 850, 900, 950 °C. The diagram also included the corresponding carbon content in austenite \({C}_{\gamma }^{0}\) calculated by the symADI program.
Changes in carbon concentration in austenite over time for various austenitizing temperatures [6] and results of computer simulation for preset austenitizing conditions
Analysis of the obtained results showed their relatively good agreement. The minimum time needed for the transformation and homogenization of carbon content in austenite as well as carbon content after austenitizing assumed similar values in both experimental tests and calculations. As demonstrated by experimental studies, for an austenitizing temperature of 850 °C, the carbon content stabilized after about 20 min. For the same temperature, simulation tests showed that the time necessary to obtain homogeneous austenite was 22 min. For a temperature of 900 °C, this time amounted to about 15 min when calculated experimentally and to 14 min when determined by simulation. The lowest consistency of results was obtained for a temperature of 950 °C, where the discrepancy between the time calculated by simulation (9 min) and the time determined by experimental tests (about 20 min) was approximately 10 min. It should be noted, however, that in the last case (950 °C), for a time longer that 10 min, the experimentally determined change in carbon concentration was small, and the scatter of results for the steady state was much larger than in the other cases. The equilibrium carbon contents calculated using the program at the considered temperatures are slightly higher compared to those determined experimentally [6]. This is due to the fact that the model does not take into account the influence of the chemical composition of cast iron on the carbon content obtained in austenite. The equilibrium carbon content in austenite is reduced by Si and Ni additions present in cast iron [25]
Figure 8 shows a part of the TTT diagram based on the results of simulation and experimental research. The solid line denotes the results of model calculations, and the dashed line—the results obtained in the experiment.
To check the validity and consistency of computational and experimental results Mean Relative Error (MRE) was calculated (14). MRE allows assessment of the average relative error between the predicted and actual values, MRE = 0% indicates a perfect fit.
where \({y}_{i}\) the actual value, \({\widehat{y}}_{i}\) the predicted value, \(n\) number of observations.
Mean relative errors calculated for the time of the beginning (y = 1%) and end of the transformation (y = 99%) are presented in Table 3.
The analysis of the results disclosed in Fig. 8 and Table 3 show a relatively good agreement between simulation results and experimental data.
The determined transformation start time (green line in Fig. 8) shows a very good agreement in the upper range of isothermal transformation temperatures (400–300 °C). In this range of transformation temperatures, the difference in the calculated and experimental time of the beginning of the transformation, Δτ1%, is in the range of 5.2–27.5 s. The discrepancy between experimental data and simulation results is visible in the lower range of temperatures (below 300 °C). In this range of transformation temperature, the value of Δτ1% is in the range of 26–200 s. In the line determining the end of transformation (red line in Fig. 8) the results of the simulation suggest an earlier end of the transformation than the analysis of the experimental TTT diagram. For the completion time of the austenite–bainite transformation τ99%, the value of the calculated and experimental time difference, Δτ99%, is in the range 579–3012 s. Therefore, in the future, it is planned to expand the scope of studies and include optimization of model parameters which may improve the obtained results, especially in the lower range of temperatures.
The verification of calculations regarding the maximum driving force of transformation \(\Delta {{\text{G}}}_{{\text{m}}}^{0}\) 0 was based on the data presented by Ławrynowicz [26]. Figure 9 shows a comparison of the results. The dashed line represents the results taken from the literature [26], while the results of own calculations are represented by the solid line.
The results presented in Fig. 9 show a consistent relationship between changes in the maximum driving force of transformation and temperature, which suggests that the model calculations are correct. Some insignificant discrepancies between these results and the results obtained by Ławrynowicz [26] may be due to the fact that other thermodynamic data were used to calculate the free energy of the occurring phases.
2.3 Model implementation
The developed models and experiments performed were used in the development and implementation of a program for studies of phase transformations occurring in ductile iron with the addition of nickel (Fig. 10). A comprehensive computer program (symADI) for simulation of the ductile iron heat treatment with isothermal holding was implemented in Visual Studio 2012 environment using C# language.
Using input data, i.e. chemical composition of the ductile iron tested and temperature of the austenitizing process \({T}_{\gamma },^\circ{\rm C} \), the program calculates:
-
time \({t}_{\gamma }\) necessary to obtain homogeneous austenite,
-
carbon concentration in austenite \({C}_{\gamma }^{0}\) after pearlite + ferrite → austenite transformation,
-
volume fraction of austenite at the stage of ausferritization,
-
time necessary for a given stage of transformation to take place at a given temperature \({T}_{i}\), ℃.
The program also allows for:
-
simulation of changes in the thickness of ferrite and austenite plates and simulation of carbon concentration changes in phases occurring on the sample cross-section at the stage of austenitization,
-
calculation of a fragment of the TTT diagram,
-
graphic presentation of:
-
o
changes in the volume fraction of ferrite \({V}_{V\alpha }\) as a function of time for the preset temperature of ausferritization \({T}_{i}\), \(^\circ{\rm C} \),
-
o
dependence of free energy on the carbon content in austenite and ferrite at a given temperature of ausferritization,
-
o
changes in the maximum driving force of transformation \(\Delta {G}_{m}^{0},\) as a function of the temperature of isothermal holding.
-
o
3 Calculation results
The developed symADI computer program was used to carry out a series of simulations for individual stages of the austenitizing and ausferritizing process.
For the pearlite → austenite transformation, the following initial conditions were adopted:
-
thickness of the pearlite plates \({x}_{{\text{perl}}}=1 \mathrm{\mu m}\),
-
thickness of the cementite layer \({l}_{\Theta }=0.1\mathrm{ \mu m}\),
-
thickness of the ferrite layer \({l}_{\alpha }={l}_{\Theta }\times 7.68\).
For the ferrite → austenite transformation, the following assumptions were made:
-
maximum size of model cell \({r}_{{\text{max}}}=50\mathrm{ \mu m}\)
-
location of boundaries \({r}_{gr}={r}_{1}=10\) μm and \({r}_{2}=30\mathrm{ \mu m}\).
It was assumed that the initial thickness of the austenite plates for both pearlite → austenite transformation and ferrite → austenite transformation was \(0.0001\) μm.
In the case of model II, sample simulations were carried out for an austenite grain size equal to \(\overline{L }=100\) μm. The calculations were carried out in the temperature range \({T}_{i}\) = 240–400 °C.
The results of the simulation of changes in the carbon concentration distribution over time as a function of the austenitizing temperature are presented in Fig. 11. The calculations were carried out for the austenitizing temperatures of 850 and 900 °C. The results were recorded after the time of 0.1; 5; 10; 60 and 300 s. On the presented diagrams (Fig. 11), the left part describes changes taking place in the \({\gamma }_{1}\) austenite formed on the graphite/ferrite interface, the central part of the diagram refers to the \({\alpha }_{3}\) ferrite layer, and the curves on the right show changes in carbon concentration in the \({\gamma }_{2}\) austenite formed as a result of pearlite transformation. The dashed line denotes changes in carbon concentration during homogenization.
Figure 12 shows changes in the thickness of the austenite and ferrite layers as a function of time for two temperatures, i.e. 850 and 900 °C. The dotted line denotes changes in the thickness of the austenite plate \({g}_{\gamma 1}\) formed on the graphite/ferrite interface, the solid line denotes changes in the thickness of the ferrite plate \({g}_{\alpha 3}\), while the dashed line denotes changes in the thickness of the austenite layer \({g}_{\gamma 2}\) formed as a result of pearlite transformation.
The results of the study of the effect of austenitizing temperature on the calculated TTT diagram for an austenite grain size equal to \(\overline{L }=100 \mathrm{\mu m}\) are shown in Fig. 13. The first (solid) line denotes the transformation start (1% ferrite), the second (dashed) line denotes the transformation end (99% of the maximum volume fraction of ferrite) for the austenitizing temperature \({T}_{\gamma }\) = 850 °C, while the third (dotted) line denotes the transformation end for the austenitizing temperature \({T}_{\gamma }\) = 900 °C
The results plotted in Fig. 13 show very clearly the effect of austenitizing temperature on transformation time. As the temperature rises, the transformation time increases.
The effect of austenite grain size on the transformation end time for an austenitizing temperature of 900℃ is shown in Fig. 14.
The results presented in Fig. 14 show very clearly the effect of austenite grain size on transformation time. With the growing size of austenite grains, the transformation time increases.
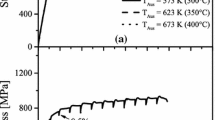
The effect of austenitizing temperature \({T}_{\gamma }\), and temperature of isothermal holding \({T}_{i}\), on the transformation time and volume fraction of ferrite is shown in Fig. 15.
The presented calculations show that the volume fraction of austenite and the austenite → ferrite transformation time depend on the austenitizing temperature \({T}_{\gamma }\), while the effect of isothermal holding temperature \({T}_{i}\) on changes in the volume fraction of individual phases is less significant. Along with the increase in the temperature \({T}_{\gamma }\), the volume fraction of ferrite increases and the time needed for the transformation to take place also increases, while with the decrease in isothermal holding temperature \({T}_{i}\), the volume fraction of austenite decreases, the differences being rather small.
4 Model validation
Model validation was performed by heat treatment of samples used in material testing, based on the model calculations. The validation scheme is shown in Fig. 16.
The austenitizing treatment was carried out under constant conditions, while for isothermal holding six variants were selected, three for the upper range (W6, W5, W4) and three for the lower range (W3, W2, W1). The scheme is shown in Fig. 17.
After heat treatment, the following examinations were carried out: X-ray examinations [27], hardness measurements and testing of mechanical properties [24].
Table 4 presents the results of model calculations, images of the microstructure and the results of experimental tests performed after heat treatment according to the variants based on the calculations.
The results show that heat treatment based on the model calculations produced an ausferritic microstructure in the upper range of temperatures, as also confirmed by hardness values obtained in the tested material, typical of the cast iron with an ausferritic structure. In the lower range of temperatures, only variant W3 produced the ausferritic structure, while tests conducted at 300 and 260 °C have indicated the possibility of the occurrence of martensite. On the other hand, according to PN-EN 1564: 2012, the mechanical properties obtained for most variants allowed classifying the tested cast iron as ADI, which is also presented in Table 4.
5 Summary and conclusions
As part of the research, a mathematical model was developed, implemented and verified to carry out the simulation of phase transformations occurring in ductile iron with a specific chemical composition during heat treatment with isothermal holding.
Based on the analysis carried out it can be concluded that the calculations carried out on the developed models allow for sufficiently exact prediction of the time needed for full austenitization with carbon homogenization and for prediction of the time needed for the austenite → ferrite transformation with the determination of the volume fraction of individual microstructural constituents.
The program developed and implemented can serve as a tool supporting the studies of phenomena that occur during phase transformations in the heat treatment process of ductile iron.
Data availability
The dataset generated during and/or analysed during the current study are available from corresponding author on responsible request.
References
Nili Ahmadabadi M, Parsa MH. Austenitisation kinetics of unalloyed and alloyed ductile iron. Mate Sci Technol. 2001;17(2):162–7.
Yazdani S, Elliot R. Influence of molybdenum on austempering behaviour of ductile iron - part 1. Mater Sci Technol. 1999;15(5):531–40.
Akbay T, Reed R, Atkinson C. Modelling reaustenitisation from ferrite/cementite mixtures in Fe-C steels. Acta Mater. 1994;42:1469–80.
Podrzucki C. Żeliwo. Struktura, własności, zastosowanie, vol. I i II. Kraków: ZG STOP; 1991.
Trepczyńska-Łent M, Dymski S. Pogląd na austenityzowanie osnowy podczas hartowania żeliwa ADI. Archiwum Odlewnictwa. 2005;5(17):353–64.
Darwish N, Elliott R. Austempering of low manganese ductile irons. Mater Sci Technol. 1993;9(7):572–602.
Dorazill E, Barta B, Crhak J, Munsterova E. High-strength bainitic cast iron with spheroidal graphite. Met Sci Heat Treat. 1978;20(7):532–5.
Moore DJ, Rouns TN, Rundman KB. Effects of manganese on structure and properties of austempered ductile iron: a processing window concept. Trans Am Foundry Soc. 1986;48:255–64.
Bayati H, Rimmer AL, Elliott R. The austempering kinetics and processing window in austempered, low-manganese compacted graphite cast iron. Cast Metals. 1994;7(1):11–24.
Rajnovic J, Eric O, Sidjanin L. The standard processing window of alloyed ADI materials. Konove Mater. 2012;50:199–208.
Dorazil E, Holzmann M, Crhák J, Kohout J. Influence of low and cryogenic temperature on deformability and fracture behaviour of austempered ductile cast irons under static and impact stresses. Giesserei-Praxis. 1985;8(9):109–23.
Wilk-Kołodziejczyk D, Mrzygłód B, Regulski K. Influence of proces parameters on the properties of austempered ductile iron (ADI) examined with the use of data mining methods. Metalurgija. 2016;55(4):849–51.
Olejarczyk-Wożeńska I, Adrian H, Wilk-Kołodziejczyk D, Kluska-Nawarecka S. Numerical model of pearlite—austenite transformation. Metal. 2014;2014:611–7.
Olejarczyk-Wożeńska I, Adrian H, Mrzygłód B. Mathematical model of the process of pearlite austenitization. Arch Metall Mater. 2014;59(3):981–6.
Rees GI, Bhadeshia HK. Bainite transformation kinetics. Part 1: modified model. Mater Sci Technol. 1992;8:985–93.
Rees GI, Bhadeshia HK. Bainite transformation kinetics. Part 2: non-uniform distribution of carbon. Mater Sci Technol. 1992;8:994–6.
Chester NA, Bhadeshia H. Mathematical modelling of bainite transformation kinetics. J Phys IV. 1997;C5(7):41–6.
Takahashi M, Bhadeshia HK. A model for the microstructure of some advanced bainitic steels. Mater Trans. 1991;32(8):689–96.
Bhadeshia HK. Thermodynamic analysis of isothermal transformation diagrams. Metal Sci. 1982;16:159–65.
Chang LC. An analysis of retained austenite in austempered ductile iron. Metall Mater Trans A. 2003;34A:211–7.
Bhadeshia HK. Bainite in steels. Transformations, microstructure and properties. 2nd ed. Cambridge: Institute of Materials; 2001.
Adrian H. Numeryczne modelowanie procesów obróbki cieplnej. Kraków: Wydawnictwa AGH; 2011.
Olejarczyk-Wożeńska I, Adrian H, Mrzygłód B, Głowacki M. Mathematical model of bainitic transformation in austempered ductile iron. Arch Foundry Eng. 2017;17(4):200–6.
Mrzygłód B, Kowalski A, Olejarczyk-Wożeńska I, Giętka T, Głowacki M. Characteristics of ADI ductile cast iron with single addition of 1.56% Ni. Arch Metall Mater. 2017;62:2273–80.
Hamid Ali A, Elliot R. Influence of austenitising temperature on austempering of an Mn-Mo-Cu alloyed ductile iron. Part 1: austempering kinetics and the processing window. Mater Sci Technol. 1996;12:1021–31.
Ławrynowicz Z. Kinetics of the bainite transformation in austempered ductile iron ADI. Adv Mater Sci. 2016;16(2):47–56.
Olejarczyk-Wożeńska I, Giętka T, Adrian H, Mrzygłód B, Głowacki M, Goły M. X-ray structural analysis of ductile iron with the addition of Ni heat-treated under isothermal conditions. Metal. 2016;2016:813–8.
Acknowledgements
This work was supported by the NCN, Project No. 2013/11/N/ST8/00326. Funding was provided by Ministerstwo Edukacji i Nauki (AGH-UST statutory research project no. 16.16.110.663.
Author information
Authors and Affiliations
Contributions
All authors have been personally and actively involved in substantive work leading to the report.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Consent to publish
We confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olejarczyk-Wożeńska, I., Mrzygłód, B. & Adrian, H. Modelling of phase transformations occurring in the process of austempered ductile iron manufacturing. Arch. Civ. Mech. Eng. 24, 195 (2024). https://doi.org/10.1007/s43452-024-00948-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-024-00948-z