Abstract
Incorporating decentralized approaches into clinical trials is a critical innovation with potential implications for improved accessibility and diversity, as well as lower burden for participants and caregivers. As we move forward in a collective effort to modernize clinical trials, we consistently hear of hurdles that interfere with the adoption of decentralized approaches. But are these hurdles really the impediments we think they are? In this commentary, we offer three perceptions that are commonly heard as impediments to the adoption of digital and decentralized clinical trials. Leveraging the Clinical Trial Transformation Initiative’s Digital Health Trial hub of work, interactions with members and regulators, and observations related to adoption, we address those perceptions and note some resources that exist to overcome them. In working through these barriers, we can instill confidence in sponsors and designers to leverage all the clinical trial design tools available to them to advance the use of decentralized approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, only a small percentage of eligible, and generally willing, patients has participated in clinical trials [1,2,3,4]. With recent attention on improving equitable access and diverse trial participation [5,6,7,8], many across the clinical trials ecosystem are seeking practical approaches to achieve this critical goal [9,10,11,12]. Given that 70 percent of potential participants in the United States live more than 2 h away from the nearest study center [13], and nearly 25 percent of trials are discontinued early (often due to recruitment challenges) [14], there is an urgent need to accommodate a participant’s real life as they sacrifice time, wages, and other responsibilities to contribute to the evidence base for new treatments. Decentralization is one promising approach to address these challenges and improve access.
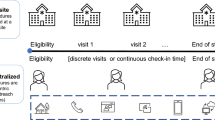
The Clinical Trials Transformation Initiative (CTTI), a public private partnership co-founded by Duke University and the U.S. Food and Drug Administration (FDA), has defined decentralized clinical trials (DCTs) as trials in which some or all study assessments or visits are conducted at locations other than the investigator site via any or all of the following: tele-visits; mobile or local healthcare providers, including local labs and imaging centers; and home delivery of investigational products. The use of digital health technologies, such as electronic sensors and computing platforms, are often important elements of decentralized trials [15, 16].
There is widespread support for decentralization, including regulatory reforms and new guidance, operational and trial design resources from public private partnerships [15, 17, 18], and government funding of innovative pilot studies, infrastructure needs, and sustainable business models [19,20,21]. The U.S. Congress recently authorized additional financial investment in health through the Advanced Research Projects Agency for Health, which has launched The Advancing Clinical Trial Readiness Initiative with a goal of enabling 90% of all eligible Americans to take part in a clinical trial within a half hour of their home: decentralization—particularly the technologies and infrastructure that will facilitate it—is one of their five focal areas [22].
Despite this momentum, real and perceived challenges remain, limiting wider implementation of decentralized elements. In this commentary the authors, all CTTI staff, describe three commonly stated barriers, comment on the extent to which perceived barriers are manageable, and discuss resources that exist to address them. Our purpose is to instill confidence in sponsors and trial designers to leverage the tools available to them to advance the use of decentralized approaches.
Overcoming Perceptions
Perception #1: Flexibility is a Threat to Data Quality
Decentralized trials often provide options to participants for where to undergo study visits and assessments. Though more work is needed to understand how decentralized designs can improve participation and participant diversity [23], small changes in trial design can be meaningful and increase access and/or retention [24]. However, concerns are often raised that such variability and flexibility can create risks to data quality [25].
It is true that “real world” factors can regularly be controlled for when on site: distractions are often less likely, technology issues or missing data can be more readily addressed, procedures are more consistent. It is also true that different sources of data may require integration.
The perception that flexibility is a threat to data quality has grounds for truth if the appropriate planning is not in place. But with proactive training, data management and oversight plans, most risks can be mitigated. CTTI’s Digital Health Trials Hub, which is a collection of resources developed from a series of evidence-driven, multi-stakeholder projects, has recommendations to help manage data and plan decentralized clinical trials [26]. (Table 1).
Perception #2. Decentralization Undermines Traditional Relationships and Accountability Structures
Previous work has highlighted concerns around roles and responsibilities in decentralized trials [27, 28]. As the field gains more experience, we continue to hear concerns about the relationship between investigator and patient, the overall role of the investigator, and the complexities for sites as they manage an increasing number of technologies and navigate oversight of home health providers.
It can be a conceptual adjustment for a clinician accustomed to meeting with patients face-to-face: how does that responsibility change when trial participants are recruited and “seen” digitally? Some worry about the erosion of these relationships. Looking forward, some evidence indicates that clinicians and investigators are becoming more comfortable with various types of technologies leveraged in DCTs [29]. Additionally, some DCTs are actively exploring ways to leverage technology to strengthen investigator-participant relationships, for instance through optional “town halls” [30].
It is also important to note that decentralization rarely means a trial without sites: sites remain partners who make decentralization possible. When used with appropriate planning, technology for DCTs should support the connection between participants and sites. For example, short virtual check-ins and collection of endpoints using wearables can reduce the number of assessments required at in-person study visits, allowing clinician-investigators to focus on required physical assessments, treatments, assessment of well-being, and building the personal connections that many site staff and patients value.
Challenges also remain around defining what constitutes a site in the context of some innovative trial designs, whether the level of oversight is clear, and who is accountable for the safety and welfare of the participant. In response, professional organizations and regulators are converging to address the issue. For example, American Society of Clinical Oncology’s recent call to action to advance patient-focused and decentralized clinical trials focuses on three key strategies: “confront challenges with the interpretation of [the Food and Drug Administration] FDA’s Form 1572 requirements (Statement of Investigator); broaden acceptance of local laboratories and imaging centers; and invest in the creation of effective, sustainable partnerships between research centers and local providers” [31].
CTTI has also developed recommendations for supporting sites to facilitate effective site-participant communication and help build site DCT infrastructure [32] (Table 2).
Moving forward, we should continue to aim to empower patients, leverage technology to improve provider-participant interactions and trust, and increasingly incorporate DCT elements that offer flexibility for participants when it makes sense to do so.
Perception #3: Regulators Haven’t Made it Clear What We Can Do
Stakeholders frequently express that that lack of clear regulations are a major impediment to widespread use of DCT elements. That said, regulators encourage “modernizing the design and conduct of clinical trials, making them more agile without compromising data integrity or participant protections” [33], and the FDA has taken strides to address these challenges in several ways. For example, the agency has offered guidance that supports decentralized approaches, including the 2023 draft guidance Decentralized Clinical Trials for Drugs, Biological Products, and Devices [34]. In October 2023, the FDA, in collaboration with CTTI, also convened a public meeting on Mitigating Clinical Study Disruptions During Disasters and Public Health Emergencies (PHEs) to share, learn from, and build on experiences during the Covid-19 pandemic, during which many trials were able to move forward in novel ways [35].
It is essential to recognize that regulations cannot account for every option and contingency. Rather, sponsors can leverage the principles of the guidance and apply them to their situation. Trial designers are also encouraged to engage early with the relevant health authorities when considering new approaches. CTTI’s recommendations for Interacting with Regulators can guide conversation during the design and conduct of DCTs [36]. Supporting resources also provide information about data processes to include in submissions to the FDA [37], data that should be made available to inspectors [38], and a guide for engaging with the FDA and/or the European Medicines Agency when develo** a digitally derived endpoint [39].
Conclusion
Despite the long arc of adoption for technologies and capabilities supporting clinical trial execution [40], the COVID-19 pandemic showed us that trials using decentralized approaches can be successfully implemented on a large scale, rapidly, while maintaining participant safety and data integrity. As we increasingly work to make “patients as partners” a reality in medicine, it is critical to both support and empower patients in their trial participation—and decentralized approaches are an increasingly valuable tool for doing so.
Decentralized approaches are not all or none, and their use should be fit for purpose. Only the appropriate DCT elements should be incorporated to ensure that data quality is not compromised and technology is used to help and not hurt. Designers must continue to consider target population and ease of use. Ongoing experimentation along these lines will help create an evidence base around the trial characteristics that encourage broader participation, including from more diverse and historically under-represented populations.
As a public–private partnership, CTTI encourages stakeholders to share their hesitations and their experiences with each other and with regulators. Harnessing our collective experiences—learning from successes and failures—can increase comfort with new methods. Change is constant. Barriers will arise. In order to foster a clinical trials ecosystem that we all want to participate in, we must continue to the leverage resources that exist and work collectively to improve it.
Data availability
Not applicable.
References
CISCRP. Perceptions and Insights Study: 2021. https://www.cis.crp.org/wp-content/uploads/2021/11/2021-PI-General-Perceptions-Report-04NOV2021-FINAL.pdf.
New National Public Opinion Poll Shows Majority of Americans Would Participate in Clinical Trials if Recommended by Their Doctor. News release. Applied Clinical Trials. June 13, 2013
Unger J. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245–55.
Getz K. Rebooting the statistic that 5% of eligible patients participate in clinical trials. Appl Clin Trials. 2023;32(3):1.
National Academies of Sciences, Engineering, and Medicine; Policy and Global Affairs; Committee on Women in Science, Engineering, and Medicine; Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research; Bibbins-Domingo K, Helman A, editors. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington (DC): National Academies Press (US); 2022 May 17. Appendix C, Improving Representativeness in Clinical Trials and Research: Facilitators to Recruitment and Retention of Underrepresented Groups. Available from: https://www.ncbi.nlm.nih.gov/books/NBK584402/
U.S. Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. https://www.fda.gov/media/157635/download.
Ahmed HR, Strauss DH, Bierer BE. Committing to the inclusion of diverse populations in clinical research. Ther Innov Regul Sci. 2020;54(4):922–4. https://doi.org/10.1007/s43441-019-00020-6.
Gottlieb S. The need for a US national clinical trial infrastructure in a public health crisis. JAMA Health Forum. 2021;2(8): e213223. https://doi.org/10.1001/jamahealthforum.2021.3223.
Government of Canada. Strategies and Initiatives: Regulatory innovation for health products: Modernizing clinical trial regulations
Medicines and Health Care products Regulatory Agency. Consultation outcome: Government response to consultation on legislative proposals for clinical trials. Updated 21 March 2023
Woodcock J. Integrating research into community practice—toward increased diversity in clinical trials. N Engl J Med. 2021;385:1351–3. https://doi.org/10.1056/NEJMp2107331.
Williams C. Demographic and health behavior factors associated with clinical trial invitation and participation in the united states. JAMA Netw Open. 2021;4(9): e2127792. https://doi.org/10.1001/jamanetworkopen.2021.27792.
Anderson, D. Digital R&D. Four ways to maximize patient engagement in clinical trials, deloitte consulting LLP. 25 Jun 2018
Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–52.
Clinical Trials Transformation Initiative. Digital Health Trials Hub. https://ctti-clinicaltrials.org/our-work/digital-health-trials/
Digital Health Technologies (DHTs) for Drug Development. https://www.fda.gov/science-research/science-and-research-special-topics/digital-health-technologies-dhts-drug-development)
Decentralized Trials and Research Alliance. https://www.dtra.org/
Innovative Medicines Initiative: Europe’s Partnership for Health. Trials@home. https://www.imi.europa.eu/projects-results/project-factsheets/trialshome
Duke Clinical and Translational Research Institute: Community Engaged Research Initiative (CERI) https://ctsi.duke.edu/community/community-engaged-research-initiative-ceri
ARPA-H. Biden-Harris Administration’s ARPA-H initiative invests to improve clinical trials and drive better health outcomes. October 20, 2023
U.S. Department of Health & Human Services. BARDA launches the D-COHRe program, seeking to enhance clinical innovation with decentralized care capabilities. Web announcement. Last Updated: July 05, 2023
Advancing Clinical Trial Readiness (ACTR) Initiative Description. Draft Announcement for feedback. October 19, 2023. https://www.customerexperiencehub.org/wp-content/uploads/2023/10/ACTR-Initiative-Description-2023-10-13.pdf
Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. Npj Digit Med. 2022;5:58.
Miyata BL, Tafuto B, Jose N. Methods and perceptions of success for patient recruitment in decentralized clinical studies. J Clin Transl Sci. 2023;7(1): e232. https://doi.org/10.1017/cts.2023.643.
de Jong AJ, van Rijssel TI, Zuidgeest MGP, van Thiel GJMW, Askin S, Fons-Martínez J, De Smedt T, de Boer A, Santa-Ana-Tellez Y, Gardarsdottir H. Opportunities and challenges for decentralized clinical trials: European regulators’ perspective. Clin Pharmacol Ther. 2022;112(2):344–52. https://doi.org/10.1002/cpt.2628.
Clinical Trials Transformation Initiative. Managing Data. https://ctti-clinicaltrials.org/our-work/digital-health-trials/managing-data/
Apostolaros M, Babaian D, Corneli A, et al. Legal, regulatory, and practical issues to consider when adopting decentralized clinical trials: recommendations from the clinical trials transformation initiative. Ther Innov Regul Sci. 2020;54(4):779–87.
Betcheva L. Applying systems thinking to inform decentralized clinical trial planning and deployment. Ther Innov Regul Sci. 2023;57(5):1081–98. https://doi.org/10.1007/s43441-023-00540-2.
Agrawal G, Xue J, Moss R, Raschke R, Wurtzer S. No place like home? Step** up the decentralization of clinical trials. McKinsey & Co. 2021. https://www.mckinsey.com/industries/life-sciences/our-insights/no-place-like-home-step**-up-the-decentralization-of-clinical-trials
Bedard, R. What Would It Mean for Scientists to Listen to Patients? New Yorker. January 23, 2024. https://www.newyorker.com/news/annals-of-inquiry/what-would-it-mean-for-scientists-to-listen-to-patients
Harvey RD, Miller TM, Hurley PA, Thota R, Black LJ, Bruinooge SS, Boehmer LM, Fleury ME, Kamboj J, Rizvi MA, Symington BE, Tap WD, Waterhouse DM, Levit LA, Merrill JK, Prindiville SA, Pollastro T, Brewer JR, Byatt LP, Hamroun L, Kim ES, Holland N, Nowakowski GS. A call to action to advance patient-focused and decentralized clinical trials. Cancer. 2024. https://doi.org/10.1002/cncr.35145.
Clinical Trials Transformation Initiative. Supporting Sites. https://ctti-clinicaltrials.org/our-work/digital-health-trials/supporting-sites/
FDA Announces Additional Steps to Modernize Clinical Trials. Press release. https://www.fda.gov/news-events/press-announcements/fda-announces-additional-steps-modernize-clinical-trials 6 Jun 2023
Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Draft Guidance Document. May 1, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/decentralized-clinical-trials-drugs-biological-products-and-devices
U.S. Food and Drug Administration. Public Meeting: Mitigating Clinical Study Disruptions During Disasters and Public Health Emergencies (PHEs). Meeting Report. Accessed from: https://ctti-clinicaltrials.org/wp-content/uploads/2024/01/OMP-DORA-Flexibility-Report-Final-240112.pdf
Clinical Trials Transformation Initiative. Recommendations for Interacting with Regulators. https://ctti-clinicaltrials.org/wp-content/uploads/2021/07/CTTI_Interacting_with_Regulators_Recommendations.pdf
Clinical Trials Transformation Initiative. Regulatory Engagement Opportunities when Develo** Digitally Derived Endpoints. https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Novel_Endpoints_FDA_Quick_Reference_Sheet.pdf
Clinical Trials Transformation Initiative. Data Availability for FDA Inspection. https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Digital_Health_Technologies_FDA_Inspection_Figure.pdf
Clinical Trials Transformation Initiative. Data Processes and Information to Provide to FDA. https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Digital_Health_Technologies_Data_to_FDA_Figure.pdf
Harper B, Florez M, Calaprice D, MacDonald C, DiCindio GK. Accelerating innovation adoption to support drug development operations. Appl Clin Trials. 2022;31(3):1.
Acknowledgements
CTTI thanks the CTTI Decentralized Clinical Trials Update Team and all the Digital Health Trials project teams (listed here: https://ctti-clinicaltrials.org/our-work/digital-health-trials/original-digital-health-trials-projects/) for their contributions to CTTI’s Digital Health Trials portfolio of work.
Funding
The CTTI work mentioned and time to prepare this commentary was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of an award totaling $3,778,241.33 with 15% financed with non-governmental sources. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, HHS, or the US Government. For more information, please visit FDA.gov. Partial funding was also provided by pooled membership fees from the Clinical Trials Transformation Initiative's (CTTI) member organizations (https://ctti-clinicaltrials.org/who_we_are/funding/).
Author information
Authors and Affiliations
Contributions
Kehoe, Hanger and Calvert: Substantial contributions to the conception or design of the work; Drafting the work and revising it critically for important intellectual content; Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Hallinan: Substantial contributions to the conception or design of the work; Revising the work critically for important intellectual content; Final approval of the version to be published; Agreement to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no competing interests for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kehoe, L., Calvert, S.B., Hallinan, Z. et al. Adoption of Decentralization: Are Our Perceptions Holding Us Back?. Ther Innov Regul Sci (2024). https://doi.org/10.1007/s43441-024-00636-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43441-024-00636-3