Abstract
Pathogenic bacteria can proliferate rapidly on porous fabrics to form bacterial plaques/biofilms, resulting in potential sources of cross-transmissions of diseases and increasing cross-infection in public environments. Many works on antibacterial modification of cotton fabrics have been reported, while very few works were reported to endow poly(ethylene terephthalate) (PET) fabrics with non-leaching antibacterial function without compromising their innate physicochemical properties though PET is the most widely used fabric. Therefore, it is urgent to impart the PET fabrics with non-leaching antibacterial activity. Herein, a novel N-halamine compound, 1-chloro-3-benzophenone-5,5-dimethylhydantoin (Cl-BPDMH), was developed to be covalently bonded onto PET fabrics, rendering non-leaching antibacterial activity while negligible cytotoxicity based on contact-killing principle. Bacterial was easily adhered to Cl-BPDMH finished PET fabrics, and then it was inactivated quickly within 10 s. Furthermore, the breaking strength, breaking elongation, tearing strength, water vapor permeability, air permeability and whiteness of Cl-BPDMH finished PET fabrics were improved obviously compared to raw PET fabrics. Hence, this work developed a facile approach to fabricate multifunctional synthetic textiles to render outstanding and rapid bactericidal activity without compromising their physicochemical properties and biocompatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
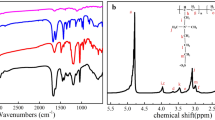
Poly(ethylene terephthalate) (PET) fabrics have been widely used in our daily clothes due to their excellent strength, easy processability, quick drying, dimensional stability, and wrinkle resistance [1]. However, pathogenic bacteria can proliferate rapidly on porous PET fabrics to form bacterial plaques/biofilms, resulting in irreversibly damaging their appearance, adversely affecting human health, and increasing cross-infection in public environments [2], since bacterial infection is one of the most serious threat to global safety especially drug-resistant bacteria [3,4,44, 2d) could be further confirmed that Cl-BPDMH was successfully bonded to the PET fabrics.
Antibacterial Activities
As shown in Fig. 3a, no live S.aureus could be observed when it was incubated with Cl-BPDMH finished PET fabrics for 24 h, while about 65% of live E.coli could be observed when it incubated with PET-2 for 24 h. Obviously, no live bacteria could be observed for both test bacteria when they incubated with PET-3 and PET-4 for 24 h.
Antibacterial activities evaluation. a The culture medium images of raw PET, PET-2, PET-3 and PET-4; b The diameters of inhibition zone (DIZs) of raw PET, PET-2, PET-3 and PET-4; c The durable antibacterial rate of PET-3 compared with the raw PET fabric (control); d Antibacterial kinetics of PET-3 as compared with raw PET against E. coli (ATCC 25,922) and S. aureus (ATCC 6538); e Ultra-high resolution fluorescence microscopy of E.coli treated with PET-3 for 5 min. Notes: the contacting time of a–b was 24 h, and error bars of c were based on the maximum and minimum values of three parallel samples
The DIZs were all zero, and no live bacteria was existed under antibacterial PET fabrics (Fig. 3b), indicating that no Cl-BPDMH was leached from the Cl-BPDMH finished PET fabrics. Therefore, the antibacterial mechanism of the Cl-BPDMH finished PET fabrics was proven to contact killing mechanism [28] by directly transferring the oxidative chlorine from N-halamine to bacterial receptors [31, 50]. Non-leaching characteristics of antibacterial fabrics usually exhibited long-lasting antibacterial activity [46]. The antimicrobial rates of PET-3 were higher than 99% against both S.aureus and E.coli after being washed for 5,10, and 20 laundering times, respectively (Fig. 3c), which were much than the reference values of AA class (FZ/T 73,023–2006 standard). The bactericidal kinetics of PET-3 were carried out compared with raw PET fabrics as shown in Fig. 3d. PET-3 could inactivate all of S. aureus and E.coli within 1 h and 3 h, respectively. Gram-positive S.aureus were more susceptible to N-halamine than Gram-negative E.coli may be the cell walls of Gram- negative E.coli were more complex than that of Gram positive S.aureus [51]. Thus, the non-leaching Cl-BPDMH finished fabrics exhibited excellent antibacterial activity and PET-3 was selected as the following experimental sample.
To further visualize the process of bacteria contacting with PET-3, and its real bactericidal speed, the in-situ dynamic process of E.coli contacting with PET-3 compared with raw PET fabrics was observed by rotating confocal fluorescence microscope as shown in Fig. 3e. The ultra-high resolution fluorescence microscopy image of E.coli treated with raw PET was full of live bacterial (green). Interestingly, E.coli was inactivated (red) after contacting with PET-3 within 10 s. Therefore, bacteria can be easily adhered to Cl-BPDMH finished PET fabrics, thereby being inactivated quickly within a few seconds, further indicating the main antibacterial mechanism of the Cl-BPDMH finished PET fabrics was contact killing. Though antiviral activity was not provided here, and it would be carried out in the future, N-haloamines have been proved to have excellent antiviral activity [52]. Therefore, Cl-BPDMH finished PET fabrics have potential applications in quick sterilization, including but not limited to water purification, biochemical protective clothing, mask, food packaging, antibacterial textiles.
Comprehensive Performance Evaluation
Mechanical property is one of the most critical requirements of the fabrics. Compared with the raw PET fabric, the breaking strength and breaking elongation, tearing strength and bursting strength of PET-3 all showed a general increase (Fig. 4a–d). Specifically, the breaking strength increased by 8.3% in the warp direction from 1450.6 to 1570.3 N, and it increased by 38.5% in the weft direction from 511.7 to 708.4 N (Fig. 4a). Additionally, the breaking elongation increased by 8.0% in the warp direction from 31.7 to 34.2%, and it increased by 69.22% in the weft direction from 11.3 to 19.2% (Fig. 4b) because the increase of the interaction between Cl-BPDMH and PET chains improved the portion of amorphous region [53]. The tearing strength of PET-3 increased by 10.1% in the warp direction from 15.4 to 17.0 N, and it increased by 20.5% in the weft direction from 7.0 to 8.5 N (Fig. 4c). Therefore, the breaking strength, breaking elongation and tearing strength of PET-3 increased obviously compared with raw PET fabrics because the intermolecular forces could increase by introducing polar chlorine group [54] after binding Cl-BPDMH, and extracting an active hydrogen to generate free radicals by mild light irradiating on the surface of PET fabric for a short time would not damage to PET structure [53]. The bursting strength remained basically unchanged as compared with raw PET (Fig. 4d), while the bending stiffness decreased obviously (Fig. 4e), indicating improved wear comfort property due to its enhanced softness.
Comprehensive performance of raw PET and Cl-BPDMH finished PET fabrics. a Breaking strength; b Breaking elongation; c Tearing strength; d Bursting strength; e Bending stiffness; f Whiteness; g Water vapor permeability (WVP); and h Air permeability of raw PET and PET-3; i Water contact angle (WCA) of the raw PET, PET-2, PET-3 and PET-4 fabrics. Error bars were based on the maximum and minimum values of five parallel samples
The whiteness of Cl-BPDMH finished PET fabrics was well remained without obvious changes as compared with raw PET (Fig. 4f). The water vapor transmission (Fig. 4g) and air permeability (Fig. 4h) of PET-3 both increased slightly may be the pore size of the Cl-BPDMH finished PET fabric were slightly increased during the finishing process, resulting in improving the ability of water vapor and air flow to penetrate the PET fabrics [2]. Compared to raw PET, the water contact angle (WCA) of PET-2, PET-3 and PET-4 all increased, meaning that Cl-BPDMH finished PET fabrics can improve the hydrophobicity of PET fabrics due to the mainly influence of the diaryl group (hydrophobic group) on the chain of Cl-BPDMH (Fig. 4i), which was conducive to adhering bacteria, and thereby inactivated them quickly (Fig. 3e). This strategy has obvious advantages over hydrophilic antibacterial surfaces for medical surgical clothing and masks with waterproof requirements.
Cytotoxicity and Skin Irritation
Cytotoxicity of PET-2, PET-3 and PET-4 compared with raw PET were shown in Fig. 5a. After being co-cultured with PET fabrics for 24 h, the viability of NIH 3T3 cell treated with Cl-BPDMH finished PET fabrics showed a slight decrease. Interestingly, compared with raw PET fabrics, the cell viability of PET-2, PET-3 and PET-4 were all more than 95%. Almost no cell was inactivated (red) after incubated with both raw PET and PET-3 for 24 h compared with NIH 3T3 cell only (Fig. 5b). Therefore, the Cl-BPDMH finished PET fabrics exhibited negligible cytotoxicity.
Biocompatibility evaluation. a Quantitative cell survival rate of NIH 3T3 cell incubated with raw PET and Cl-BPDMH finished PET fabrics for 24 h; b The fluorescence microscope photos of NIH 3T3 cell only, or NIH 3T3 cell incubated with raw PET and PET-3 for 24 h; Error bars of the fluorescence microscope photos were all 50 μm. c The images of rabbits’ back skin contacting with raw PET and PET-3 at different contact time; d HE staining images of skin contacted with raw PET and PET-3, the scale bars were all 250 μm
Raw PET and PET-3 were further exposed to the back of rabbit’s skin to test their irritation responses. After being contacted for 12, 24, 48, and 72 h, respectively, no erythema and edema of rabbit’s skin treated with both raw PET and PET-3 was found on (Fig. 5c). Besides, no distinct histopathological abnormal was found in hematoxylin & eosin (H&E) stained images at any evaluated areas masked by raw PET and PET-3 (Fig. 5d).
Conclusion
Cl-CBPDMH was synthesized to fabricate non-leaching bactericidal PET fabrics by photocatalytic hydrogen abstraction reaction. The Cl-CBPDMH finished PET fabrics can inactivate bacteria within 10 s, indicating their outstanding bactericidal activity against both Gram-positive S.aureus and Gram-negative E.coli. Moreover, PET fabrics can maintain even improve their mechanical properties, comfort properties and biocompatibility after being finished with Cl-BPDMH. Therefore, the Cl-BPDMH was a preferred antimicrobial to fabricate excellent bactericidal PET fabrics while negligible toxicity. Selecting C–H bond as the modified active site, this strategy can be widely applied to finish synthetic fiber fabrics, including but not limited to polyester, polyamide, polypropylene, and polyacrylonitrile. This work can promote the development of permanent antibacterial finishing strategy of synthetic fibers. And Cl-BPDMH finished synthetic fiber fabrics has promising applications including but not limited to water purification, masks, and medical textiles, socks, curtains, carpet and so on.
References
Wang ML, Zhang MJ, Pang LJ, Yang CG, Zhang YM, Hu JT, Wu GZ. Fabrication of highly durable polysiloxane-zinc oxide (ZnO) coated polyethylene terephthalate (PET) fabric with improved ultraviolet resistance, hydrophobicity, and thermal resistance. J Colloid Interf Sci. 2019;537:91.
Chen SG, Zhang SB, Galluzzi M, Li F, Zhang XC, Yang XH, Liu XY, Cai XH, Zhu XL, Du B, Li JN, Huang P. Insight into multifunctional polyester fabrics finished by one-step eco-friendly strategy. Chem Eng J. 2019;358:634.
Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336.
Li N, Pranantyo D, Kang ET, Wright DS, Luo HK. In situ self-assembled polyoxotitanate cages on flexible cellulosic substrates: multifunctional coating for hydrophobic, antibacterial, and uv-blocking applications. Adv Funct Mater. 2018;28:1800345.
**a GX, Wu YM, Bi YF, Chen K, Zhang WW, Liu SQ, Zhang WJ, Liu RH. Antimicrobial properties and application of polysaccharides and their derivatives. Chinese J Polym Sci. 2021;39:133.
Zhou M, Qian Y, **e J, Zhang W, Jiang W, **ao X, Chen S, Dai C, Cong Z, Ji Z, Shao N, Liu L, Wu Y, Liu R. Poly(2-Oxazoline)-based functional peptide mimics: eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angew Chem Int Ed. 2020;59:6412.
Sreeja S, Muraleedharan CV, Varma PRH, Sailaja GS. Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater Sci Eng C. 2020;109:16.
Wu M, Ma B, Pan T, Chen S, Sun J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv Funct Mater. 2016;26:569.
Liu QX, Huang J, Zhang JM, Hong Y, Wan YB, Wang Q, Gong ML, Wu ZG, Guo CF. Thermal, waterproof, breathable, and antibacterial cloth with a nanoporous structure. ACS Appl Mater Interfaces. 2018;10:2026.
Yun G, Pan S, Wang TY, Guo J, Richardson JJ, Caruso F. Synthesis of metal nanoparticles in metal-phenolic networks: Catalytic and antimicrobial applications of coated textiles. Adv Healthcare Mater. 2018;7:1700934.
Amani A, Montazer M, Mahmoudirad M. Low starch/corn silk/ZnO as environmentally friendly nanocomposites assembling on PET fabrics. Int J Biol Macromol. 2021;170:780.
Zhang T, Yu H, Li J, Song H, Wang S, Zhang Z, Chen S Green light–triggered antimicrobial cotton fabric for wastewater disinfection, Mater Today Phys, 2020;15:100254.
Gao D, Li Y, Lyu B, Lyu L, Chen S, Ma J. Construction of durable antibacterial and anti-mildew cotton fabric based on P(DMDAAC-AGE)/Ag/ZnO composites. Carbohyd Polym. 2019;204:161.
Rehan M, El-Naggar ME, Mashaly HM, Wilken R. Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohyd Polym. 2018;182:29.
Ding S, Wang Y, Li J, Chen S.Progress and prospects in chitosan derivatives: Modification strategies and medical applications, J Mater Sci Technol 2021; in press https://doi.org/10.1016/j.jmst.2020.12.008.
Chen SG, Yuan LJ, Li QQ, Li JN, Zhu XL, Jiang YG, Sha O, Yang XH, **n JH, Wang JX, Stadler FJ, Huang P. Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small. 2016;12:3516.
Chen SG, Chen SJ, Jiang S, **ong ML, Luo JX, Tang JN, Ge ZC. Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl Mater Interfaces. 2011;3:1154.
Xu J, Zhao H, **e Z, Ruppel S, Zhou X, Chen S, Liang JF, Wang X. Stereochemical strategy advances microbially antiadhesive cotton textile in safeguarding skin flora. Adv Healthcare Mater. 2019;8:1900232.
Li X, Bai H, Yang Y, Yoon J, Wang S, Zhang X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv Mater. 2019;31:1805092.
Zhang SB, Yang XH, Tang B, Yuan LJ, Wang K, Liu XY, Zhu XL, Li JN, Ge ZC, Chen SG. New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem Eng J. 2018;336:123.
Zhang SM, Li R, Huang D, Ren XH, Huang TS. Antibacterial modification of PET with quaternary ammonium salt and silver particles via electron-beam irradiation. Mater Sci Eng C. 2018;85:123.
Xu J, **e Z, Du F, Wang X. One-step anti-superbug finishing of cotton textiles with dopamine-menthol. J Mater Sci Technol. 2021;69:79.
Han H, Zhu J, Wu DQ, Li FX, Wang XL, Yu JY, Qin XH. Inherent guanidine nanogels with durable antibacterial and bacterially antiadhesive properties. Adv Funct Mater. 2019;29:1806594.
Cao YH, Gu JW, Wang S, Zhang ZC, Yu HL, Li JN, Chen SG. Guanidine-functionalized cotton fabrics for achieving permanent antibacterial activity without compromising their physicochemical properties and cytocompatibility. Cellulose. 2020;27:6027.
Chen W, Zhu Y, Zhang Z, Gao Y, Liu W, Borjihan Q, Qu H, Zhang Y, Zhang Y, Wang Y, Zhang L, Dong A. Engineering a multifunctional N-halamine-based antibacterial hydrogel using a super-convenient strategy for infected skin defect therapy. Chem Eng J. 2020;379:122238.
Tang X, Xu H, Shi Y, Wu M, Tian H, Liang J. Porous antimicrobial starch particles containing N-halamine functional groups. Carbohyd Polym. 2020;229:115546.
Bai R, Kang J, Simalou O, Liu WX, Ren H, Gao TY, Gao YY, Chen WJ, Dong A, Jia R. Novel N-Br bond-containing N-halamine nanofibers with antibacterial activities. ACS Biomater Sci Eng. 2018;4:2193.
Dong A, Wang Y, Gao Y, Gao T, Gao G. Chemical insights into antibacterial N-halamines. Chem Rev. 2017;117:4806.
Liang M, Wang F, Liu M, Yu J, Si Y, Ding B N-halamine functionalized electrospun poly(vinyl alcohol-co-ethylene) nanofibrous membranes with rechargeable antibacterial activity for bioprotective applications, Adv Fiber Mater 2019; 1:126.
Chen YJ, Worley SD, Kim J, Wei CI, Chen TY, Santiago JI, Williams JF, Sun G. Biocidal poly(styrenehydantoin) beads for disinfection of water. Ind Eng Chem Res. 2003;42:280.
Chen Y, Wang YY, Feng CY, He QK, Chen Q, Wang ZD, Han QX. Novel quat/di-N-halamines silane unit with enhanced synergism polymerized on cellulose for development of superior biocidability. Int J Biol Macromol. 2020;154:173.
Tsao TC, Williams DE, Worley CG, Worley SD. Novel N-halamine disinfectant compounds. Biotech Prog. 1991;7:60.
Kovacic P, Lowery MK, Field KW. Chemistry of N-bromamines and N-chloramines. Chem Rev. 1970;70:639.
Ren T, Hayden M, Quo M, Huang TS, Ren X, Weesel J. Absorbent pads containing N-halamine compound for potential antimicrobial use for chicken breast and ground chicken. J Agric Food Chem. 2018;66:1941.
Ma Y, Li J, Si Y, Huang K, Nitin N, Sun G. Rechargeable antibacterial N-halamine films with antifouling function for food packaging applications. ACS Appl Mater Interfaces. 2019;11:17814.
Wang YF, Yin ML, Ma W, Li ZG, Xu ZZ, Ren XH N-halamine modified mesoporous silica coated cotton as multipurpose protective fibrous materials, Cellulose, 2020; 27: 10461.
Sun X, Zhang L, Cao Z, Deng Y, Liu L, Fong H, Sun Y. Electrospun composite nanofiber fabrics containing uniformly dispersed antimicrobial agents as an innovative type of polymeric materials with superior antimicrobial efficacy. ACS Appl Mater Interfaces. 2010;2:952.
Liang J, Chen Y, Barnes K, Wu R, Worley SD, Huang TS N-halamine/quat siloxane copolymers for use in biocidal coatings, Biomaterials, 2006; 27:2495.
Liu Y, Li J, Li L, McFarland S, Ren X, Acevedo O, Huang TS. Characterization and mechanism for the protection of photolytic decomposition of N-halamine siloxane coatings by titanium dioxide. ACS Appl Mater Interfaces. 2016;8:3516.
Liu C, Shan H, Chen X, Si Y, Yin X, Yu J, Ding B. Novel inorganic-based N-halamine nanofibrous membranes as highly effective antibacterial agent for water disinfection. ACS Appl Mater Interfaces. 2018;10:44209.
Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS. Polymeric antimicrobial N-halamine epoxides. ACS Appl Mater Interfaces. 2011;3:2845.
Lv J, Zhou Q, Zhi T, Gao D, Wang C. Environmentally friendly surface modification of polyethylene terephthalate (PET) fabric by low-temperature oxygen plasma and carboxymethyl chitosan. J Clean Prod. 2016;118:187.
Del Hoyo GS, Pérez Álvarez L, Gómez Galván F, Lizundia E, Kuritka I, Sedlarik V, Laza JM, Vila Vilela JL. Construction of antibacterial poly(ethylene terephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohyd Polym. 2016;143:35.
Zhang S, Kai C, Liu B, Zhang S, Wei W, Xu X, Zhou Z. Preparation, characterization and antibacterial properties of cellulose membrane containing N-halamine. Cellulose. 2019;26:5621.
Ma W, Li L, **ao X, Du H, Ren X, Zhang X, Huang T. Construction of chlorine labeled ZnO–chitosan loaded cellulose nanofibrils film with quick antibacterial performance and prominent UV stability. Macromol Mater Eng. 2020;305:2000228.
Gu JW, Yuan LJ, Zhang Z, Yang XH, Luo JX, Gui ZF, Chen SG. Non-leaching bactericidal cotton fabrics with well-preserved physical properties, no skin irritation and no toxicity. Cellulose. 2018;25:5415.
Zhang T, Gu JW, Liu XY, Wei D, Zhou HL, **ao HH, Zhang ZC, Yu HL, Chen SG. Bactericidal and antifouling electrospun PVA nanofibers modified with a quaternary ammonium salt and zwitterionic sulfopropylbetaine. Mater Sci Eng C. 2020;111:110855.
Chen YX, Li JN, Li QQ, Shen YY, Ge ZC, Zhang WW, Chen SG. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohyd Polym. 2016;143:246.
Dong A, Lan S, Huang J, Wang T, Zhao T, Wang W, **ao L, Zheng X, Liu F, Gao G, Chen Y. Preparation of magnetically separable N-halamine nanocomposites for the improved antibacterial application. J Colloid Interf Sci. 2011;364:333.
Zheng Y, Pan N, Liu Y, Ren X. Novel porous chitosan/N-halamine structure with efficient antibacterial and hemostatic properties. Carbohyd Polym. 2021;253:117205.
Kell AJ, Stewart G, Ryan S, Peytavi R, Boissinot M, Huletsky A, Bergeron MG, Simard B. Vancomycin-modified nanoparticles for efficient targeting and preconcentration of Gram-positive and Gram-negative bacteria. ACS Nano. 2008;2:1777.
Zhou J, Hu Z, Zabihi F, Chen Z, Zhu M. Progress and perspective of antiviral protective material. Adv Fiber Mater. 2020;2:123.
Chen X, Wang Y, Dai GL, Peng J, Li JQ, Shi MW, Zhai ML. Radiation grafting of glycidyl methacrylate and divinylbenzene onto polyethylene terephthalate fabrics for improving anti-drip** performance. Radiat Phys Chem. 2016;127:256.
Chao P, Chen H, Zhu Y, Zheng N, Meng H, He F. Chlorination of conjugated side chains to enhance intermolecular interactions for elevated solar conversion. Macromolecules. 2020;53:165.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.51773117), the Science and Technology Project of Shenzhen city (No. JSGG20201102154400001), the Collaborative Innovation and Nanshan District Key lab for Biopolymers and Safety Evaluation (No. KC2014ZDZJ0001A).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no financial and non-financial competing interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Li, J., Cao, Y. et al. Non-Leaching, Rapid Bactericidal and Biocompatible Polyester Fabrics Finished with Benzophenone Terminated N-halamine. Adv. Fiber Mater. 4, 119–128 (2022). https://doi.org/10.1007/s42765-021-00100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-021-00100-z