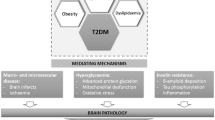
Abstract
Diabetes mellitus (DM) is a metabolic disease that activates several molecular pathways involved in neurodegenerative disorders. Metformin, an anti-hyperglycemic drug used for treating DM, has the potential to exert a significant neuroprotective role against the detrimental effects of DM. This review discusses recent clinical and laboratory studies investigating the neuroprotective properties of metformin against DM-induced neurodegeneration and the roles of various molecular pathways, including mitochondrial dysfunction, oxidative stress, inflammation, apoptosis, and its related cascades. A literature search was conducted from January 2000 to December 2022 using multiple databases including Web of Science, Wiley, Springer, PubMed, Elsevier Science Direct, Google Scholar, the Core Collection, Scopus, and the Cochrane Library to collect and evaluate peer-reviewed literature regarding the neuroprotective role of metformin against DM-induced neurodegenerative events. The literature search supports the conclusion that metformin is neuroprotective against DM-induced neuronal cell degeneration in both peripheral and central nervous systems, and this effect is likely mediated via modulation of oxidative stress, inflammation, and cell death pathways.








Similar content being viewed by others
Data availability
This manuscript is a review article with no unpublished research data included. All references are available upon request to the corresponding author.
Abbreviations
- AChE/Ach:
-
Acetylcholinesterase/acetylcholine
- AD:
-
Alzheimer disease
- AGE:
-
Advanced glycation end products
- ALS:
-
Amyotrophic lateral sclerosis
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- AMPK/Tet2:
-
Adenosine monophosphate-activated protein kinase/Ten-eleven translocation-2
- APP:
-
Amyloid precursor protein
- AOPPs:
-
Advanced oxidation protein products
- Bcl-2:
-
B-cell lymphoma 2
- BDNF:
-
Brain derived neurotropic factor
- BiP:
-
Binding immunoglobulin protein
- CAT:
-
Catalase
- CCL 2 and 20:
-
C–C Motif chemokine ligand 2 and 20
- Cdk5:
-
Cyclin-dependent kinase 5
- CHOP:
-
CCAAT-enhancer-binding protein homologous protein
- ChREBP:
-
Carbohydrate-responsive element-binding protein
- CMA:
-
Chaperone-mediated autophagy
- CNS:
-
Central nervous system
- CoA-SH:
-
Coenzyme A
- COX-PG:
-
Cyclooxygenase-prostaglandin
- cPLA2:
-
Cytosolic phospholipases A2
- CREB:
-
CAMP response element-binding protein
- CRP:
-
C-reactive protein
- CXCL 5 or 7 or 12:
-
C-X-C motif chemokine ligand 5 or 7 or 5
- DAP12:
-
12-Kilodalton DNAX activating protein (also known as TYROBP and KARAP)
- DM:
-
Diabetes mellitus
- eIF2α:
-
Eukaryotic initiation factor-2α
- eNOS:
-
Endothelial nitric oxide synthase
- EPM:
-
Elevated plus maze
- ERK:
-
Extracellular signal-related kinase
- EZM:
-
Elevated zero maze
- FAAD:
-
Fas-associated death domain protein
- FRAP:
-
FKBP-12-rapamycin associated protein (also known as mTOR and RAFT-1)
- FST:
-
Forced swim test
- GABAA:
-
γ-Aminobutyric acid A
- GFAP:
-
Glial fibrillary acidic protein
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon-like peptide-1
- GM-CSF:
-
Colony-stimulating factor
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GRP94:
-
Glucose-regulated protein of 94 kDa
- GSH:
-
Reduced form of glutathione
- GSK3:
-
Glycogen synthase kinase 3 beta
- GSSG:
-
Oxidized form of glutathione
- HD:
-
Huntington disease
- GST:
-
Glutathione S-transferase
- HDL:
-
High density lipoprotein
- HNE:
-
4-Hydroxynonenal
- HPA:
-
Hypothalamic–pituitary–adrenal
- IFN-γ:
-
Interferon gamma
- IGF-1:
-
Insulin-like growth factor-1
- IL:
-
Interleukin (1,2,3,4,5,6,7,8,9,10,11,12)
- iNOS:
-
Inducible nitric oxide synthase
- IRE1α:
-
Inositol-requiring transmembrane kinase/endoribonuclease 1α
- IsoLGs:
-
Isolevuglandins
- JAK/STAT:
-
Janus kinase (JAK)/signal transducer and activator of transcription (STAT)
- LCAT:
-
Lecithin-cholesterol acyltransferase
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- LDL:
-
Low density lipoprotein
- LKB1:
-
Liver kinase B1
- MDA:
-
Malondialdehyde
- MDD:
-
Major depressive disorder
- MiR-141:
-
MicroRNA 141
- MND:
-
Motor neuron disease
- MOMP:
-
Mitochondrial outer membrane permeabilization
- MS:
-
Multiple sclerosis
- MSA:
-
Multiple system atrophy
- mTORC1:
-
Mammalian target of rapamycin complex 1
- MWM:
-
Morris water maze
- NADPH/NAD+ :
-
Nicotinamide adenine dinucleotide phosphate/nicotinamide adenine dinucleotide
- NGF:
-
Neural growth factor
- NF-kb:
-
Nuclear factor κB
- NO:
-
Nitric oxide
- NORT:
-
Novel object recognition task
- NOS-NO:
-
Nitric oxide synthase-nitric oxide
- nNOS:
-
Neuronal nitric oxide synthase
- NRF-1:
-
Nuclear respiratory factor 1
- NSF:
-
Novelty suppressed feeding
- OFT:
-
Open field test
- OGT:
-
O-linked β-N-acetylglucosamine (O-GlcNAc) transferase
- ONE:
-
4-Oxononenal
- OSI:
-
Oxidative stress index
- PARP:
-
Poly-ADP ribose polymerase
- PD:
-
Parkinson disease
- PDIA:
-
Protein disulfide-isomerase
- PERK:
-
Protein kinase RNA-like endoplasmic reticulum kinase
- Pit-1:
-
Pituitary transcriptional factor-1
- PGC1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator
- PNS:
-
Peripheral nervous system
- PON 1:
-
Serum paraoxonase and arylesterase 1
- PP2A:
-
Protein phosphatase 2
- PRD:
-
Pelvic radiation disease
- Preb:
-
Prolactin regulatory element-binding
- PTP:
-
Permeability transition pore
- RAGE:
-
Receptor for advanced glycation end products
- RAM:
-
Radial arm maze
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SCA:
-
Spinocerebellar ataxia
- SIRT-SirT1:
-
Silent mating type information regulation 2 homolog
- SMAC:
-
Second mitochondria-derived activator of caspase
- SMA:
-
Spinal muscular atrophy
- SOD:
-
Super oxide dismutase
- ST:
-
Splash test
- STZ :
-
Streptozotocin
- TAC:
-
Total antioxidant capacity
- TAS:
-
Total antioxidant status
- TBARS:
-
Thiobarbituric acid reactive substances
- TC:
-
Total cholesterol
- Tfam:
-
Transcription factor A mitochondrial
- TGF-β:
-
Transforming growth factor beta
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor alpha
- TOS:
-
Total oxidant status
- TREM-1:
-
Triggering receptor expressed on myeloid cells 1
- TST:
-
Tail suspension tests
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- TXNIP:
-
Thioredoxin-interacting protein
- ULK:
-
Unc-51-like autophagy activating kinase
- VEGF:
-
Vascular endothelial growth factor
- VLDL:
-
Very low density protein
- XIAP:
-
X-linked inhibitor of apoptosis
References
Perneczky R, Kempermann G, Korczyn AD, Matthews FE, Ikram MA, Scarmeas N, et al. Translational research on reserve against neurodegenerative disease: consensus report of the International Conference on Cognitive Reserve in the Dementias and the Alzheimer’s Association Reserve, Resilience and Protective Factors Professional Interest Area working groups. BMC Med. 2019;17:1–15.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69.
Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–83.
Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012: 428010.
**ang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis. 2017;22:1–26.
Cai W, Zhang K, Li P, Zhu L, Xu J, Yang B, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77–87.
Weis S, Büttner A. Neurotoxicology and drug-related disorders. Handb Clin Neurol. 2017;145:181–92.
Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12:267–77.
Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1037–45.
Ochoa-Sanchez R, Tamnanloo F, Rose CF. Hepatic encephalopathy: from metabolic to neurodegenerative. Neurochem Res. 2021;46:2612–25.
Kelly DM, Rothwell PM. Disentangling the relationship between chronic kidney disease and cognitive disorders. Front Neurol. 2022;13: 830064.
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–81.
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harbor Perspect Biol. 2016;9: a028035.
Toth C. Diabetes and neurodegeneration in the brain. Handb Clin Neurol. 2014;126:489–511.
Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38.
Kozakova M, Morizzo C, Goncalves I, Natali A, Nilsson J, Palombo C. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol. 2019;18:1–11.
Li X, Jiao Y, **ng Y, Gao P. Diabetes mellitus and risk of hepatic fibrosis/cirrhosis. BioMed Res Int. 2019;2019:5308308.
Mazzucco G, Bertani T, Fortunato M, Bernardi M, Leutner M, Boldorini R, et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis. 2002;39:713–20.
Visca D, Pignatti P, Spanevello A, Lucini E, La Rocca E. Relationship between diabetes and respiratory diseases—clinical and therapeutic aspects. Pharmacol Res. 2018;137:230–5.
Soni D, Sagar P, Takkar B. Diabetic retinal neurodegeneration as a form of diabetic retinopathy. Int Ophthalmol. 2021;41:3223–48.
Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60–84.
Morsi M, Maher A, Aboelmagd O, Johar D, Bernstein L. A shared comparison of diabetes mellitus and neurodegenerative disorders. J Cell Biochem. 2018;119:1249–56.
Iqbal K, Liu F, Gong C-X. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol. 2016;12:15–27.
Cheng H, Gang X, Liu Y, Wang G, Zhao X, Wang G. Mitochondrial dysfunction plays a key role in the development of neurodegenerative diseases in diabetes. Am J Physiol Endocrinol. 2020;318:E750–64.
Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111:431–3.
Mule NK, Singh JN. Diabetes mellitus to neurodegenerative disorders: is oxidative stress fueling the flame? CNS Neurol Disord Drug Targets. 2018;17:644–53.
Buyuktepe TC, Demirel S, Batıoğlu F, Özmert E. The correlation of inflammation and microvascular changes with diabetic retinal neurodegeneration. Curr Eye Res. 2021;46:1559–66.
Farhadi A, Vosough M, Zhang J-S, Tahamtani Y, Shahpasand K. A possible neurodegeneration mechanism triggered by diabetes. Trends Endocrinol Metab. 2019;30:692–700.
Mayeda ER, Whitmer RA, Yaffe K. Diabetes and cognition. Clin Geriatr Med. 2015;31:101–15.
Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93:56–62.
Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of type 2 diabetic embryopathy. Diabetes. 2015;64:2526–36.
Nna VU, Abu Bakar AB, Ahmad A, Eleazu CO, Mohamed M. Oxidative stress, NF-κb-mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: combined protective effects of Malaysian propolis and metformin. Antioxidants. 2019;8:465.
Oliveira WH, Nunes AK, França MER, Santos LA, Los DB, Rocha SW, et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016;1644:149–60.
Kim YS, Kim M, Choi MY, Lee DH, Roh GS, Kim HJ, et al. Metformin protects against retinal cell death in diabetic mice. Biochem Biophys Res Commun. 2017;492:397–403.
Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321:1926–7.
Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122:253–70.
Khazaei H, Pesce M, Patruno A, Aneva IY, Farzaei MH. Medicinal plants for diabetes associated neurodegenerative diseases: a systematic review of preclinical studies. Phytother Res. 2021;35:1697–718.
Ebokaiwe AP, Okori S, Nwankwo JO, Ejike CE, Osawe SO. Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 2021;394:591–602.
Mousavi F, Eidi A, Khalili M, Roghani M. Metformin ameliorates learning and memory deficits in streptozotocin-induced diabetic rats. J Basic Clin Physiol. 2018;6:17–22.
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146–57.
El-Mir M-Y, Detaille D, Gloria R, Delgado-Esteban M, Guigas B, Attia S, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008;34:77–87.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–76.
Moreira PI. Metformin in the diabetic brain: friend or foe? Ann Transl Med. 2014;2:54.
Mendonça IP, de Paiva IHR, Duarte-Silva EP, de Melo MG, da Silva RS, de Oliveira WH, et al. Metformin and fluoxetine improve depressive-like behavior in a murine model of Parkinson’s disease through the modulation of neuroinflammation, neurogenesis and neuroplasticity. Int Immunopharmacol. 2022;102: 108415.
Wang Y-W, He S-J, Feng X, Cheng J, Luo Y-T, Tian L, et al. Metformin: a review of its potential indications. Drug Des Devel Ther. 2017;11:2421.
Holt RI, De Groot M, Golden SH. Diabetes and depression. Curr Diabetes Rep. 2014;14:1–9.
Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–60.
Rebolledo-Solleiro D, Crespo-Ramírez M, Roldán-Roldán G, Hiriart M, de la Mora MP. Role of thirst and visual barriers in the differential behavior displayed by streptozotocin-treated rats in the elevated plus-maze and the open field test. Physiol Behav. 2013;120:130–5.
Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–8.
Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8.
Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Mood and anxiety related phenotypes in mice. New Jersey: Humana Press; 2009. p. 1–20.
Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav. 2013;118:227–39.
Smith KJ, Béland M, Clyde M, Gariépy G, Pagé V, Badawi G, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99.
Gupta D, Radhakrishnan M, Kurhe Y. Insulin reverses anxiety-like behavior evoked by streptozotocin-induced diabetes in mice. Metab Brain Dis. 2014;29:737–46.
Gambeta E, de Souza CP, de Morais H, Zanoveli JM. Reestablishment of the hyperglycemia to the normal levels seems not to be essential to the anxiolytic-like effect induced by insulin. Metab Brain Dis. 2016;31:563–71.
Guangpin C, Chuntao L, ** Q, Yue H, **anfang M. Pyrrolidine dithiocarbamate alleviated anxiety in diabetic mice. Indian J Pharm Sci. 2017;79:149–54.
Husain GM, Chatterjee SS, Singh PN, Kumar V. Beneficial effect of Hypericum perforatum on depression and anxiety in a type 2 diabetic rat model. Acta Pol Pharm. 2011;68:913–8.
Volchegorskii I, Miroshnichenko IY, Rassokhina L, Faizullin R, Pryakhina K. Anxiolytic and antidepressant effects of emoxipine, reamberin and mexidol in experimental diabetes mellitus. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117:52–7.
Mersha AG, Tollosa DN, Bagade T, Eftekhari P. A bidirectional relationship between diabetes mellitus and anxiety: a systematic review and meta-analysis. J Psychosom Res. 2022;162:110991.
Lin EH, Von Korff M, Consortium WWs. Mental disorders among persons with diabetes—results from the World Mental Health Surveys. J Psychosom Res. 2008;65:571–80.
Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res. 2007;62:31–8.
de Ornelas Maia ACC, de Azevedo BA, Brouwers A, Nardi AE, Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry. 2012;53:1169–73.
Maia ACC, Braga AA, Paes F, Machado S, Nardi AE, Silva AC. Psychiatric comorbidity in diabetes type 1: a cross-sectional observational study. Rev Assoc Med Bras. 2014;60:59–62.
Kontoangelos K, Raptis AE, Papageorgiou CC, Papadimitriou GN, Rabavilas AD, Dimitriadis G, et al. The association of the metabolic profile in diabetes mellitus type 2 patients with obsessive-compulsive symptomatology and depressive symptomatology: new insights. Int J Psychiatry Clin Pract. 2013;17:48–55.
Grassi G, Figee M, Pozza A, Dell’Osso B. Obsessive-compulsive disorder, insulin signaling and diabetes—a novel form of physical health comorbidity: the sweet compulsive brain. Compr Psychiatry. 2022;117:152329.
Ludman E, Katon W, Russo J, Simon G, Von Korff M, Lin E, et al. Panic episodes among patients with diabetes. Gen Hosp Psychiatry. 2006;28:475–81.
Pontow I-M, Theil J, Diefenbacher A. Comorbidity of hypoglycaemia anxiety and panic disorder in a patient with type-1 diabetes-Combined treatment with cognitive-behavioral therapy and Continuous Glucose Monitoring (CGM) in a psychosomatic day-treatment center. Dtsch Med Wochenschr. 1946;2020(145):314–7.
Bădescu S, Tătaru C, Kobylinska L, Georgescu E, Zahiu D, Zăgrean A, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9:120.
Campayo A, De Jonge P, Roy JF, Saz P, De la Cámara C, Quintanilla MA, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–8.
Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20:47.
Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–86.
Rajashree R, Kholkute SD, Goudar SS. Effects of duration of diabetes on behavioural and cognitive parameters in streptozotocin-induced juvenile diabetic rats. Malays J Med Sci MJMS. 2011;18:26.
Farzin D, Fathiazad F, Fazellian M. Antidepressant effect of methanolic ginger extract in diabetic mice using forced-swim test. J Mazandaran Univ Med Sci. 2013;23:208–20.
da Silva HA, Sitta A, Barschak AG, Deon M, Barden AT, Schmitt GO, et al. Oxidative stress parameters in diabetic rats submitted to forced swimming test: the clonazepam effect. Brain Res. 2007;1154:137–43.
Wayhs CAY, Manfredini V, Sitta A, Deon M, Ribas G, Vanzin C, et al. Protein and lipid oxidative damage in streptozotocin-induced diabetic rats submitted to forced swimming test: the insulin and clonazepam effect. Metab Brain Dis. 2010;25:297–304.
Bhutada P, Mundhada Y, Bansod K, Bhutada C, Tawari S, Dixit P, et al. Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol Learn Mem. 2010;94:293–302.
Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121:S8–15.
Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract. 2013;99:98–104.
Mosili P, Mkhize BC, Ngubane P, Sibiya N, Khathi A. The dysregulation of the hypothalamic–pituitary–adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr Metab. 2020;17:1–12.
Tirabassi G, Chelli F, Ciommi M, Lenzi A, Balercia G. Influence of the hypothalamic–pituitary–adrenal axis dysregulation on the metabolic profile of patients affected by diabetes mellitus-associated late onset hypogonadism. Nutr Metab Cardiovasc Dis. 2016;26:53–9.
Tirabassi G, Muscogiuri G, Colao A, Balercia G. Dysregulation of the hypothalamic–pituitary–adrenal axis increases central body fat accumulation in males affected by diabetes mellitus and late-onset hypogonadism. Endocr Pract. 2016;22:427–33.
Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–62.
Yamato T, Misumi Y, Yamasaki S, Kino M, Aomine M. Diabetes mellitus decreases hippocampal release of neurotransmitters: an in vivo microdialysis study of awake, freely moving rats. Diabetes Nutr Metab. 2004;17:128–36.
Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, et al. Regulatory mechanism of hypothalamo-pituitary–adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010;40:130–9.
Ramasubbu K, Devi RV. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem. 2022;1:1–18.
Shpakov A, Chistyakova O, Derkach K, Bondareva V. Hormonal signaling systems of the brain in diabetes mellitus. In: Chang RC-C, editor. Neurodegenerative diseases—processes, prevention, protection and monitoring. USA: InTech; 2011. p. 349–86.
Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes. 2016;7:412.
Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511.
Ahmadi M, Rajaei Z, Hadjzadeh M, Nemati H, Hosseini M. Crocin improves spatial learning and memory deficits in the Morris water maze via attenuating cortical oxidative damage in diabetic rats. Neurosci Lett. 2017;642:1–6.
Farbood Y, Rashno M, Ghaderi S, Khoshnam SE, Sarkaki A, Rashidi K, et al. Ellagic acid protects against diabetes-associated behavioral deficits in rats: possible involved mechanisms. Life Sci. 2019;225:8–19.
Roberts RO, Geda YE, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–73.
Ebady S, Arami M, Shafigh M. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2008;82:305–9.
Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and cognitive impairment. Curr Diabetes Rep. 2016;16:1–11.
Vijayakumar T, Sirisha G, Farzana Begam M, Dhanaraju M. Mechanism linking cognitive impairment and diabetes mellitus. Eur J Appl Sci. 2012;4:01–5.
Luchsinger JA. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimer’s Dis. 2012;30:S185–98.
Martins LB, Braga Tibaes JR, Berk M, Teixeira AL. Diabetes and mood disorders: shared mechanisms and therapeutic opportunities. Int J Psychiatry Clin Pract. 2022;26(2):183–95.
McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–9.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Kowluru RA, Chan P-S. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603.
Yang H, ** X, Lam CWK, Yan S-K. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011;49:1773–82.
Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014: 102158.
Babizhayev MA, Strokov IA, Nosikov VV, Savel’yeva EL, Sitnikov VF, Yegorov YE, et al. The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem Biophys. 2015;71:1425–43.
Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45.
Kahya MC, Nazirolu M, Övey İS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ sup2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54:2345.
Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, et al. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radical Biol Med. 2013;65:978–87.
Kumawat M, Singh N, Singh S. Status of antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus with neuropathy. Ann Neurosci. 2010;12:49–52.
Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85:713–20.
Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–28.
Calderon G, Juarez O, Hernandez G, Punzo S, De la Cruz Z. Oxidative stress and diabetic retinopathy: development and treatment. Eye. 2017;31:1122–30.
Silva KC, Rosales MA, Biswas SK, Lopes de Faria JB, Lopes de Faria JM. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382–90.
Rosales-Corral S, Tan D-X, Manchester L, Reiter RJ. Diabetes and Alzheimer disease, two overlap** pathologies with the same background: oxidative stress. Oxid Med Cell Longevity. 2015;2015: 985845.
Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330.
Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:283–90.
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J. 2016;24:547–53.
Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes/Metab Res Rev. 2006;22:257–73.
Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev. 2013;2013: 168039.
Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I, et al. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012;39:8669–78.
Pan H-Z, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92:548–51.
Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311:174–90.
Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61:187–96.
West IC. Radicals and oxidative stress in diabetes. Diabetic Med. 2000;17:171–80.
Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol Res. 2010;59:147–56.
Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, et al. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012: 696215.
Tiwari BK, Pandey KB, Abidi A, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013: 378790.
Pereira RVF, Tronchini EA, Tashima CM, Alves EPB, Lima MM, Zanoni JN. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci. 2011;56:3507–16.
Xue HY, ** L, ** LJ, Li XY, Zhang P, Ma YS, et al. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res. 2009;23:980–6.
Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015: 515042.
Aruoma OI, Neergheen VS, Bahorun T, Jen L-S. Free radicals, antioxidants and diabetes: embryopathy, retinopathy, neuropathy, nephropathy and cardiovascular complications. Neuroembryol Aging. 2006;4:117–37.
Hassan A, Kandel RS, Mishra R, Gautam J, Alaref A, Jahan N. Diabetes mellitus and Parkinson’s disease: shared pathophysiological links and possible therapeutic implications. Cureus. 2020;12: e9853.
Domínguez R, Pagano M, Marschoff E, González S, Repetto M, Serra J. Alzheimer disease and cognitive impairment associated with diabetes mellitus type 2: associations and a hypothesis. Neurologia. 2014;29:567–72.
Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851.
Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci. 2016;351:380–6.
Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–61.
Song Y, Ding W, Bei Y, **ao Y, Tong H-D, Wang L-B, et al. Insulin is a potential antioxidant for diabetes-associated cognitive decline via regulating Nrf2 dependent antioxidant enzymes. Biomed Pharmacother. 2018;104:474–84.
Ceretta LB, Réus GZ, Abelaira HM, Ribeiro KF, Zappellini G, Felisbino FF, et al. Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp Diabetes Res. 2012;2012: 302682.
Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–13.
Younus H. Therapeutic potentials of superoxide dismutase. Int J Health Sci. 2018;12:88.
Iranzo O. Manganese complexes displaying superoxide dismutase activity: a balance between different factors. Bioorg Chem. 2011;39:73–87.
Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res. 2003;37:471–80.
Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–71.
Shanmugam KR, Mallikarjuna K, Kesireddy N, Reddy KS. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:893–7.
Alipour M, Salehi I, Soufi FG. Effect of exercise on diabetes-induced oxidative stress in the rat hippocampus. Iran Red Crescent Med J. 2012;14:222.
Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15:1–10.
Sytze van Dam P. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2002;18:176–84.
Obrosova IG. Update on the pathogenesis of diabetic neuropathy. Curr Diab Rep. 2003;3:439–45.
Ola M, Alhomida A. Neurodegeneration in diabetic retina and its potential drug targets. Curr Neuropharmacol. 2014;12:380–6.
Simó R, Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25:23–33.
Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:425–33.
Infante-Garcia C, Garcia-Alloza M. Review of the effect of natural compounds and extracts on neurodegeneration in animal models of diabetes mellitus. Int J Mol Sci. 2019;20:2533.
Sofic E, Salkovic-Petrisic M, Tahirovic I, Sapcanin A, Mandel S, Youdim M, et al. Brain catalase in the streptozotocin-rat model of sporadic Alzheimer’s disease treated with the iron chelator–monoamine oxidase inhibitor, M30. J Neural Transm. 2015;122:559–64.
Giordano CR, Roberts R, Krentz KA, Bissig D, Talreja D, Kumar A, et al. Catalase therapy corrects oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Invest Ophthalmol Visual Sci. 2015;56:3095–102.
Kwong-Han K, Zunaina E, Hanizasurana H, Che-Badariah AA, Che-Maraina CH. Comparison of catalase, glutathione peroxidase and malondialdehyde levels in tears among diabetic patients with and without diabetic retinopathy. J Diabetes Metab Disord. 2022;21:681–8.
Djordjević GM, Djurić SS, Djordjević VB, Apostolski S, Zivkovic M. The role of oxidative stress in pathogenesis of diabetic neuropathy: erythrocyte superoxide dismutase, catalase and glutathione peroxidase level in relation to peripheral nerve conduction in diabetic neuropathy patients. In: Croniger C, editor. Role of the adipocyte in development of type 2 diabetes, vol. 2. USA: Intech; 2011. p. 153–72.
Piotrowski P, Wierzbicka K, Smialek M. Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol. 2001;39:147–54.
Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–14.
Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6:551–9.
Chowdhury SKR, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56–65.
Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–8.
Montgomery MK. Mitochondrial dysfunction and diabetes: is mitochondrial transfer a friend or foe? Biology (Basel). 2019;8:33.
Kempuraj D, Thangavel R, Natteru P, Selvakumar G, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurosurg Spine. 2016;1:1003.
Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, **e XS, et al. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:1–12.
Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014: 674987.
Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13:2699–712.
Nagayach A, Patro N, Patro I. Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front Cell Neurosci. 2014;8:355.
Zeng H-Y, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–32.
Zhang T-T, Xue R, Fan S-Y, Fan Q-Y, An L, Li J, et al. Ammoxetine attenuates diabetic neuropathic pain through inhibiting microglial activation and neuroinflammation in the spinal cord. J Neuroinflammation. 2018;15:1–13.
Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–65.
Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabetes Res. 2003;4:303–12.
Cheung CMG, Vania M, Ang M, Chee SP, Li J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis. 2012;18:830.
Chatzigeorgiou A, Harokopos V, Mylona-Karagianni C, Tsouvalas E, Aidinis V, Kamper E. The pattern of inflammatory/anti-inflammatory cytokines and chemokines in type 1 diabetic patients over time. Ann Med. 2010;42:426–38.
King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–34.
Saleh A, Smith DR, Balakrishnan S, Dunn L, Martens C, Tweed CW, et al. Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: Diminished expression in diabetes may contribute to sensory neuropathy. Brain Res. 2011;1423:87–95.
Purwata TE. High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res. 2011;4:169.
Mendiola AS, Cardona AE. The IL-1β phenomena in neuroinflammatory diseases. J Neural Transm. 2018;125:781–95.
Andriambeloson E, Baillet C, Vitte PA, Garotta G, Dreano M, Callizot N. Interleukin-6 attenuates the development of experimental diabetes-related neuropathy. Neuropathol. 2006;26:32–42.
Herder C, Carstensen M, Ouwens D. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes Metab. 2013;15:39–50.
Zhang W, Liu H, Rojas M, Caldwell RW, Caldwell RB. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3:609–28.
Carbonetto P, Stephens M. Integrated enrichment analysis of variants and pathways in genome-wide association studies indicates central role for IL-2 signaling genes in type 1 diabetes, and cytokine signaling genes in Crohn’s disease. PLoS Genet. 2013;9: e1003770.
Creusot RJ, Chang P, Healey DG, Tcherepanova IY, Nicolette CA, Fathman CG. A short pulse of IL-4 delivered by DCs electroporated with modified mRNA can both prevent and treat autoimmune diabetes in NOD mice. Mol Ther. 2010;18:2112–20.
Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26:685–98.
Rodrigues KF, Pietrani NT, Bosco AA, Campos FMF, Sandrim VC, Gomes KB. IL-6, TNF-α, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch Endocrinol Metab. 2017;61(5):438–46.
Hanifi-Moghaddam P, Kappler S, Seissler J, Müller-Scholze S, Martin S, Roep B, et al. Altered chemokine levels in individuals at risk of type 1 diabetes mellitus. Diabetic Med. 2006;23:156–63.
Herder C, Haastert B, Müller-Scholze S, Koenig W, Thorand B, Holle R, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Diabetes. 2005;54(Suppl 2):S11–7.
Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435–44.
Laaksonen D, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen T-P, Valkonen V-P, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403–10.
Chase HP, Cooper S, Osberg I, Stene LC, Barriga K, Norris J, et al. Elevated C-reactive protein levels in the development of type 1 diabetes. Diabetes. 2004;53:2569–73.
De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–72.
Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:942.
Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:1–15.
Debnath M, Agrawal S. Diabetic neuropathy: oxidative stress and neuroinflammation. Med Res. 2016;3:237–41.
Madonna R, Giovannelli G, Confalone P, Renna FV, Geng Y-J, De Caterina R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: implications for diabetic retinopathy. Cardiovasc Diabetol. 2016;15:1–14.
Wong WT, Tian XY, Huang Y. Endothelial dysfunction in diabetes and hypertension: cross talk in RAS, BMP4, and ROS-dependent COX-2–derived prostanoids. J Cardiovasc Pharmacol. 2013;61:204–14.
Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, et al. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol. 2005;187:37–44.
Costagliola C, Romano V, De Tollis M, Aceto F, Romano MR, Pedicino C, et al. TNF-alpha levels in tears: a novel biomarker to assess the degree of diabetic retinopathy. Mediators Inflamm. 2013;2013: 629529.
Serasanambati M, Chilakapati SR. Function of nuclear factor kappa B (NF-kB) in human diseases—a review. South Indian J Biol Sci. 2016;2:368–87.
Najafi R, Hosseini A, Ghaznavi H, Mehrzadi S, Sharifi AM. Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res Bull. 2017;131:117–22.
Patel S, Santani D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61:595–603.
Li J, Tang Y, Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012.
Granic I, Dolga AM, Nijholt IM, van Dijk G, Eisel UL. Inflammation and NF-κB in Alzheimer’s disease and diabetes. J Alzheimer’s Dis. 2009;16:809–21.
Yun JH, Lee DH, Jeong HS, Kim HS, Ye SK, Cho CH. STAT3 activation in microglia exacerbates hippocampal neuronal apoptosis in diabetic brains. J Cell Physiol. 2021;236:7058–70.
Chowdhury SR, Saleh A, Akude E, Smith DR, Morrow D, Tessler L, et al. Ciliary neurotrophic factor reverses aberrant mitochondrial bioenergetics through the JAK/STAT pathway in cultured sensory neurons derived from streptozotocin-induced diabetic rodents. Cell Mol Neurobiol. 2014;34:643–9.
Cho C-H, Roh K-H, Lim N-Y, Park SJ, Park S, Kim HW. Role of the JAK/STAT pathway in a streptozotocin-induced diabetic retinopathy mouse model. Graefes Arch Clin Exp Ophthalmol. 2022;260:3553–63.
Abdul Y, Abdelsaid M, Li W, Webb RC, Sullivan JC, Dong G, et al. Inhibition of toll-like receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: possible role of vascular endothelial TLR-4. Mol Neurobiol. 2019;56:1607–17.
Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci. 2012;122:203–14.
Wong FS, Wen L. Toll-like receptors and diabetes. Ann N Y Acad Sci. 2008;1150:123–32.
Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–83.
Muranyi M, Fujioka M, He Q, Han A, Yong G, Csiszar K, et al. Diabetes activates cell death pathway after transient focal cerebral ischemia. Diabetes. 2003;52:481–6.
Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–6.
Li Z-G, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–31.
Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Visual Sci. 2011;52:1156–63.
Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z. The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med. 2016;7:57.
Adamiec-Mroczek J, Zajac-Pytrus H, Misiuk-Hojlo M. Caspase-dependent apoptosis of retinal ganglion cells during the development of diabetic retinopathy. Adv Clin Exp Med. 2015;24:531–5.
Soufi FG, Mohammad-Nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress—nuclear factor κb—apoptosis pathway. Pharmacol Rep. 2012;64:1505–14.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6.
Lavrik IN. Systems biology of apoptosis signaling networks. Curr Opin Biotechnol. 2010;21:551–5.
Kong F-J, Ma L-L, Guo J-J, Xu L-H, Li Y, Qu S. Endoplasmic reticulum stress/autophagy pathway is involved in diabetes-induced neuronal apoptosis and cognitive decline in mice. Clin Sci. 2018;132:111–25.
Liu Y-P, Shao S-J, Guo H-D. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459.
Liu J, Liu L, Han YS, Yi J, Guo C, Zhao HQ, et al. The molecular mechanism underlying mitophagy-mediated hippocampal neuron apoptosis in diabetes-related depression. J Cell Mol Med. 2021;25:7342–53.
Zhang X, Xu L, He D, Ling S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. Biomed Res Int. 2013;2013: 924327.
Nazarnezhad S, Rahmati M, Shayannia A, Abbasi Z, Salehi M, Khaksari M. Nesfatin-1 protects PC12 cells against high glucose-induced cytotoxicity via inhibiting oxidative stress, autophagy and apoptosis. Neurotoxicology. 2019;74:196–202.
Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, Karmakar P. Is autophagy associated with diabetes mellitus and its complications? A review? EXCLI J. 2018;17:709.
Wang X, Zhang B, **a R, Jia Q. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020;24:9601–14.
Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–5.
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: Mechanisms and diseases. Signal Transduction Targeted Ther. 2021;6:1–21.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Zhang X, Wang N, Barile GR, Bao S, Gillies M. Diabetic retinopathy: neuron protection as a therapeutic target. Int J Biochem Cell Biol. 2013;45:1525–9.
Chen X, Famurewa AC, Tang J, Olatunde OO, Olatunji OJ. Hyperoside attenuates neuroinflammation, cognitive impairment and oxidative stress via suppressing TNF-α/NF-κB/caspase-3 signaling in type 2 diabetes rats. Nutr Neurosci. 2022;25:1774–84.
Park S-H, Park J-W, Park S-J, Kim K-Y, Chung J-W, Chun M-H, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–8.
Shamsaei N, Abdi H, Shamsi M. The effect of a continuous training on necrosis and apoptosis changes in the hippocampus of diabetic rats. J Ilam Univ Med Sci. 2017;25:1–11.
Yang J-S, Lu C-C, Kuo S-C, Hsu Y-M, Tsai S-C, Chen S-Y, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei). 2017;7:8.
Zemdegs J, Martin H, Pintana H, Bullich S, Manta S, Marqués MA, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39:5935–48.
Fatemi I, Khaluoi A, Kaeidi A, Shamsizadeh A, Heydari S. Protective effect of metformin on D-galactose-induced aging model in mice. Iran J Basic Med Sci. 2018;21:19.
Fatemi I, Heydari S, Kaeidi A, Shamsizadeh A, Hakimizadeh E, Khaluoi A, et al. Metformin ameliorates the age-related changes of d-galactose administration in ovariectomized mice. Fundam Clin Pharmacol. 2018;32:392–9.
Fan J, Li D, Chen HS, Huang JG, Xu JF, Zhu WW, et al. Metformin produces anxiolytic-like effects in rats by facilitating GABAA receptor trafficking to membrane. Br J Pharmacol. 2019;176:297–316.
Ji S, Wang L, Li L. Effect of metformin on short-term high-fat diet-induced weight gain and anxiety-like behavior and the gut microbiota. Front Endocrinol. 2019;10:704.
Chen F, Wei G, Wang Y, Liu T, Huang T, Wei Q, et al. Risk factors for depression in elderly diabetic patients and the effect of metformin on the condition. BMC Public Health. 2019;19:1–9.
Li W, Chaudhari K, Shetty R, Winters A, Gao X, Hu Z, et al. Metformin alters locomotor and cognitive function and brain metabolism in normoglycemic mice. Aging Dis. 2019;10:949.
Baptista LC, Machado-Rodrigues AM, Martins RA. Exercise but not metformin improves health-related quality of life and mood states in older adults with type 2 diabetes. Eur J Sport Sci. 2017;17:794–804.
Chen J-L, Luo C, Pu D, Zhang G-Q, Zhao Y-X, Sun Y, et al. Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Exp Neurol. 2019;311:44–56.
Turan I, Özaçmak HS, Erdem S, Ergenc M, Ozacmak VH. Protective effect of metformin against ovariectomy induced depressive-and anxiety-like behaviours in rats: role of oxidative stress. NeuroReport. 2021;32:666–71.
Pak HM, Hassanipour M, Kaeidi A, Saeed AP, Fatemi I, Rahmani MR, et al. Effect of metformin on some cognitive functions in old rats. J Shahid Sadoughi Univ Med Sci. 2020;28:2479–89.
Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis. 2017;8:17.
Nguyena HD, Hoanga NMH, Joa WH, Hamb JR, Leeb M-K, Kima MS. Associations among the TREM-1 pathway, tau hyperphosphorylation, prolactin expression, and metformin in diabetes mice. NeuroImmunoModulation. 2022;29:359–68.
Wang Y, Liu B, Yang Y, Wang Y, Zhao Z, Miao Z, et al. Metformin exerts antidepressant effects by regulated DNA hydroxymethylation. Epigenomics. 2019;11:655–67.
Wang Y, Zhao J, Guo F-L, Gao X, **e X, Liu S, et al. Metformin ameliorates synaptic defects in a mouse model of AD by inhibiting Cdk5 activity. Front Cell Neurosci. 2020;14:170.
Alhowail A, Chigurupati S, Sajid S, Mani V. Ameliorative effect of metformin on cyclophosphamide-induced memory impairment in mice. Eur Rev Med Pharmacol Sci. 2019;23:9660–6.
Qin Z, Zhou C, **ao X, Guo C. Metformin attenuates sepsis-induced neuronal injury and cognitive impairment. BMC Neurosci. 2021;22:1–10.
Chen J, Zhou T, Guo A-M, Chen W-B, Lin D, Liu Z-Y, et al. Metformin ameliorates lipopolysaccharide-induced depressive-like behaviors and abnormal glutamatergic transmission. Biology. 2020;9:359.
Yang S, Chen X, Xu Y, Hao Y, Meng X. Effects of metformin on lipopolysaccharide-induced depressive-like behavior in mice and its mechanisms. NeuroReport. 2020;31:305–10.
Fang W, Zhang J, Hong L, Huang W, Dai X, Ye Q, et al. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J Affect Disord. 2020;260:302–13.
Shivavedi N, Kumar M, Tej GNVC, Nayak PK. Metformin and ascorbic acid combination therapy ameliorates type 2 diabetes mellitus and comorbid depression in rats. Brain Res. 2017;1674:1–9.
Keshavarzi S, Kermanshahi S, Karami L, Motaghinejad M, Motevalian M, Sadr S. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 2019;72:74–84.
Hammad AM, Ibrahim YA, Khdair SI, Hall FS, Alfaraj M, Jarrar Y, et al. Metformin reduces oxandrolone-induced depression-like behavior in rats via modulating the expression of IL-1β, IL-6, IL-10 and TNF-α. Behav Brain Res. 2021;414: 113475.
Kotagale N, Rahangdale S, Borkar A, Singh K, Ikhar A, Takale N, et al. Possible involvement of agmatine in neuropharmacological actions of metformin in diabetic mice. Eur J Pharmacol. 2021;907: 174255.
Delanogare E, Bullich S, Barbosa LAS, Barros WM, Braga SP, Kraus SI, et al. Metformin improves neurobehavioral impairments of streptozotocin-treated and western diet-fed mice: beyond glucose-lowering effects. Fundam Clin Pharmacol. 2022. https://doi.org/10.1111/fcp.12825.
Li G-F, Zhao M, Zhao T, Cheng X, Fan M, Zhu L-L. Effects of metformin on depressive behavior in chronic stress rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chinese J Appl Physiol. 2019;35:245–9.
Oliveira WH, Braga CF, Lós DB, Araújo SMR, França MR, Duarte-Silva E, et al. Metformin prevents p-tau and amyloid plaque deposition and memory impairment in diabetic mice. Exp Brain Res. 2021;239:2821–39.
Chen Y, Zhao S, Fan Z, Li Z, Zhu Y, Shen T, et al. Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice. Alzheimer’s Res Ther. 2021;13:1–13.
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimer’s Dis. 2014;41:61–8.
Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–7.
Son SM, Shin H-J, Byun J, Kook SY, Moon M, Chang YJ, et al. Metformin facilitates amyloid-β generation by β-and γ-secretases via autophagy activation. J Alzheimer’s Dis. 2016;51:1197–208.
Xu X, Sun Y, Cen X, Shan B, Zhao Q, **e T, et al. Metformin activates chaperone-mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model. Protein Cell. 2021;12:769–87.
Picone P, Vilasi S, Librizzi F, Contardi M, Nuzzo D, Caruana L, et al. Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging (Albany NY). 2016;8:1718.
Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41:650–6.
Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, et al. Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: the Sydney Memory and Ageing Study. Diabetes Care. 2020;43:2691–701.
Hofmann P. Treatment of patients with comorbid depression and diabetes with metformin and milnacipran. Neuropsychiatr Dis Treat. 2010;6:9.
Soldevila-Domenech N, Cuenca-Royo A, Babio N, Forcano L, Nishi S, Vintró-Alcaraz C, et al. Metformin use and cognitive function in older adults with type 2 diabetes following a Mediterranean diet intervention. Front Nutr. 2021;8: 742586.
Koo BK, Kim LK, Lee JY, Moon MK. Taking metformin and cognitive function change in older patients with diabetes. Geriatr Gerontol Int. 2019;19:755–61.
Campbell JM, Stephenson MD, De Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimer’s Dis. 2018;65:1225–36.
Ying M, Maruschak N, Mansur R, Carvalho A, Cha D, McIntyre R. Metformin: repurposing opportunities for cognitive and mood dysfunction. CNS Neurol Disord Drug Targets. 2014;13:1836–45.
Rezano A, Khairinnisa A, Ekawardhani S. Metformin as an antidepressant in type 2 diabetes mellitus patients. Depression. 2020;1101:11–2.
Ha J, Choi D-W, Kim KJ, Cho SY, Kim H, Kim KY, et al. Association of metformin use with Alzheimer’s disease in patients with newly diagnosed type 2 diabetes: a population-based nested case–control study. Sci Rep. 2021;11:1–9.
Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM. Metformin—a future therapy for neurodegenerative diseases. Pharm Res. 2017;34:2614–27.
Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, et al. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220:30–41.
Saliu JA, Oboh G, Omojokun OS, Rocha JB, Schetinger MR, Guterries J, et al. Effect of dietary supplementation of Padauk (Pterocarpus soyauxii) leaf on high fat diet/streptozotocin induced diabetes in rats’ brain and platelets. Biomed Pharmacother. 2016;84:1194–201.
Arafa NM, Marie M-AS, Al Azimi SAM. Effect of canagliflozin and metformin on cortical neurotransmitters in a diabetic rat model. Chem Biol Interact. 2016;258:79–88.
Mostafa DK, Ismail CA, Ghareeb DA. Differential metformin dose-dependent effects on cognition in rats: role of Akt. Psychopharmacology. 2016;233:2513–24.
Pilipenko V, Narbute K, Pupure J, Langrate IK, Muceniece R, Kluša V. Neuroprotective potential of antihyperglycemic drug metformin in streptozocin-induced rat model of sporadic Alzheimer’s disease. Eur J Pharmacol. 2020;881: 173290.
Sridhar GR, Lakshmi G, Nagamani G. Emerging links between type 2 diabetes and Alzheimer’s disease. World J Diabetes. 2015;6:744–51.
Sciannimanico S, Grimaldi F, Vescini F, De Pergola G, Iacoviello M, Licchelli B, et al. Metformin: up to date. Endocr Metab Immune Disord Drug Targets. 2020;20:172–81.
Ma J, Yu H, Liu J, Chen Y, Wang Q, **ang L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur J Pharmacol. 2015;764:599–606.
Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192:233–42.
Mehta V, Verma P, Sharma N, Sharma A, Thakur A, Malairaman U. Quercetin, ascorbic acid, caffeine and ellagic acid are more efficient than rosiglitazone, metformin and glimepiride in interfering with pathways leading to the development of neurological complications associated with diabetes: a comparative in-vitro study. Bull Fac Pharm Cairo Univ. 2017;55:115–21.
Correia S, Carvalho C, Santos MS, Proença T, Nunes E, Duarte AI, et al. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358–64.
Peña-Bautista C, Vento M, Baquero M, Chafer-Pericas C. Lipid peroxidation in neurodegeneration. Clin Chim Acta. 2019;497:178–88.
Hall ED. The contributing role of lipid peroxidation and protein oxidation in the course of CNS injury neurodegeneration and neuroprotection. In: Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. 2015.
Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci. 2013;14:21021–44.
Delkhosh-Kasmaie F, Farshid AA, Tamaddonfard E, Imani M. The effects of safranal, a constitute of saffron, and metformin on spatial learning and memory impairments in type-1 diabetic rats: behavioral and hippocampal histopathological and biochemical evaluations. Biomed Pharmacother. 2018;107:203–11.
Sangi SMA, Al Jalaud NA. Prevention and treatment of brain damage in streptozotocin induced diabetic rats with metformin, Nigella sativa, Zingiber officinale, and Punica granatum. Biomed Res Ther. 2019;6:3274–85.
Mousavi SM, Niazmand S, Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, et al. Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int J Alzheimers Dis. 2015;2015: 493729.
Shiming Z, Mak K-K, Balijepalli MK, Chakravarthi S, Pichika MR. Swietenine potentiates the antihyperglycemic and antioxidant activity of metformin in Streptozotocin induced diabetic rats. Biomed Pharmacother. 2021;139: 111576.
Salman ZK, Refaat R, Selima E, El Sarha A, Ismail MA. The combined effect of metformin and L-cysteine on inflammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. Eur J Pharmacol. 2013;714:448–55.
Chukwunonso Obi B, Chinwuba Okoye T, Okpashi VE, NonyeIgwe C, Olisah AE. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J Diabetes Res. 2016;2016:1635361.
Kashyap H, Gupta S. Analysis of metformin on endogenous antioxidants and oxidative stress in mice brain tissue of alloxan-induced diabetes. Int J Pharm Sci Res. 2019;11:727–37.
Esteghamati A, Eskandari D, Mirmiranpour H, Noshad S, Mousavizadeh M, Hedayati M, et al. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin Nutr. 2013;32:179–85.
Abdulkadir AA, Thanoon IA. Comparative effects of glibenclamide and metformin on C-reactive protein and oxidant/antioxidant status in patients with type II diabetes mellitus. Sultan Qaboos Univ Med J. 2012;12:55.
Signorini AM, Fondelli C, Renzoni E, Puccetti C, Gragnoli G, Giorgi G. Antioxidant effects of gliclazide, glibenclamide, and metformin in patients with type 2 diabetes mellitus. Curr Ther Res. 2002;63:411–20.
Abdul-Hadi MH, Naji MT, Shams HA, Sami OM, Al-Harchan NA-A, Al-Kuraishy HM, et al. Oxidative stress injury and glucolipotoxicity in type 2 diabetes mellitus: the potential role of metformin and sitagliptin. Biomed Biotechnol Res J. 2020;4:166.
Memişoğullari R, Tuerkeli M, Bakan E, Akcay F. Effect of metformin or gliclazide on lipid peroxidation and antioxidant levels in patients with diabetes mellitus. Turk J Med Sci. 2008;38:545–8.
Los DB, de Oliveira WH, Duarte-Silva E, Sougey WWD, de Freitas ESR, de Oliveira AGV, et al. Preventive role of metformin on peripheral neuropathy induced by diabetes. Int Immunopharmacol. 2019;74:105672.
Min HK, Kim SH, Choi JH, Choi K, Kim H-R, Lee S-H. Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean health insurance data. World J Clin Cases. 2021;9:10198.
Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective effects of metformin in diabetic nephropathy. J Cell Physiol. 2017;232:731–42.
Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–68.
Garimella S, Seshayamma V, Rao HJ, Kumar S, Kumar U, Saheb SH. Effect of metformin on lipid profile of type II diabetes. Int J Intg Med Sci. 2016;3:449–53.
Waisundara VY, Hsu A, Huang D, Tan BK-H. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chinese Med. 2008;36:517–40.
Zhang S, Xu H, Yu X, Wu Y, Sui D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med. 2017;14:383–90.
Demaré S, Kothari A, Calcutt NA, Fernyhough P. Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system. Expert Rev Neurother. 2021;21:45–63.
Vial G, Detaille D, Guigas B. Role of mitochondria in the mechanism (s) of action of metformin. Front Endocrinol. 2019;10:294.
Andrzejewski S, Gravel S-P, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:1–14.
Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabetic Med. 2004;21:203–7.
Szablewski L. Role of immune system in type 1 diabetes mellitus pathogenesis. Int Immunopharmacol. 2014;22:182–91.
Docrat TF, Nagiah S, Chuturgoon AA. Metformin protects against neuroinflammation through integrated mechanisms of miR-141 and the NF-ĸB-mediated inflammasome pathway in a diabetic mouse model. Eur J Pharmacol. 2021;903: 174146.
Vuong B, Odero G, Rozbacher S, Stevenson M, Kereliuk SM, Pereira TJ, et al. Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J Neuroinflammation. 2017;14:1–13.
Mudgal J, Nampoothiri M, Basu Mallik S, Kinra M, Hall S, Grant G, et al. Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology. 2019;27:941–8.
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, et al. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci. 2020;255: 117861.
Tanokashira D, Kurata E, Fukuokaya W, Kawabe K, Kashiwada M, Takeuchi H, et al. Metformin treatment ameliorates diabetes-associated decline in hippocampal neurogenesis and memory via phosphorylation of insulin receptor substrate 1. FEBS Open Bio. 2018;8:1104–18.
Li Y, Gappy S, Liu X, Sassalos T, Zhou T, Hsu A, et al. Metformin suppresses pro-inflammatory cytokines in vitreous of diabetes patients and human retinal vascular endothelium. PLoS ONE. 2022;17: e0268451.
Dehkordi AH, Abbaszadeh A, Mir S, Hasanvand A. Metformin and its anti-inflammatory and anti-oxidative effects; new concepts. J renal inj prev. 2018;8:54–61.
Zhang Y, Zhang Y, Shi X, Han J, Lin B, Peng W, et al. Metformin and the risk of neurodegenerative diseases in patients with diabetes: a meta-analysis of population-based cohort studies. Diabetic Med. 2022;39: e14821.
Zhang W, Zhao L, Zhang J, Li P, Lv Z. Metformin improves cognitive impairment in diabetic mice induced by a combination of streptozotocin and isoflurane anesthesia. Bioengineered. 2021;12:10982–93.
Jiang L-L, Liu L. Effect of metformin on stem cells: molecular mechanism and clinical prospect. World J Stem Cells. 2020;12:1455.
Sartoretto JL, Melo GA, Carvalho MH, Nigro D, Passaglia RT, Scavone C, et al. Metformin treatment restores the altered microvascular reactivity in neonatal streptozotocin-induced diabetic rats increasing NOS activity, but not NOS expression. Life Sci. 2005;77:2676–89.
Liu Y, Huang C, Ceng C, Zhan H, Zheng D, Han W. Metformin enhances nitric oxide production and diminishes Rho kinase activity in rats with hyperlipidemia. Lipids Health Dis. 2014;13:1–7.
HasanpourDehkordi A, Abbaszadeh A, Mir S, Hasanvand A. Metformin and its anti-inflammatory and anti-oxidative effects; new concepts. J Renal Inj Prev. 2019;8:54–61.
Khezri MR, Yousefi K, Mahboubi N, Hodaei D, Ghasemnejad-Berenji M. Metformin in Alzheimer’s disease: an overview of potential mechanisms, preclinical and clinical findings. Biochem Pharmacol. 2022;197:114945.
Alomar SY, Barakat BM, Eldosoky M, Atef H, Mohamed AS, Elhawary R, et al. Protective effect of metformin on rat diabetic retinopathy involves suppression of toll-like receptor 4/nuclear factor-k B expression and glutamate excitotoxicity. Int Immunopharmacol. 2021;90: 107193.
Zhang Q-Q, Li W-S, Liu Z, Zhang H-L, Ba Y-G, Zhang R-X. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: a meta-analysis and systematic review. Medicine (Baltimore). 2020;99: e19378.
Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep. 2019;29(1511–23): e5.
Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte–endothelium interactions. Redox Biol. 2020;34:101517.
Belosludtsev KN, Belosludtseva NV, Dubinin MV. Diabetes mellitus, mitochondrial dysfunction and Ca2+-dependent permeability transition pore. Int J Mol Sci. 2020;21:6559.
El-Mir M-Y, Detaille D, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008;34:77–87.
Zhao M, Li XW, Chen DZ, Hao F, Tao SX, Yu HY, et al. Neuro-protective role of metformin in patients with acute stroke and type 2 diabetes mellitus via AMPK/mammalian target of rapamycin (mTOR) signaling pathway and oxidative stress. Med Sci Monit. 2019;25:2186.
Zhao X, Zeng Z, Gaur U, Fang J, Peng T, Li S, et al. Metformin protects PC12 cells and hippocampal neurons from H2O2-induced oxidative damage through activation of AMPK pathway. J Cell Physiol. 2019;234:16619–29.
Gorgich EAC, Parsaie H, Yarmand S, Baharvand F, Sarbishegi M. Long-term administration of metformin ameliorates age-dependent oxidative stress and cognitive function in rats. Behav Brain Res. 2021;410: 113343.
Mohamed MAE, Abdel-Rahman RF, Mahmoud SS, Khattab MM, Safar MM. Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav. 2020;104: 106893.
Kim SH, Park TS, ** HY. Metformin preserves peripheral nerve damage with comparable effects to alpha lipoic acid in streptozotocin/high-fat diet induced diabetic rats. Diabetes Metab J. 2020;44:842–53.
Nahar N, Mohamed S, Mustapha NM, Lau S, Ishak NIM, Umran NS. Metformin attenuated histopathological ocular deteriorations in a streptozotocin-induced hyperglycemic rat model. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:457–67.
Sanati M, Aminyavari S, Afshari AR, Sahebkar A. Mechanistic insight into the role of metformin in Alzheimer’s disease. Life Sci. 2022;291:120299.
Paudel YN, Angelopoulou E, Piperi C, Shaikh MF, Othman I. Emerging neuroprotective effect of metformin in Parkinson’s disease: a molecular crosstalk. Pharmacol Res. 2020;152: 104593.
Nandini H, Paudel YN, Krishna K. Envisioning the neuroprotective effect of metformin in experimental epilepsy: a portrait of molecular crosstalk. Life Sci. 2019;233: 116686.
Chiang M-C, Cheng Y-C, Chen S-J, Yen C-H, Huang R-N. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res. 2016;347:322–31.
Sharma S, Nozohouri S, Vaidya B, Abbruscato T. Repurposing metformin to treat age-related neurodegenerative disorders and ischemic stroke. Life Sci. 2021;274: 119343.
Tang BL. Could metformin be therapeutically useful in Huntington’s disease? Rev Neurosci. 2020;31:297–317.
Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab. 2013;24:40–7.
Malaguarnera L, Zorena K. Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol. 2016;14:831–9.
Akinola O, Gabriel M, Suleiman A-A, Olorunsogbon F. Treatment of alloxan-induced diabetic rats with metformin or glitazones is associated with amelioration of hyperglycaemia and neuroprotection. Open Diabetes J. 2012;5:8–12.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: MM. Data curation: FK, HJ and MSZ. Formal analysis: AWH and NC-F. Funding acquisition: None declared. Investigation and methodology: MG and MD. Project administration: MM and HJ. Resources and software: MG. Design of figures: MG. Preparation of tables: MS. Supervision: MM. Validation, visualization, writing, review, and editing: MM, AWH, and NC-F.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
The manuscript is a narrative review article and does not require ethical approval. No unpublished studies involving animals or humans were included in the review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karami, F., Jamaati, H., Coleman-Fuller, N. et al. Is metformin neuroprotective against diabetes mellitus-induced neurodegeneration? An updated graphical review of molecular basis. Pharmacol. Rep 75, 511–543 (2023). https://doi.org/10.1007/s43440-023-00469-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00469-1