Abstract
Hydrochar has potential applications in soil improvement and heavy metal remediation. Hydrochar would undergo the process of aging when introduced into the soil, altering its properties. However, recent studies have focused mainly on the artificial aging of hydrochar, which could not reveal the cumulative effect of multiple environmental factors. Therefore, the periodical monitoring of the property and sorption behavior of hydrochar after amending soils is necessary to better understand the multifaceted mechanisms associated with the natural aging of hydrochar. This study selected the sludge-derived hydrochar (SLHC) as a typical hydrochar and applied a 16-month rice–wheat–rice rotation to mimic the natural aging of hydrochar, focusing on changing properties and cadmium (Cd) sorption and literature contrast between aging strategies and biochar types. The porosity, O abundance, and ash content of 16-month aged SLHC increased by 37%, 47%, and 8.5%, respectively, facilitating Cd sorption due to surface complexation, pore sorption, and precipitation. The sorption percentage of Cd to SLHC was in the range of 11–14% for SLHC-A0 and increased to 17–31% for SLHC-A4 and 20–32% for SLHC-A16 after natural aging. The natural aging of SLHC induced by ash content played an essential role in Cd sorption site heterogeneity. Linear regression analysis showed that aging strategies on sorption behavior significantly differed between biochars. Thus, studies involving natural aging with multiple environmental factors are preferred over those involving chemical or biological aging. Future studies should continue to explore the mechanisms of natural aging-induced heavy metal sorption between hydrochar and pyrochar. These results improve insights to appraise the potential of SLHC as soil amendments to alleviate the adverse effects of heavy metal contamination and provide an essential basis for researchers and staff in soil management and environmental prevention.
Graphical Abstract

Highlights
-
-
Aged hydrochar undergoes surface oxidation, mineral complexation, and leaching.
-
-
-
Ash content and SSA play the essential role in enhancing Cd sorption and site heterogeneity.
-
-
-
Aging degree and sorption behavior differ markedly between pyrochar and hydrochar.
-
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the main byproduct in biological wastewater treatment, waste-activated sludge is an important secondary environmental pollution source and a public hazard (Luo et al. 2016). In China, nearly 6 million tons (wet) of waste-activated sludge is produced annually (Wang et al. 2018a). Increasingly, sludge is considered an abundant and renewable resource rather than a waste given its constituents (Zheng et al. 2019).
Using carbonization technologies, including pyrolysis and hydrothermal carbonization, to carbonize these waste-activated sludge is a topic of current interest (De Carvalho Gomes et al. 2022; Zhuang et al. 2017). Pyrochar (pyrogenic biochar) prepared by pyrolysis technology is carbon-rich with unique properties (e.g. high porosity, larger surface area, more surface charge) belonging to hard carbon. The diverse applications of pyrochar in climate change mitigation, soil remediation, soil improvement and other positive environmental as well as socio-economic effects have become a consensus (De Carvalho Gomes et al. 2022; Yao et al. 2021). Recently, HTC technology is an energy-efficient alternative for sludge as no dehydration is needed and high yields are obtained (Wang et al. 2019a). The HTC solid product hydrochar has received particular attention in various fields, such as soil amendment and energy production (Khiari et al. 2019). Moreover, hydrochar as a soil amendment also has various benefits in improving carbon sequestration, contaminant immobilization, and overall soil quality and fertility (Guan et al. 2021). Therefore, hydrochar may be used as a green material to promote sustainable remediation (Wang et al. 2021b). Because of the hydrophobic–hydrophilic core–shell structure, hydrochar is also investigated as a potential low-cost sorbent of various contaminants, especially for heavy metals. Cadmium (Cd) is a potentially toxic and carcinogenic heavy metal that accumulate in soil and poses a threat to ecosystems and public health (El Rasafi et al. 2020). The type of feedstock used for hydrochar primarily controls its physicochemical properties and affect the sorption of metals by hydrochar the hydrochar. Sludge-derived hydrochar (SLHC) has a relatively high levels of inorganic compounds, including carbonates and phosphates, which precipitate metal ions (Brnardic et al. 2017). These properties make SLHC a potential low-cost sorbent or soil amendment for heavy metals (Cd).
However, hydrochar or pyrochar introduced into the soil undergoes the process of aging, altering the properties of the fresh one. Considering the distinct physicochemical properties of hydrochar and pyrochar, hydrochar as a soft carbon, such as lower carbon content, higher oxygen content, and smaller specific surface area, was speculated to be less stable, which may render a more apparent aging process in the soil than pyrochar, e.g. more leaching of labile water-soluble carbon, stronger mineral complexation, or newly formed porosity. Recent studies have focused mainly on pyrochar natural aging (Yi et al. 2020) and artificial aging of hydrochar, including physical aging (Hou et al. 2021), chemical aging (Wang et al. 2022), and biological aging (Hua et al. 2020). Compared with physical aging (freeze–thaw cycles or high temperature), chemical aging, e.g., acidification or oxidation, has more substantial effects on the physicochemical properties (elemental composition, ash content, (O + N)/C, O abundance, and dissolved organic matter of plant-derived hydrochar (Guan et al. 2021). The sorption capacity of hydrochar increased after chemical aging because of increased C-OR bonds and oxygenated functional groups (Liu et al. 2019b). It has also been shown that oxygenated functional groups increase after physical aging (Guan et al. 2021). Hua et al. (2020) also found that the sorption performance of biologically aged wheat straw hydrochar for Cd was better because of the enhanced functional group complexation, surface electrostatic interaction, ion exchange, and coordination (Hua et al. 2020). The soil environment involves multiple factors, including variations in temperature and water content, redox cycles, farming disturbance, and associated biological and microbial activity (Mia et al. 2017), however, it is worth noting that laboratory experiments often fail to reveal the cumulative effects of multiple environmental factors on the physicochemical properties of hydrochar. Therefore, long-term monitoring of the properties and sorption behavior of hydrochar after amending soils is necessary to better understand the multifaceted mechanisms associated with the natural aging of hydrochar.
This study aimed to elaborate on the natural aging effect on physicochemical properties and the Cd sorption behavior of SLHC. Besides, the correlation of heavy metal sorption capacity with the critical properties of hydrochar and pyrochar under different aging effects was compared with literature and experiment data via linear regression. The main objectives in this study were (1) to determine the natural aging effect on the physicochemical properties of SLHC, (2) to assess the influence of natural aging effects on Cd sorption by SLHC, and (3) to understand the different effects of aging on the properties and sorption behavior of biochar from the aging strategy and biochar types (pyrochar and hydrochar). This study will provide a reference for the effective utilization of SLHC as a soil amendment.
2 Materials and methods
2.1 Natural aging of sludge-derived hydrochar
Waste-activated sludge was collected from Nan**g east sewage treatment plant in Jiangsu Province, China. It was converted to SLHC in a closed self-pressurized hydrothermal reactor for one hour at 260 °C (8 MP). The SLHC was subsequently dried in an oven at 90 °C and sieved through 2 mm (10 mesh), denoted as SLHC-A0. The natural aging experiment was carried out at the Jiangsu Academy of Agricultural Sciences, Nan**g, Jiangsu Province, China (32°2′16″N, 118°51′58″E). According to a previous study, 20 g SLHC-A0 was subsequently placed in a 100-mesh size bag and buried below the soil surface at a 10 cm depth of soil column (30 cm in diameter, 50 cm in height) under a rice–wheat-rice rotation system for a natural aging experiment on July 12, 2018 (Wang et al. 2021a). The mesh bag method guaranteed the SLHC layer as thin as possible (2–5 mm), facilitating more contact with the surrounding soil (Weyers and Spokas 2014). The aged SLHCs were isolated from the soil after 4-month (November 23, 2018) and 16-month (November 14, 2019) of aging and are referred to SLHC-A4 and SLHC-A16, respectively. Subsequently, the aged SLHCs were taken out of the mesh bag and dried naturally without water washing. The details of the rice–wheat–rice rotation system can be found in Additional file 1.
2.2 Characterization
The surface element contents of C, O, Si, N, Al, and Ca in SLHC-A0, SLHC-A4, and SLHC-A16 were measured with X-ray photoelectron spectroscopy (XPS, ESCALAB 250 ** XPS system, Thermo Fisher, UK). The surface functional groups (O abundance) of SLHC samples were analyzed through XPS and Fourier transform infrared spectroscopy (FTIR, a Nicolet iS5 FTIR spectrometer, Thermo Fisher, USA). The total content (C, O, H, N) and ash content of SLHC samples were determined by the Elemental analyzer (EA, a Vario EL cube, Elementar, Germany) and combustion method. The mineral ingredients of SLHC samples were measured with X-ray fluorescence spectrometry (XRF, an ARL 9800XP + XRF, Thermo Fisher, USA). The specific surface area (SSA), total pore volume (Vt), and micropore volume (Vmir) of SLHC samples were measured at 77 K using a surface area and porosimetry analyzer (ASAP 2020, Micromeritics, USA). The surface morphology of SLHC samples was obtained by scanning electron microscope (SEM, a JSM-IT500HR, JEOL, USA). A TG analyzer measured thermogravimetric (TG, TG5500, TA, USA) analysis of SLHC samples in a nitrogen and air atmosphere at a heating rate of 10 °C min−1 from 50 to 800 °C. The recalcitrance index of R50 based on air atmosphere TG was calculated as the equation: R50 = T50,hydrochar/T50,graphite, where T50,hydrochar is the corrected temperatures corresponding to 50% oxidation of the hydrochar and T50,graphite is used as 886 °C according to the reference (Harvey et al. 2012). The dissolved organic matter of water extracts from SLHC samples was analyzed using a total organic carbon (TOC) analyzer (multi N/C 2100, Analytik Jena, Germany). The zeta potential of SLHC samples under different cation concentration solutions was measured using a zeta potential analyzer (Nano ZS 90, Malvern, UK). All the SLHC samples were single sample characterization except for mineral ingredients with average ± standard deviation due to the function of Thermo XRF, and the detailed characterization methods can be found in Additional file 1.
2.3 Sorption experiment
2.3.1 Sorption kinetics
Batch sorption kinetics experiment of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 was conducted with repeated sampling using a 250-mL plastic conical flask. Each flask received 200 mg of SLHC-A0, SLHC-A4, or SLHC-A16 and added the 200 mL aliquot of Cd solution with 18 mg L−1 at pH 7 in triplicate. The sampling times are 0.5, 1, 2, 4, 7, 12, 24, 36, 54, and 72 h.
2.3.2 Sorption isotherm
Sorption isotherm experiment was conducted using 50-mL plastic centrifuge tubes with the addition of 50 mL aliquot of different concentrations (15, 18, 21.6, 26, and 32 mg L−1) Cd solution and 50 mg of SLHC-A0, SLHC-A4, or SLHC-A16 in duplicate, which was conducted on a constant temperature oscillating chamber with 120 rpm at 25 °C for 72 h.
2.3.3 Site energy distribution
The site energy distribution of hydrochar can be deduced from the Langmuir model based on an assumption of the distribution of site energies, expressed as Eq. (1).
where Ce is the equilibrium aqueous-phase concentration, Qe (Ce) is the total sorbed solid-phase concentration, Qh (E, Ce) is energetically homogeneous isotherm with sorption energy E, and F(E) is the site energy frequency function of homogeneous sorption site energies.
Based on the condensation theory of Cerofolini, the equilibrium aqueous-phase concentration (Ce) of sorbate is related to the sorption site energy (E) given by Eq. (2).
where Cs is the maximum solubility of solute, Es is the lowest physically realizable sorption energy when Ce = Cs, R is the universal gas constant, T is the absolute temperature, and calculated E* is the divergence of sorption energies between the sorbent surface in the solvent and the sorbate, determined by substituting the Ce and Cs into Eq. (2).
Incorporating Eq. (2) into the Langmuir model (Zhou et al. 2019), Qe (E*) was expressed by Eq. (3).
where KL (L/kg) is the Langmuir affinity coefficient, Qmax (mg/kg) is the maximum sorption capacity, and Cs (mg/L) is the maximum solublity of solute.
The approximate site energy distribution F(E*) can be obtained by differentiating the isotherm Qe (E*) with respect to E* as shown by Eq. (4).
2.3.4 Temperature and coexisting cation effect
Two sets of temperature and coexisting ions effects experiments were conducted using 50-mL plastic centrifuge tubes with the addition of 50 mL aliquot of Cd solution with 21.6 mg L−1 and 50 mg of SLHC-A0, SLHC-A4, or SLHC-A16 in triplicate, which was conducted on a constant temperature oscillating chamber with 120 rpm at 25 °C for 72 h. In the temperature effect experiments, sorption was conducted at 278.15 K, 298.15 K, and 318.15 K, respectively. This study selected two common cations in the environment (sodium ion/Na+ and calcium ion/Ca2+) for coexisting cations effect experiments; single-point batch experiments were conducted using background solutions of 0, 0.01, 0.05, and 0.1 mol L−1 of Na+ or Ca2+. The initial concentration of Cd was 21.6 mg L−1, and the equilibrium pH was 7.
2.3.5 Analytical method
The Cd concentration was determined by an inductively coupled plasma mass spectrometer (ICP-OES). The supernatant was filtered through a 0.45-µm membrane into a 10-mL ICP tube for analysis. The calibration curves were six standard concentration levels, covering the test concentration range in the sorption experiment of Cd. The calibration curves for Cd were obtained by measuring the absorbance values at 265 nm. The sorbed Cd was equal to the initial subtracting the final aqueous-phase concentration based on the obtained calibration curve.
2.4 Data collection and category
An extensive literature search was performed using keywords such as “biochar OR hydrochar” AND “aging OR ageing OR chemical aging OR oxidation OR microbial aging OR physical aging” AND “heavy metals” AND “sorption” and the literature was collected from Web of Science and Google Scholar. The included literature was further screened to meet the following criteria: (1) the research is on heavy metals sorption to biochar/hydrochar; (2) the original data of heavy metals sorption capacity and each specific physicochemical property of fresh and aged biochar/hydrochar are measured and can be available in the manuscript or supporting information. From the 13 selected research, detailed information on biochar types (pyrochar or hydrochar), aging strategy (physical aging or chemical aging or biological aging), SSA, (O + N)/C, ash, O abundance, and sorption capacity of pyrochar or hydrochar before and after aging were collected (Additional file 1: Tables S1-S4). The extracted data (sorption capacity and specific physicochemical property value after aging) from literature and this study were divided by the control value of fresh pyrochar/hydrochar to standardize to compare different research.
2.5 Statistical analyses
All statistical analyses were performed using Microsoft Excel 2013 software. One-way analysis of variance analysis was used to evaluate the significant difference at a P < 0.05 probability level with Duncan’s multiple range tests. GraphPad Prism 8 was used for linear regression analysis. Meanwhile, the goodness of fit (R2) and significance (P) of the model were analyzed.
3 Results
3.1 Element and ash/mineral ingredients of fresh and aged SLHC
The surface element composition, bulk element composition, and ash content of SLHC-A0, SLHC-A4, and SLHC-A16 are shown in Table 1. The surface elements contained in the SLHC-A0, SLHC-A4, and SLHC-A16 are mainly C (44.84%–52.28%) and O (34.33%–38.27%), with a small amount of N, Si, and metal elements Ca as well as Al. A decrease was seen in C content (from 52.28% to 44.84%) and Ca content (from 2.26% to 1.80%) with natural aging. In contrast, the O, Si, N, and Al content increased from 34.33% to 38.27%, from 2.42% to 2.68%, from 1.36% to 2.32% and from 8.35% to 10.08%. A uniform downward trend was seen from the bulk elemental composition of SLHC-A0, SLHC-A4, and SLHC-A16 (Table 1). For example, C content dropped from 11.43% to 8.55%, O content decreased from 15.42% to 13.28%, H content reduced from 2.27% to 1.77%, and N content declined from 1.28% to 0.90%. Compared with SLHC-A0, the higher O/C and (O + N)/C indicated that oxygen-containing functional groups gained in the aged hydrochar (SLHC-A4 and SLHC-A16) with higher hydrophilicity and polarity. The C/N of SLHC-A16 was much higher than that of SLHC-A0. In comparison, the C/N of SLHC-A4 was slightly lower than that of SLHC-A0 (Table 1). The TOC of water extracts from SLHC was 432.30 mg L−1 for SLHC-A0, 75.26 mg L−1 for SLHC-A4, and 45.25 mg L−1 for SLHC-A16, which fell off with natural aging.
The ash content of SLHC gradually increased from 69.60% to 75.49% over natural aging (Table 1). The ash content of SLHC-A4 and SLHC-A16 increased by 7.84% and 8.46% compared with SLHC-A0, respectively. Ash refers to the residue after calcination, mainly inorganic components, reflected in the mineral ingredients contained in the hydrochar. The primary mineral composition in Table 1 showed that most mineral ingredients, including silicon dioxide/SiO2 (from 12.53 ± 0.18% to 13.56 ± 0.18%), calcium oxide/CaO (11.17 ± 0.17% to 11.62 ± 0.17%), ferric oxide/Fe2O3 (2.58 ± 0.08% to 3.93 ± 0.10%), magnesium oxide/MgO (1.91 ± 0.07% to 2.15 ± 0.07%), aluminum oxide/Al2O3 (16.89 ± 0.21% to 19.01 ± 0.21%), and phosphorus pentoxide/P2O5 (4.49 ± 0.11% to 5.10 ± 0.11%), were enriched after natural aging. Only potassium oxide/K2O decreased from 0.49 ± 0.24% to 0.45 ± 0.02% after natural aging.
3.2 Oxygen functional groups of fresh and aged SLHC
FTIR spectra in Fig. 1 characterized the surface oxygen functional groups of SLHC-A0, SLHC-A4, and SLHC-A16, showing bands mainly from O–H stretching (3400 cm–1), aliphatic –CH2– (2925 cm–1), C≡N (2370 cm–1), C=O (1720 cm–1), C=C (1590 cm–1), and C–O–C (1023 cm–1). The FTIR spectra of hydrochar before and after sorption of Cd were compared. It was found that the intensity of C–O–C, C=O, and C=C significantly reduced after sorption of Cd. In addition, the intensity of C≡N in hydrochar also slightly lowered after sorption.
Fourier transformed infrared spectra of different hydrochars. a SLHC series hydrochars. b SLHC-A0 before and after sorption of Cd. c SLHC-A4 before and after sorption of Cd. d SLHC-A16 before and after sorption of Cd. SLHC-A0 represents fresh sludge-derived hydrochar. SLHC-A4 represents 4-month aged sludge-derived hydrochar. SLHC-A16 represents 16-month aged sludge-derived hydrochar. For the FTIR analysis, hydrochar was measured by a Nicolet iS5 FTIR spectrometer equipped with an iD7 diamond attenuated total reflectance optical base. Each spectrum was taken in the range of 500 to 4000 cm–1 with a resolution of 2 cm–1
The C1s deconvoluted XPS data in Fig. 2a–c and Table 2 further show that the surface functional groups of SLHC have noticeable changes after aging. It can be seen that as the aging time increases, the relative content of C–C/C=C gradually decreases, from 82.10% to 79.70% and then to 73.50%. The O abundance of C–OH in SLHC shows an upward trend, rising from 8.90% to 11.40% and 17.50%. However, compared with O abundance (C=O) in the fresh SLHC (SLHC-A0) of 9.00%, the C=O content of the aged SLHC is essentially unchanged, the C=O content of SLHC-A4 is 8.80%, and the C=O content of SHLC-A16 is 8.90%. The total O abundance (C–OH and C=O) of SHLC-A16 increased by 47% compared with SLHC-A0.
C1s X-ray photoelectron spectroscopy (XPS) spectra, scanning electron microscope (SEM) images, and thermogravimetric (TG) profiles of fresh and aged hydrochars. The peaks with the binding energy of 284.8, 286.1, and 288.0 eV are assigned to the carbon atoms in aromatic rings (C–C/C=C), hydroxyl (C–OH), and carbonyl (C=O), respectively. a, d SLHC-A0; b, e SLHC-A4; c and f SLHC-A16; g in air atmosphere; h in nitrogen atmosphere. SLHC-A0 represents fresh sludge-derived hydrochar. SLHC-A4 represents 4-month aged sludge-derived hydrochar. SLHC-A16 represents 16-month aged sludge-derived hydrochar
3.3 Pore structure of fresh and aged hydrochar
It can be seen from the SEM image (Fig. 2d–f) that the surface morphology of the fresh and aged hydrochar is relatively loose. As shown in Table 6, the pore structure of SLHC developed with natural aging. For SLHC-A0, the SSA is 43.88 m2 g−1 and Vt is 0.27 m3 g−1. After natural aging, the SSA increased to 80.40 m2 g−1 for SLHC-A4 and 77.82 m2 g−1 for SLHC-A16, respectively. The total pore volume changes during the aging process have a similar trend, and Vt of SLHC-A16 is 0.37 cm3 g−1, which has increased by 37.0% compared with SLHC-A0.
3.4 Stability of fresh and aged SLHC
Figure 2g and h shows the TG analysis of SLHC-A0, SLHC-A4, and SLHC-A16. The weight loss of SLHC-A4 and SLHC-A16 in the air atmosphere was much less than that of SLHC-A0, indicating that refractory substances increase after natural aging. To prevent additional reactions with air atmosphere (such as oxygen), we also investigated TG analysis in an inert atmosphere (N2). All SLHC decomposed less in the N2 atmosphere than in the air. The relatively low weight loss observed under the N2 atmosphere indicated that the aged hydrochar has higher thermal stability after natural aging.
3.5 Sorption kinetics and isotherm
Figure 3a compares sorption kinetics of Cd among the SLHC-A0, SLHC-A4, and SLHC-A16, and the sorption kinetics of Cd to SLHC was generally accelerated after natural aging. Two kinetic sorption models (pseudo-first-order kinetic and pseudo-second-order models) could be utilized to explore the sorption kinetic behavior. The detailed kinetic sorption models can be found in the SI. The kinetic fitting parameters are shown in Tables 3, 4, 5, and the pseudo-second-order kinetic model fitted the sorption kinetics well (R2 > 0.99). The pseudo-second-order kinetic sorption rate constants k2 of SLHC-A4 and SLHC-A16 increased 6.00 and 7.00 times, respectively.
a Sorption kinetics, b sorption isotherm, c Langmuir model based-site energy on Cd solid-phase sorption, d Langmuir model based-site energy distribution of Cd to fresh and aged sludge-derived hydrochar, and the relationship between average sorption site energy (E*) and e SSA, f (O + N)/C, g ash content, and h O abundance (C–OH) of fresh and aged sludge-derived hydrochar. SLHC-A0 represents fresh sludge-derived hydrochar. SLHC-A4 represents 4-month aged sludge-derived hydrochar. SLHC-A16 represents 16-month aged sludge-derived hydrochar
To further clarify the natural aging on the sorption affinity of Cd to SLHC, sorption isotherms of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 are presented in Fig. 3b. The fitting parameters are presented in Table 6, and three sorption isotherm models (Langmuir model, Freundlich model, and Temkin model) fitted the sorption isotherm data well (R2 > 0.90). The sorption percentage of Cd to SLHC was in the range of 10.81–14.4% for SLHC-A0 and increased to 16.90–30.93% for SLHC-A4 and 19.55−32.17% for SLHC-A16 after natural aging. The sorption affinity of Cd to SLHC-A4 and SLHC-A16 was elevated by increasing pore structure and surface functional groups as shown in Fig. 3b, supported by a 5.50, 6.97, and 6.58 maximum times increase of KL, KF, and KT, respectively (Table 3). Meanwhile, the maximum sorption capacity of Cd to SLHC-A4 and SLHC-A16 was enhanced by 44.83%−68.97% after natural aging.
3.6 Sorption site energy distribution
Determination of sorption site energy E* of Cd to fresh and aged hydrochar was based on isotherm modeling and Eq. (2), as depicted in Fig. 3c. The sorption site energy E* decreased gradually with Cd solid-phase sorption concentration Qe. Cd was preferentially sorbed to the high-energy sorption sites and then to the low-energy sorption sites. As shown in the curve of F(E*) with E* in Fig. 3d, the plots of F(E*) of SLHC-A4 and SLHC-A16 was much taller than SLHC-A0.
To obtain the average site energies (E*) of Cd sorption on hydrochar, the weighted mean was obtained in the range of the experimental concentrations by merging Eqs. (3) and (4) and expressed as Eq. (5). From Eq. (5), it can be calculated that the average values of E*/μ(E*) of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 were 2.68 × 104, 2.70 × 104, and 2.71 × 104 J/mol, increasing with the aging time. The site energy distribution illustrated in Fig. 3d, characterizes site energy heterogeneity based on standard deviation σe* of site energy distribution. The standard deviation σe* of site energy distribution was able to describe the sorption site energy inhomogeneity, which could quantify by Eqs. (6) and (7) in the range of the experimental concentrations as follows. From Eq. (7), it can be calculated that the standard deviation σe* of site energy distribution for the Cd sorption to SLHC-A0, SLHC-A4, and SLHC-A16 were 586.84, 737.82, and 744.56 J/mol. It was observed that the average sorption site energy μ(E*) with standard deviation σe* on SLHC significantly increased as their SSA (R2 = 0.96), (O + N)/C (R2 = 0.72), ash content (R2 = 0.99), and O abundance (C–OH) (R2 = 0.66) increased (Fig. 3e–h).
3.7 Temperature effect on Cd sorption
The temperature effect (278.15 K, 298.15 K, and 318.15 K) on the sorption of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 was revealed in Fig. 4. The sorption distribution constants (Kd) of Cd to the aged hydrochar gradually increased with Different degrees of temperatures effects were observed in fresh and aged SLHC. For SLHC-A0, the Kd value increased by 1.46 folds at 298.15 K and 3.41 folds at 318.15 K, respectively. For SLHC-A4 and SLHC-A16, the Kd value increased by 1.47 and 1.49 folds at 298.15 K and 4.64 and 5.44 folds at 318.15 K, respectively.
Effect of temperature on the Cd sorption (a) and their linear regression curve (b); effect of coexisting cations on the Cd sorption (histogram) and zeta potential (scatter diagram) to fresh and aged sludge-derived hydrochar. c SLHC-A0, fresh sludge-derived hydrochar. d SLHC-A4, 4-month aged sludge-derived hydrochar. e SLHC-A16, 16-month aged sludge-derived hydrochars. A probability level of P < 0.05 (*) was used to indicate statistical significance, which is compared with control (278.15 K data in temperature effect and CK data in cation effect)
To estimate whether the sorption of Cd to SLHC is spontaneous, thermodynamic considerations are needed to conclude. Table 3 represents the thermodynamic parameters calculated using the thermodynamic model. The ΔG0 is negative, indicating that sorption of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 is a spontaneous process. ΔG0 becomes more negative with with increasing temperature, indicating that the tendency to sorption increases at the higher temperatures. ΔH0 is positive, indicating that all the sorption of Cd to SLHC-A0, SLHC-A4, and SLHC-A16 is an endothermic process. ΔS0 is positive, demonstrating that SLHC spontaneously sorbs Cd in water.
3.8 Cation effect on Cd sorption
For SLHC-A0, increasing concentrations of Na+ and Ca2+ significantly enhanced the sorption of Cd (P < 0.05) (Fig. 4). Inversely, for SLHC-A4 and SLHC-A16, Ca2+ significantly inhibited the sorption of Cd on the aged SLHC (P < 0.05). The zeta potential of SLHC-A0 was obviously increased from − 9.76 ± 0.64 mV to − 0.43 ± 0.31 ~ − 0.04 ± 0.03 mV for 0.01 ~ 0.10 mol L−1 of Na+ and to − 0.26 ± 0.10 ~ − 0.02 ± 0.08 mV for 0.01 ~ 0.10 mol L−1 of Ca2+ (P < 0.05). However, the zeta potential of SLHC-A4 only was slightly decreased from − 1.46 ± 0.26 mV to − 1.79 ± 0.12 ~ − 1.53 ± 0.72 mV for 0.01 ~ 0.10 mol L−1 of Na+ and increased to − 1.06 ± 0.32 ~ − 0.33 ± 0.28 mV for 0.01 ~ 0.10 mol L−1 of Ca2+. Likewise, SLHC-A16 also had no obvious change in zeta potential under different Na+ and Ca2+ concentration solutions.
4 Discussion
4.1 Natural aging effect on physicochemical properties of SLHC
The SLHC applied to the soil with a 16-month rice–wheat–rice rotation simultaneously underwent physical, biological, and chemical processes during natural aging. For the physical processes, loss of dissolved organic carbon (82.59–89.53%) from SLHC caused a 9.06–14.23% decrease in surface C content (Table 1), a 2.92–10.47% reduction in the abundance of C–C/C=C (Table 2), and a 77.34–83.23% increase in SSA (Table 6), resulting in a well-developed pore structure which were consistent with previous studies (Liu et al. 2019b). A decrease in surface N content in Table 1 occurred in SLHC-A4 due to the decomposition of labile N components. However, the surface N content of SLHC-A16 in Table 1 significantly increased after 16-month aging, probably caused by preferential surface C-losses, consistent with previous studies (de la Rosa et al. 2018). Besides, some N-containing compounds from nitrogen fertilizer, root exudates, or microbial byproducts might be sorbed to the surface of SLHC. However, they are not the main factors for the alternation of N during the 16 months aging period. With the longer aging time, the labile components in SLHC gradually decomposed, and the durable components, such as the carbon skeleton and minerals, remained in the aged SLHC. Accordingly, the bulk C/N of aged SLHC (SLHC-A16) in Table 1 was higher than that of the fresh SLHC (SLHC-A0). On the one hand, the agricultural potential of aged SLHC (SLHC-A16) might be negatively impacted compared to fresh SLHC because a high C/N ratio makes available soil N a limiting factor for microbial decomposition, resulting in deficient available N level (Liu et al. 2017). On the other hand, enhancing SSA and Vt (Table 2) for aged SLHC is beneficial in increasing soil nutrient fixation, positively affecting its agricultural potential.
Biological processes in this study were not negligible during natural aging; thus, the enhancement of microbial digestion also facilitated decomposing labile components (Liu et al. 2019b), which decreased the content of C and N elements (Table 1) and the abundance of C–C/C=C (Table 2) for the aged SLHCs. Besides, the root exudates or microbial byproducts may also be accessible to the SLHC (de la Rosa et al. 2018). Biological processes were associated with the rise of oxygen functional groups (−COO− and −OH, Table 2) and (O + N)/C in Table 1 on aged SLHC, consistent with previous studies (Hua et al. 2020). It should be noted that the effect of microorganisms on hydrochar natural aging was based on reasonable speculation from previous studies, and more direct evidence for bioprocess-induced aging of hydrochar along with their contributions to sorption behavior change is needed in the future.
Chemical processes were much more complex than the other two processes. First, there were complexation and precipitation processes. A relatively high surface Al content in aged SLHC (Table 1) has been observed due to the interaction with Al-containing mineral ingredients (Lin et al. 2012). The slight decrease in surface Ca content of aged SLHC (Table 1) was attributed to Ca-humate precipitation during natural aging (Yao et al. 2010). The aged SLHC contains more mineral ingredients than fresh SLHC in Table 1 due to the complexation with soil mineral ingredients or phosphate fertilizer interaction, which is also supported by increased surface oxygen functional groups over natural aging. The SEM images in Fig. 2 also confirmed complexation with soil mineral ingredients, showing that the surface of aged SLHC was rough with enhanced ash. Previous studies showed that wealthy surface functional groups enhance the complexing ability of biochar with soil mineral ingredients (Zhao and Zhou 2019). The second was the surface oxidation process. The surface oxidation processes increased the number of oxygen functional groups (−COO− and −OH) and (O + N)/C on aged SLHC, consistent with the results of the previous study (Liu et al. 2019b).
Under the above three processes during natural aging, the essential role of ash or mineral ingredients also affected the SLHC properties. The pH of the hydrochar increased from 7.13 to 7.46 after natural aging in Table 2, which was attributed to the decomposition of small organic acids (acetic acid) and an increase of alkaline ions coming from the complexation with soil minerals (Reza et al. 2015). Moreover, the zeta potential of SLHC (Table 2) increased from − 9.76 ± 0.64 mV for SLHC-A0 to − 1.46 ± 0.26 mV and − 1.52 ± 0.09 mV for SLHC-A4 and SLHC-A16 after natural aging, coinciding with the increasing ash and mineral ingredients (Qian and Chen 2014), suggesting that surface complexation was the dominated sorption force for metal cations to SLHC-A0. It was well known that the surface functional groups and mineral ingredients should both potentially contribute to the surface charge of hydrochar. Thus, the enhanced surface functional groups have a more negligible effect on the zeta potential of aged SLHC than the increased mineral ingredients due to the diminished negative surface charge during natural aging. The higher ash content (75.0–75.49%) and mineral ingredients content facilitated the thermal stability of aged SLHC in Table 1. The recalcitrance index (R50) has been frequently used to quantify the degradability of biochar. If the R50 of biochar is higher than 0.7 (Class 1), it means that this biochar is highly resistant to degradation; an R50 of biochar in the range of 0.5–0.7 (Class 2) indicates that this biochar is slightly degradable; with R50 of biochar lower than 0.5 (Class 3), the biochar is most degradable. The R50 value increased from 0.45 to 0.47–0.48 for aged SLHC in Table 2, suggesting a higher ability to resist degradation. This result was also consistent with the study of Han et al. (2021), which has shown that the addition of inorganic metals increased the R50 value of biochar (Han et al. 2021).
4.2 Natural aging effect on the sorption behavior of SLHC
The natural aging process affects the surface physicochemical characteristics of hydrochar; thus, it would also affect the sorption behavior of Cd. The pseudo-second-order kinetic model fitted the Cd sorption kinetics to SLHC-A0, SLHC-A4, and SLHC-A16 well (R2 > 0.99) in Table 3, consistent with our previous studies (Li et al. 2021). It can be proposed that the acceleration of sorption rate after natural aging may be due to increased surface sorption sites on the aged SLHC, such as increased pore structure and surface functional groups, which were easy to interact with Cd due to the alleviative pore-clogging effect (Wang et al. 2017). From a practical view of soil remediation, this result implied that the agriculture application of SLHC could affect the heavy metal mobility differently over natural aging, and the acceleration of sorption rate could enhance the immobilization of heavy metals.
The promotion of sorption affinity of aged SLHC may be due to the larger Vt, higher SSA, and more rich surface oxygen-containing functional groups of the aged SLHC, all leading to more readily accessible sites on the hydrochar surface for Cd sorption. The FTIR spectra in Fig. 1 of SLHC with/without Cd illustrated that the intensity of C–O–C, C=O, and C=C was weakened after Cd sorption, indicating that Cd is sorbed by these rich surface oxygen-containing functional groups and rich π electron region. Moreover, the maximum sorption capacity of Cd also increased after natural aging, as reflected in an increase of Qmax (Table 4). For the aged SLHC, the zeta potential significantly increased after natural aging (Table 2); however, the sorption capacity also increased obviously, which indicated that electrostatic attraction was not the primary sorption mechanism for the aged SLHC (Liu et al. 2020). In contrast, the rich mineral ingredients might also facilitate the surface complexation of aged hydrochar (Liu et al. 2020). Besides, precipitation of Cd with anions liberated from mineral ingredients, such as carbonate, hydroxyl, phosphate, and sulfate ions, also may be the prevailing factor for the enhanced sorption of Cd to aged hydrochar (Vijayaraghavan 2020). Therefore, pore sorption, surface complexation, and precipitation may be the predominant sorption mechanisms of Cd to aged SLHC, and electrostatic attraction was the primary sorption mechanism for fresh SLHC.
Due to the complex surface topography of hydrochar, the surface of hydrochar was heterogeneous; generally, sorption sites with high surface energy became the sorption center of environmental pollutants playing an essential role in the sorption process (Shen et al. 2015). The sorption site energy in fresh and aged SLHC (E*) decreased gradually with Qe in Fig. 3c, firstly indicating heterogeneous sorption site energy distribution in the fresh and aged SLHC surface and further suggesting Cd was preferentially sorbed to the high-energy sorption site and then to the low-energy sorption site (Yang et al. 2021; Zhou et al. 2019). Comparing the average site energy μ(E*) of Cd between fresh and aged SLHC, it was found that the longer aging time, the more considerable the average site energy μ(E*), illustrating that natural aging promoted more significant Cd sorption on SLHC. The relationship between average sorption sites energy μ(E*) and SSA or ash content was positive (R2 = 0.97–0.99) in Fig. 3g, noting that the natural aging of SLHC induced by SSA and ash content played an essential role in their sorption site heterogeneity. The F(E*) of aged SLHC was much higher than fresh SLHC, indicating more high-energy and low-energy sites for Cd sorption to aged SLHC. This could be explained by the fact that the sorption site energy increased with natural aging, resulting in more available binding sites for Cd sorption. The heterogeneous sorption sites originate from the defect structure, disordered arrangement, and grafting functional groups of carbonaceous materials, described with standard deviation (σe*) (Shen et al. 2015). In this study, the increased ash content and surface O abundance of SLHC after natural aging caused the enhanced Cd sorption site heterogeneity of SLHC (a larger value of σe* for aged SLHC). Moreover, the enhanced Cd sorption site heterogeneity could also be explained by the greater contribution of pore diffusion, allowing Cd to interact with more heterogeneous pore sorption sites due to the developed porosity with larger SSA and pore volume (Liu et al. 2019a).
Temperature is an essential factor that affects the environmental behavior of metal ions, especially their sorption behavior (Verma and Singh 2019). The observation of positive ΔH0 (Table 5) verifies that the sorption of Cd to SLHC was a spontaneous and endothermic process (Van et al. 2019). Besides, aged SLHC reveals larger values of ΔG0, ΔH0, and ΔS0 in Table 5, which points to the more significant temperature effect in aged SLHC (Elaigwu et al. 2014). The results show that the temperature effect on SLHC is positive. The longer the aging time of SLHC, the more significant the temperature effect.
The sorption behavior of Cd in the natural environment was usually affected by similarly charged ions (Shaheen et al. 2018). For SLHC-A0, it was found that the increasing concentrations of Na+ and Ca2+ enhanced the sorption of Cd in Fig. 4c probably due to the compressed electric double layer with lower absolute zeta potential (Wang et al. 2013). Inversely, for SLHC-A4 and SLHC-A16 in Fig. 4d, e, the increasing concentrations of Ca2+ inhibited the sorption of Cd on the aged hydrochar mainly due to the competitive sorption (Wang et al. 2018b). However, the increased concentration of Na+ enhanced the sorption of Cd, which may be related to the decreased hydration radius of Cd (He et al. 2009; Wang et al. 2013). Thus, natural aging could alter the property of SLHC and affect the different ionic strength effects on metal sorption.
4.3 Correlation of sorption capacity with aging strategy and biochar type
Different aging methods and preparation methods may have different effects on the aging process and the sorption capacity of heavy metals. This section also analyzed the aging effect on biochar's key physicochemical properties and heavy metal sorption capacity with different aging strategies and preparation methods based on literature investigation and data from this study. According to the literature reviewed (Additional file 1: Tables S1–S4), the aging effect on heavy metal sorption to pyrochar focuses on chemical aging and physical aging. In contrast, chemical and biological aging was the research emphasis of heavy metal sorption to hydrochar. Before and after aging, the ratio of heavy metal sorption capacity was plotted against the ratio of physicochemical properties (SSA, (O + N)/C, Ash, and O abundance). If the ratio was greater than 1, the aging enhanced SSA, (O + N)/C, Ash, O abundance, and sorption capacity, and vice versa.
4.3.1 Correlation of sorption capacity with SSA
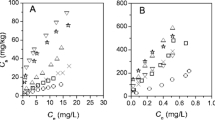
Different biochar types (pyrochar or hydrochar) significantly affect their aging degree on SSA and positively correlate with its sorption behavior, as shown in Fig. 5a. The correlation result of linear regression analysis of biochar types and aging method with SSA is listed in Additional file 1: Table S5 (P < 0.05).
Correlation of heavy metal sorption capacity with SSA, (O + N)/C, Ash, and O abundance of hydrochar and pyrochar under different aging effects, respectively. The X-axis represents the ratio of each physicochemical property before and after aging. The Y-axis represents the ratio of the corresponding sorption capacity of heavy metals before and after aging. Linear regression (solid line) and 95% confidence intervals (dotted lines) are shown. Hollow symbols represent the data in the research literature, while the red solid symbol symbolizes the data in this study
For pyrochar, chemical aging and physical aging could either enhance (SSAaged: SSAfresh > 1) or reduce (SSAaged: SSAfresh < 1) pyrochar SSA to some extent in Figs. 5a and 6a. The main reason for physical aging to reduce pyrochar SSA was the pore destruction or pore-clogging during freeze–thaw or dry–wet cycles with a 0.70–0.86 ratio (Tan et al. 2020a). Similarly, other studies of reducing pyrochar SSA involving chemical aging reported a 0.80–0.92 ratio attributed to the damaged porous structure (Nie et al. 2019) or partial clogging by the new formation of oxygen-containing functional groups (Chang et al. 2018). In contrast, an enhanced SSA caused by physical aging (a 1.00–1.65 ratio) or chemical aging (a 1.00–2.59 ratio) may stem from the dissolution of labile carbon (Tan et al. 2020b) or the formation of new pores by the aggregation of pyrochar and pyrochar-derived organic substances (Tan et al. 2020a). Biological aging may reduce pyrochar SSA due to the clogging by forming microbial layers (Wang et al. 2020).
Box diagram distribution of the SSA ratio, (O + N)/C ratio, ash ratio, O abundance ratio, and heavy metal sorption capacity ratio (Q ratio) of hydrochar and pyrochar under different aging strategies. The dotted line represents that the corresponding parameters remain unchanged before and after aging. Those above the dotted line indicate the positive effect and those under the dotted line represent the negative effect. A probability level of P < 0.05 (*) or P < 0.01 (**) was used to indicate statistical significance
Unlike pyrochar, biological aging (a 2.04–3.00 ratio) enhances hydrochar SSA due to the well-developed pore structure (Hua et al. 2020) or digestion or leached organic carbon compounds of hydrochar by soil microbes (Fu et al. 2021). Chemical aging (a 2.54–7.50 ratio) has a more obvious hydrochar SSA enhancement than biological aging in Fig. 6a, resulting from the formation of macro and micro pores by chemical oxidation (Li et al. 2021). Further, biological aging and chemical aging of hydrochar can lead to significantly higher SSA ratios than natural aging in this study (P < 0.05), as shown in Fig. 6a. However, 4-month or 16-month natural aging (a 1.77–1.83 ratio) in this study was relatively closer to biological aging, which illustrated that hydrochar SSA enhancement was due to the digestion or removal of organic carbon by leaching to form the well-developed pore structure.
Hence, different preparation methods of biochar (pyrolysis or hydrothermal carbonization) significantly affect their aging, and accordingly sorption behavior. The enhancement of sorption capacity after natural aging of hydrochar was significantly lower (P < 0.05) than that of biological aging and chemical aging, as shown in Fig. 6e due to unconspicuous increased SSA compared with biological aging and chemical aging hydrochar.
4.3.2 Correlation of sorption capacity with (O + N)/C
Biochar types significantly affect their aging degree on (O + N)/C (based on bulk elemental composition) and positively correlate with its sorption behavior, as displayed in Fig. 5b. The correlation result of linear regression analysis of biochar types and aging method with (O + N)/C is listed in Additional file 1: Table S5 (P < 0.05 or P < 0.1).
For pyrochar, chemical aging and physical aging could mainly enhance pyrochar (O + N)/C with an (O + N)/Caged: (O + N)/Cfresh > 1, as shown in Fig. 6b. The chemical aging (a 0.95–2.42 ratio) approach can lead to significantly higher (O + N)/C ratios due to over-oxidation as much as twice that of physical aging (a 0.86–1.25 ratio) (P < 0.05) (Qian and Chen 2014). The highest response of (O + N)/C to the heavy metal sorption capacity (slope of linear regression in Table S5) was by pyrochar physical aging, which indicated that the contribution of (O + N)/C on the enhanced sorption of heavy metal was essential to physically aged pyrochar.
For hydrochar, the chemical aging could either enhance (a 1.03–1.60 ratio) or reduce (a 0.55–0.99 ratio) hydrochar (O + N)/C to some extent as shown in Fig. 6b. A decrease in the (O + N)/C reflected the background loss of hydrochar and transformation (carbonization) in the process of chemical aging (Chang et al. 2019; Zheng et al. 2021). In contrast, the increasing (O + N)/C of hydrochar reflected the chemical oxidation (Li et al. 2021). Interestingly, biological aging changed hydrochar (O + N)/C by 0.90–1.64 times as shown in Fig. 6b, further evidencing that biological aging is associated with increasing surface oxygen functional groups on hydrochar (Hua et al. 2020). Among these, the highest response of (O + N)/C to the heavy metal sorption capacity (slope of linear regression in Additional file 1: Table S5) was hydrochar chemical aging. The (O + N)/C was essential for the enhanced sorption of chemically aged hydrochar.
In this study, the natural aging (a 1.05–1.14 ratio) of hydrochar was also closer to biological aging as shown in Fig. 6b, which illustrated that (O + N)/C enhancement was due to increasing surface oxygen functional groups on hydrochar. However, the enhancement of sorption capacity (slope of linear regression in Additional file 1: Table S5) as shown in Fig. 6e (P < 0.05) was much less than biological aging, even chemical aging, which illustrated that artificial aging, such as biological or chemical aging, exaggerated the aging effect of (O + N)/C on sorption capacity.
4.3.3 Correlation of sorption capacity with ash
The different aging strategies may affect the correlation between metal sorption capacity of pyrochar/hydrochar and ash content as shown in Fig. 5c. The correlation result of linear regression analysis of biochar types and aging method with ash content is listed in Additional file 1: Table S5 (P < 0.05 or P < 0.1).
The changing trend of metal sorption capacity to pyrochar and hydrochar was positively correlated with ash after chemical aging. Moreover, chemical aging can cause the dissolution of pyrochar minerals during oxidation (a decreasing 0.04–0.99 ratio) (Fan et al. 2018; Wang et al. 2019b), while chemical aging might also result in the background loss of hydrochar (an increasing 1.02–1.58 ratio) or pyrochar (an increasing 1.32–1.59 ratio) as shown in Fig. 6c, which thus lead to the increase in relative ash content (Li et al. 2021; Zheng et al. 2021). Chemical aging of hydrochar can lead to significantly higher SSA ratios than biological aging (P < 0.05) as shown in Fig. 6c. In contrast, different aging strategies (chemical aging and physical aging) for pyrochar have fewer ash effects. However, for non-chemical aging, the changing trend of heavy metal sorption capacity was negatively correlated with ash after biological aging for hydrochar or physical aging for pyrochar as shown in Fig. 5c, indicating that ash content is not the main factor affecting sorption in biologically aged hydrochar and physically aged pyrochar.
In this study, the ash content in naturally aged hydrochar increased with a 1.08 ratio due to the complexation with soil mineral ingredients, which facilitated the sorption of heavy metals to the aged hydrochar. For natural aging, the correlation of ash content and sorption capacity of hydrochar significantly differs between chemical aging and biological aging as shown in Fig. 5c, showing that laboratory experiments may not reveal the cumulative effect of multiple environmental factors on the physicochemical properties of hydrochar thoroughly. Thus, chemical or biological aging instead of natural aging remains discussed to study the aging biochar effect.
4.3.4 Correlation of sorption capacity with O abundance
The different aging strategies and biochar types may affect the correlation between heavy metal sorption capacity of pyrochar/hydrochar and O abundance (the intensity of surface oxygen-containing functional groups, such as −COO− and −OH) (Fig. 5d). The correlation result of linear regression analysis of biochar types and aging method with O abundance is listed in Additional file 1: Table S5 (P < 0.05).
The O abundance after physical aging for pyrochar or biological/chemical aging for hydrochar was positively correlated with the changing trend in heavy metal sorption capacity as shown in Fig. 5d. Compared with natural aging (a 1.06–1.47 ratio) of hydrochar in this study, the chemical aging (a 1.45–2.45 ratio) of hydrochar approach can lead to significantly higher O abundance ratios due to over-oxidation as shown in Fig. 6d (P < 0.05). Thus, the highest response of O abundance ratio to the heavy metal sorption capacity in Fig. 5d is hydrochar chemical aging, confirming that the higher O abundance was essential for the enhanced sorption of chemically aged hydrochar.
In contrast, only the changing trend of heavy metal sorption capacity was negatively correlated with O abundance after chemical aging for pyrochar, indicating that O abundance is not the main sorption effect factor in chemically aged pyrochar. The negative correlation might be the over-oxidation of pyrochar resulting in the damaged porous structure (Nie et al. 2019) or partial clogging by the new formation of oxygen-containing functional groups (Chang et al. 2018). The different correlation of sorption between O abundance and (O + N)/C came from two aspects: (1) O abundance represented the surface property, and the (O + N)/C was based on the bulk property; (2) data sources of O abundance and (O + N)/C were collected from different literature.
In this study, the change of O abundance natural aging (a 1.06–1.47 ratio) of hydrochar was closer to biological aging in Fig. 6d. However, the sorption capacity enhancement was much less than biological aging in Fig. 6e, even chemical aging, which illustrated that artificial aging, such as biological or chemical aging, exaggerated the natural aging effect of O abundance on sorption capacity. For pyrochar, different aging strategies have a more negligible effect on O abundance and sorption capacity.
4.3.5 Environment implication
Since natural aging is a complex process involving simultaneously physical, biological, and chemical processes, long-term monitoring of the property and sorption behavior of hydrochar is conducive to understanding the multifaceted natural aging mechanisms. Although single chemical aging or biological aging instead of complex natural aging to study the hydrochar aging effect remains discussed, quantitative artificial aging in the laboratory produces engineered hydrochar with excellent heavy immobilization performance. It is also noteworthy that current studies mainly focus on the chemical aging- and physical aging-induced heavy metal sorption to pyrochar and chemical and biological aging-induced heavy metal sorption to hydrochar. However, the linear regression analysis combined with box diagram distribution in this study has revealed that artificial aging (such as chemical aging or biological aging) had inconsistent effects on the properties of different types of biochar (hydrochar or pyrochar) compared with natural aging and overstated the natural aging effect on the heavy metal sorption capacity of hydrochar (Fig. 6). Future studies should continue to explore the mechanisms of natural aging-induced heavy metal sorption between hydrochar and pyrochar.
5 Conclusion
The natural aging process, which involves physical, biological, and photochemical processes, enhances the polarity, porosity, ash content, surface functional groups, and stability of SLHC. The 16-month aged SLHC has a stronger sorption affinity and faster sorption kinetic to Cd due to the enhancive porosity, surface functional groups, and ash content. Natural aging promoted more significant Cd sorption site heterogeneity on SLHC, controlled by ash content. The sorption of Cd to SLHC was spontaneous and endothermic, and sorption on aged SLHC was more strongly influenced by temperature. The Na+ promoted the sorption of Cd to the fresh and aged hydrochar, while Ca2+ inhibited the sorption of Cd on aged hydrochar. From the linear regression analysis, it was pointed out that artificial aging, such as biological or chemical aging, exaggerated the aging effect or reveal the inconsistent aging effect on the properties and sorption of hydrochar compared with natural aging. Moreover, the biochar types were also made a marked difference between pyrochar and hydrochar in the aging degree and sorption alternation. For example, chemical or physical aging could either enhance pyrochar SSA and (O + N)/C or reduce pyrochar SSA. In contrast, biological or chemical aging enhanced hydrochar SSA or reduced hydrochar (O + N)/C, thus positively affecting metal sorption with SSA and (O + N)/C. The metal sorption to chemically aged pyrochar or hydrochar was positively correlated with ash, while non-chemical aged pyrochar or hydrochar was negatively correlated with ash. The O abundance for chemically aged pyrochar only negatively affects metal sorption and facilitates the sorption to physically aged pyrochar or biological/chemical aged hydrochar. Thus, where possible, studies involving natural aging with multiple environmental factors are preferred over these single involving chemical or biological aging.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Brnardic I, Curkovic L, Sofilic T et al (2017) Removal of heavy metals and pharmaceuticals from contaminated water using waste sludge - Kinetics and mechanisms. Clean-Soil Air Water 45(7):1600509. https://doi.org/10.1002/clen.201600509
Chang Z, Tian L, Wu M et al (2018) Molecular markers of benzene polycarboxylic acids in describing biochar physiochemical properties and sorption characteristics. Environ Pollut 237:541–548. https://doi.org/10.1016/j.envpol.2018.02.071
Chang R, Sohi SP, **g F et al (2019) A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ Pollut 254:113123. https://doi.org/10.1016/j.envpol.2019.113123
De Carvalho Gomes S, Zhou JL, Zeng X et al (2022) Water treatment sludge conversion to biochar as cementitious material in cement composite. J Environ Manage 306:114463. https://doi.org/10.1016/j.jenvman.2022.114463
El Rasafi T, Oukarroum A, Haddioui A et al (2020) Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2020.1835435
Elaigwu SE, Rocher V, Kyriakou G et al (2014) Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. Ind Eng Chem Res 20(5):3467–3473. https://doi.org/10.1016/j.jiec.2013.12.036
Fan Q, Sun J, Chu L et al (2018) Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 207:33–40. https://doi.org/10.1016/j.chemosphere.2018.05.044
Fu H, Wang B, Li D et al (2021) Anaerobic fermentation treatment improved Cd2+ adsorption of different feedstocks based hydrochars. Chemosphere 263:127981. https://doi.org/10.1016/j.chemosphere.2020.127981
Guan J, Liu Y, **g F et al (2021) Contrasting impacts of chemical and physical ageing on hydrochar properties and sorption of norfloxacin with coexisting Cu2+. Sci Total Environ 772:145502. https://doi.org/10.1016/j.scitotenv.2021.145502
Han L, Sun H, Sun K et al (2021) Effect of Fe and Al ions on the production of biochar from agricultural biomass: Properties, stability and adsorption efficiency of biochar. Renew Sust Energ Rev 145:111133. https://doi.org/10.1016/j.rser.2021.111133
Harvey OR, Kuo L-J, Zimmerman AR et al (2012) An index-based approach to assessing recalcitrance and soil carbon sequestration potential of engineered black carbons (biochars). Environ Sci Technol 46(3):1415–1421. https://doi.org/10.1021/es2040398
He J, Xue H-X, Lue C-W et al (2009) The impacts of common ions on the adsorption of heavy metal. Environ Geol 58(7):1499–1508. https://doi.org/10.1007/s00254-008-1651-z
Hou R, Wang L, Shen Z et al (2021) Simultaneous reduction and immobilization of Cr(VI) in seasonally frozen areas: Remediation mechanisms and the role of ageing. J Hazard Mater 415:125650. https://doi.org/10.1016/j.jhazmat.2021.125650
Hua Y, Zheng X, Xue L et al (2020) Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresour Technol 300:122708. https://doi.org/10.1016/j.biortech.2019.122708
Khiari B, Jeguirim M, Limousy L et al (2019) Biomass derived chars for energy applications. Renew Sust Energ Rev 108:253–273. https://doi.org/10.1016/j.rser.2019.03.057
Li D, Cui H, Cheng Y et al (2021) Chemical aging of hydrochar improves the Cd2+ adsorption capacity from aqueous solution. Environ Pollut 287:117562. https://doi.org/10.1016/j.envpol.2021.117562
Lin Y, Munroe P, Joseph S et al (2012) Nanoscale organo-mineral reactions of biochars in ferrosol: An investigation using microscopy. Plant Soil 357(1–2):369–380. https://doi.org/10.1007/s11104-012-1169-8
Liu Y, Yao S, Wang Y et al (2017) Bio- and hydrochars from rice straw and pig manure: Inter-comparison. Bioresour Technol 235:332–337. https://doi.org/10.1016/j.biortech.2017.03.103
Liu CH, Chuang YH, Li H et al (2019a) Long-term sorption of lincomycin to biochars: The intertwined roles of pore diffusion and dissolved organic carbon. Water Res 161:108–118. https://doi.org/10.1016/j.watres.2019.06.006
Liu Y, Sohi SP, **g F et al (2019b) Oxidative ageing induces change in the functionality of biochar and hydrochar: Mechanistic insights from sorption of atrazine. Environ Pollut 249:1002–1010. https://doi.org/10.1016/j.envpol.2019.03.035
Liu Y, Wang L, Wang X et al (2020) Oxidative ageing of biochar and hydrochar alleviating competitive sorption of Cd(II) and Cu(II). Sci Total Environ 725:138419. https://doi.org/10.1016/j.scitotenv.2020.138419
Luo JY, Chen YG, Feng LY (2016) Polycyclic aromatic hydrocarbon affects acetic acid production during anaerobic fermentation of waste activated sludge by altering activity and viability of acetogen. Environ Sci Technol 50(13):6921–6929. https://doi.org/10.1021/acs.est.6b00003
de la Rosa J M, Rosado M, Paneque M et al (2018) Effects of aging under field conditions on biochar structure and composition: Implications for biochar stability in soils. Sci Total Environ 613:969–976. https://doi.org/10.1016/j.scitotenv.2017.09.124
Mia S, Dijkstra FA, Singh B (2017) Long-term aging of biochar: A molecular understanding with agricultural and environmental implications. In: Sparks DL (ed) Adv Agron, vol 141. Sringer, New York, pp 1–51
Nie T, Hao P, Zhao Z et al (2019) Effect of oxidation-induced aging on the adsorption and co-adsorption of tetracycline and Cu2+ onto biochar. Sci Total Environ 673:522–532. https://doi.org/10.1016/j.scitotenv.2019.04.089
Qian L, Chen B (2014) Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J Agr Food Chem 62(2):373–380. https://doi.org/10.1021/jf404624h
Reza MT, Rottler E, Herklotz L et al (2015) Hydrothermal carbonization (HTC) of wheat straw: influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour Technol 182:336–344. https://doi.org/10.1016/j.biortech.2015.02.024
Shaheen SM, Niazi NK, Hassan NEE et al (2018) Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int Mater Rev 64(4):216–247. https://doi.org/10.1080/09506608.2018.1473096
Shen X, Guo X, Zhang M et al (2015) Sorption mechanisms of organic compounds by carbonaceous materials: Site energy distribution consideration. Environ Sci Technol 49(8):4894–4902. https://doi.org/10.1021/es506034e
Tan L, Ma Z, Yang K et al (2020a) Effect of three artificial aging techniques on physicochemical properties and Pb adsorption capacities of different biochars. Sci Total Environ 699:134223. https://doi.org/10.1016/j.scitotenv.2019.134223
Tan LS, Sun CC, Wang Y et al (2020b) Changes in biochar properties in typical loess soil under a 5-year field experiment. J Soil Sediment 20(1):340–351. https://doi.org/10.1007/s11368-019-02398-0
Van HT, Nguyen LH, Nguyen VD et al (2019) Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: Batch and column studies. J Environ Manage 241:535–548. https://doi.org/10.1016/j.jenvman.2018.09.079
Verma L, Singh J (2019) Synthesis of novel biochar from waste plant litter biomass for the removal of Arsenic (III and V) from aqueous solution: A mechanism characterization, kinetics and thermodynamics. J Environ Manage 248:109235. https://doi.org/10.1016/j.jenvman.2019.07.006
Vijayaraghavan K (2020) The importance of mineral ingredients in biochar production, properties and applications. Crit Rev Environ Sci Technol 51(2):113–139. https://doi.org/10.1080/10643389.2020.1716654
Wang T, Liu W, **ong L et al (2013) Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chem Eng J 215:366–374. https://doi.org/10.1016/j.cej.2012.11.029
Wang B, Zhang W, Li H et al (2017) Micropore clogging by leachable pyrogenic organic carbon: A new perspective on sorption irreversibility and kinetics of hydrophobic organic contaminants to black carbon. Environ Pollut 220:1349–1358. https://doi.org/10.1016/j.envpol.2016.10.100
Wang W, Yu Q, Meng H et al (2018a) Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent. Bioresour Technol 249:361–367. https://doi.org/10.1016/j.biortech.2017.09.205
Wang Y-Y, Liu Y-X, Lu H-H et al (2018b) Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J Solid State Chem 261:53–61. https://doi.org/10.1016/j.jssc.2018.02.010
Wang L, Chang Y, Li A (2019a) Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew Sust Energ Rev 108:423–440. https://doi.org/10.1016/j.rser.2019.04.011
Wang X, **g Y, Cao Y et al (2019b) Effect of chemical aging of Alternanthera philoxeroides-derived biochar on the adsorption of Pb(II). Water Sci Technol 80(2):329–338. https://doi.org/10.2166/wst.2019.276
Wang L, O’Connor D, Rinklebe J et al (2020) Biochar aging: Mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54(23):14797–14814. https://doi.org/10.1021/acs.est.0c04033
Wang B, Fu H, Han L et al (2021a) Physicochemical properties of aged hydrochar in a rice-wheat rotation system: A 16-month observation. Environ Pollut 272:116037. https://doi.org/10.1016/j.envpol.2020.116037
Wang L, Rinklebe J, Tack FMG et al (2021b) A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manage 00:1–28. https://doi.org/10.1111/sum.12717
Wang L, Huang J, Li G et al (2022) Long-term immobilization of soil metalloids under simulated aging: Experimental and modeling approach. Sci Total Environ 806:150501. https://doi.org/10.1016/j.scitotenv.2021.150501
Weyers SL, Spokas KA (2014) Crop residue decomposition in Minnesota biochar-amended plots. Solid Earth 5(1):499–507. https://doi.org/10.5194/se-5-499-2014
Yang S, Zhao F, Sang Q et al (2021) Investigation of 3-aminopropyltriethoxysilane modifying attapulgite for Congo red removal: Mechanisms and site energy distribution. Powder Technol 383:74–83. https://doi.org/10.1016/j.powtec.2021.01.046
Yao FX, Arbestain MC, Virgel S et al (2010) Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 80:724–732. https://doi.org/10.1016/j.chemosphere.2010.05.026
Yao B, Luo Z, Du S et al (2021) Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms. Bioresour Technol 340:125698. https://doi.org/10.1016/j.biortech.2021.125698
Yi Q Q, Liang B Q, Nan Q et al (2020) Temporal physicochemical changes and transformation of biochar in a rice paddy: Insights from a 9-year field experiment. Sci Total Environ 721:137670. https://doi.org/10.1016/j.scitotenv.2020.137670
Zhao Z, Zhou W (2019) Insight into interaction between biochar and soil minerals in changing biochar properties and adsorption capacities for sulfamethoxazol. Environ Pollut 245:208–217. https://doi.org/10.1016/j.envpol.2018.11.013
Zheng C, Ma X, Yao Z et al (2019) The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour Technol 285:121347. https://doi.org/10.1016/j.biortech.2019.121347
Zheng X, Ma X, Hua Y et al (2021) Nitric acid-modified hydrochar enhance Cd2+ sorption capacity and reduce the Cd2+ accumulation in rice. Chemosphere 284:131261. https://doi.org/10.1016/j.chemosphere.2021.131261
Zhou Y, He Y, He Y et al (2019) Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration. Sci Total Environ 650(Pt 2):2260–2266. https://doi.org/10.1016/j.scitotenv.2018.09.393
Zhuang X, Huang Y, Song Y et al (2017) The transformation pathways of nitrogen in sewage sludge during hydrothermal treatment. Bioresour Technol 245:463–470. https://doi.org/10.1016/j.biortech.2017.08.195
Acknowledgements
We appreciate Miss. Shan Yu, Mr. Detian Li, and Miss Gulizhati Yeerjiang for assisting part of the experiments.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 41877090 and 42107398), Natural Science Foundation of Jiangsu Province (BK20181165 and BK20210358), China Postdoctoral Science Foundation (2020M68618), and Yunnan Branch of China National Tobacco Corporation (2022530000241022).
Author information
Authors and Affiliations
Contributions
YF as the corresponding author contributed to the conception, supervision and funding of the study. Material preparation, data collection and analysis were performed by BW and CS. The first draft of the manuscript was written by BW and CS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Shang, C., **e, H. et al. Unraveling natural aging-induced properties change of sludge-derived hydrochar and enhanced cadmium sorption site heterogeneity. Biochar 4, 34 (2022). https://doi.org/10.1007/s42773-022-00159-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00159-w