Abstract
Background
Sweet oranges are a vital agricultural commodity, but they are susceptible to various pathogenic fungi that can cause significant economic losses. This study aimed to evaluate the antifungal activity of plant extracts against pathogenic fungi attacking sweet oranges in Jimma, Ethiopia.
Materials and methods
Various plant extracts were obtained from locally available plant species and tested against selected pathogenic fungi using standard in vitro antifungal assays. The results revealed varying degrees of antifungal activity among the plant extracts, with some showing promising inhibitory effects against the targeted fungi. Statistical analysis indicated significant differences in antifungal efficacy among the tested extracts.
Results
The study found that Penicilium spp., Aspergillus spp., Rhizopus spp., Mucor spp., and Fusarium spp, were the predominant fungal species. The study found that the extracts of Vernonia amygdalina, Ginger oficealae, and Pterolobium stalatam were effective in inhibiting mycelial growth against these pathogens. This suggests the use of these medicinal plants for the preservation and control of sweet orange fruit in most of the Ethiopian region.
Conlusion
These findings suggest the potential of certain plant extracts as natural alternatives to synthetic fungicides for managing fungal diseases in sweet orange cultivation. Further research is needed to elucidate the mechanisms of action of these plant extracts and optimize their application in agricultural practices. This study contributes to the development of sustainable and eco-friendly strategies for plant disease management in the region.
Article Highlights
-
1.
The researchers identified various plant extracts that exhibit significant antifungal properties against fungi.
-
2.
The study conducted a comparative analysis between natural plant extracts and conventional chemical fungicides.
-
3.
Detailed phytochemical analysis identified the active compounds for the antifungal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background of the study
Fruits play a crucial role in human nutrition, offering essential carbohydrates, vitamins, and minerals. Among these, their high vitamin C content and antioxidant potential make them globally valuable. Citrus fruits, a major commercial crop, are cultivated extensively in over 137 countries, predominantly in subtropical and tropical regions. Brazil leads as the world’s largest citrus producer, followed by the USA, China, and Mexico. The leading exporters of citrus include Spain, the USA, and South Africa. Citrus fruits are not only significant for international trade but also for local economies, such as Ethiopia, where agriculture contributes over 85% of the national income [1]. Concurrently, there is a growing trend in the food industry to use polyphenol-rich spices and herb extracts due to their antifungal, anti-aflatoxigenic, and antioxidant properties. This shift is driven by the increasing preference for natural alternatives to synthetic chemicals. The potential of these natural compounds to act as antifungal agents is particularly relevant for preserving fruit crops and ensuring food safety [1]. The use of polyphenol-rich spices and herbs extracts in food industries is gaining importance due to their antifungal, anti-aflatoxigenic, and antioxidant properties, and growing interest in natural alternatives [2].
There is an urgent need for natural alternatives to synthetic fungicides due to health and environmental concerns. Polyphenol-rich spices and herb extracts have shown potential in inhibiting fungal growth and protecting against aflatoxins. Quantitative data on these plant-based solutions is essential. Sweet oranges, prone to fungal spoilage, face significant economic losses. Identifying fungal pathogens and evaluating the antifungal properties of medicinal plant extracts could provide sustainable, safe alternatives. This research is crucial for Jimma Zone in Ethiopia, where agriculture is vital to the economy. Fungal spoilage of sweet oranges in Jimma Zone causes significant post-harvest losses, impacting local farmers and the economy. Synthetic fungicides used to combat these pathogens pose health and environmental risks. This study aims to isolate and identify the fungal pathogens and evaluate the antifungal activity of medicinal plant extracts as safer, sustainable alternatives.
2 Materials and methods
2.1 Sampling and sample collection
The tudy was conducted in Jimma Zone, Jimma town, Western Ethiopia. The data collection was conducting from June to July 2021. Totally about, 6 fruits farms were selected based on size, variety of citrus cultivated, and historical incidence of fungal spoilage. The fruits samples were collected based on their high history of fungal spoilage,minimal use of synthetic fungicides to reflect natural fungal prevalence, and accessibility and cooperation of orchard owners. About 10 sweet orange fruits per orchard, totaling 60 fruits for the study. 10 fruits randomly picked from each tree (from different parts: top, middle, bottom; north, south, east, west sides). Fruits harvested using clean, sterilized gloves and tools to prevent contamination. Each fruit placed in a sterile plastic bag and labeled with date, location, and tree number. Samples transported to the laboratory in insulated cool boxes to prevent fungal growth or death during transit. Samples stored at 4 °C and processed within 24 h to maintain freshness and viability of fungal pathogens.
2.2 Isolation of fungi from sweet orange
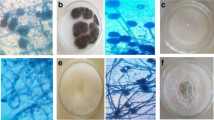
The process of isolating the mold-causing organism from sweet oranges involved several critical steps to ensure the purity and viability of the fungal culture for further studies. Initially, blue mold lesions on infected sweet oranges were selected as the source of the fungal isolates. The fruits underwent surface sterilization by rinsing with distilled water, immersion in a disinfectant solution, and thorough rinsing with sterile distilled water. Sterilized fruits were aseptically cut into small segments, which were then placed onto solidified Potato Dextrose Agar (PDA) plates to promote fungal growth. These plates were incubated under controlled conditions, allowing different fungal isolates to develop. Individual fungal colonies were carefully isolated and transferred to fresh PDA plates to obtain pure cultures.
The colonies were characterized based on morphological features, and the pure cultures were transferred again to maintain their growth and purity. For long-term preservation, the pure cultures were stored at 4 °C, slowing down metabolic activity and preventing overgrowth. The preserved pure cultures were intended for in vivo tests to study the organism’s pathogenicity on living host plants and in vitro tests to understand its growth characteristics, pathogenic mechanisms, and potential control methods. This meticulous process successfully isolated and preserved a pure culture of the mold-causing organism, providing a reliable source for further scientific investigation and testing [2].
2.3 Characterization and identification of the pathogen
Characterization and identification of the pathogen isolated from sweet oranges involve a series of detailed procedures to accurately determine the fungal species causing blue mold lesions. Initially, macroscopic characterization is performed by observing colony morphology on Potato Dextrose Agar (PDA) plates, noting color, texture, growth pattern, and measuring colony diameter to assess growth rate under various conditions. Microscopic characterization follows, where slides prepared from the fungal culture are stained and examined under a microscope to observe hyphal structure, spore size, shape, color, and arrangement, as well as spore-producing structures like conidiophores [15].
2.4 Pathogenicity test
A decay test was conducted to determine the cause of spoilage in citrus fruits. Healthy fruits were sterilized, and agar discs containing fungal cultures were placed in the fruit. The fungi were then re-isolated and compared to the initial isolates. The fungi’s point of inoculation was examined and measured. Pathogenicity tests confirm the pathogen’s identity by inoculating healthy sweet oranges with the fungus and observing symptom development. Integrating macroscopic and microscopic observations, biochemical tests, molecular identification, and pathogenicity tests provides a comprehensive characterization and identification of the pathogen, crucial for understanding its biology and develo** effective control strategies.
2.5 Testing for antagonistic activity of plant extracts
The study involved the collection of three different plant materials from farms in Jimma Town and the selected plants included: Pterolobium stellatum (root), Zingiber officinale (ginger root) and Vernonia amygdalina (leaf). The preparation of plant extracts required various apparatus and instruments, including a separator funnel, oven, filter paper, electric blender, pipettes, cuvettes, water bath, electronic balance, and conical flasks. The collected plant materials were initially separated to remove any unwanted parts such as stems, seeds, or other extraneous matter. Each plant material was then thoroughly washed under running tap water to remove soil, dust, and other contaminants. This was followed by rinsing with sterile distilled water to ensure sterility. The cleaned plant materials were spread out on clean, dry trays and placed in an oven set at a controlled temperature (typically around 40–50 °C) to dry. The drying process was monitored regularly to prevent overheating and degradation of the active compounds. Alternatively, the plant materials could be air-dried in a well-ventilated, shaded area if an oven was not available. Once fully dried, the plant materials were pounded into a fine powder using an electric blender. This step increased the surface area of the plant material, facilitating the extraction of bioactive compounds. The powdered plant material was accurately weighed using an electronic balance. A measured amount of the powder was placed in a conical flask, and a solvent (such as ethanol, methanol, or water) was added in a specific ratio. The choice of solvent depended on the polarity of the compounds to be extracted. The mixture was then subjected to continuous stirring or shaking to enhance the extraction process. This could be done using a mechanical shaker or magnetic stirrer for several hours (typically 24–48 h) at room temperature or in a water bath at a slightly elevated temperature. After the extraction period, the mixture was filtered using filter paper to remove the solid residues, collecting the filtrate containing the dissolved bioactive compounds in a clean container The extraction process could be repeated multiple times with fresh solvent to ensure maximum extraction of the active compounds. The filtrate was concentrated by evaporating the solvent under reduced pressure using a rotary evaporator or by placing it in a water bath at a controlled temperature. This step resulted in a concentrated extract containing the active plant compounds. The concentrated extracts were then dried to remove any remaining solvent, yielding a dry, stable extract. The final plant extracts were stored in airtight containers, preferably in a cool, dark place or a refrigerator, to preserve their stability and bioactivity until further use in antagonistic activity tests [2].
2.6 Preparation of solvent extracts
The study encompassed a meticulous process for handling powdered plant material and extracting bioactive compounds using various solvents. Initially, the powdered plant material underwent air-drying to eliminate moisture content, ensuring optimal conditions for subsequent extraction. Three different solvents—ether, ethanol, and chloroform—were employed in separate extraction procedures to extract a diverse range of bioactive compounds from the plant material. After air-drying, the powdered plant material was subjected to extraction with each solvent. This process involved mixing the plant material with the solvent and allowing them to interact to extract the bioactive compounds. Following extraction, filtration was carried out to separate the liquid extract, which contained the dissolved bioactive compounds, from the solid residue. The next step involved concentration of the extracted compounds. This was achieved by removing the solvent from the liquid extract, typically through methods like evaporation under reduced pressure or gentle heating. The concentrated crude extracts were then obtained. Subsequently, the residue remaining after solvent removal underwent drying to ensure complete removal of any residual solvent and to obtain a pure extract. Once dried, the extracts were diluted with sterilized distilled water to achieve the desired concentration suitable for testing. Finally, the diluted crude extracts were stored in the refrigerator under controlled conditions. Refrigeration helps maintain the stability and potency of the extracts by slowing down chemical reactions and microbial growth, ensuring the integrity of the bioactive compounds for subsequent analyses [2].
2.7 In vitro evaluation of plant extract
The study delved into assessing the antifungal properties of plant extracts sourced from sweet oranges against fungal pathogens. This investigation was crucial in determining the potential efficacy of these extracts in inhibiting fungal growth, particularly in agricultural and food preservation contexts. To conduct the assessment, a series of in vitro tests were meticulously carried out. Initially, the plant extracts were incorporated into Potato Dextrose Agar (PDA) medium, which serves as a nutrient-rich substrate for fungal growth. The addition of these extracts aimed to create an environment conducive to observing the effects of the extracts on fungal mycelial growth. The in vitro test involved inoculating the PDA plates with fungal pathogens known to cause diseases in sweet oranges. These pathogens were carefully selected based on their relevance to agricultural settings and their potential to cause detrimental effects on citrus crops. After inoculation, the plates were incubated under controlled conditions, including optimal temperature and humidity, to promote fungal growth. Simultaneously, the presence of plant extracts within the PDA medium allowed for the assessment of their inhibitory effects on fungal mycelial growth.
Additionally, the extent of growth inhibition was quantified by measuring the diameter of the growth inhibition zones surrounding the extract-treated areas. Comparative analyses were conducted between plates treated with different concentrations of the plant extracts and control plates without extracts to evaluate the dose-dependent nature of the antifungal activity. Furthermore, microscopic examination of the fungal mycelia in the presence of plant extracts provided insights into any morphological changes or abnormalities induced by the extracts, further supporting the assessment of their antifungal efficacy. By meticulously conducting these in vitro tests and analyses, the study aimed to comprehensively evaluate the antifungal activity of plant extracts from sweet oranges. The results obtained from these experiments would contribute valuable insights into the potential use of these extracts as natural alternatives for controlling fungal pathogens in agricultural and food-related applications. The control groups would include PDA plates inoculated with the same fungal pathogens as the treatment groups but without the addition of plant extracts. These plates represent the standard conditions for fungal growth without any inhibitory treatment.
2.8 Determination of minimum inhibitory concentration (MIC)
The MIC of plant extracts was determined by dissolving them in ethanol and water, then inoculating them on a mycelial disc. The plate with the least concentration of extract and standard fungicides showed no growth, indicating the highest antimicrobial activity.
2.9 Data analysis
SPSS version 26.0 statistical software package was used for statistical analysis of percentage inhibition in each case. The data analysis was performed with One-Way ANOVA followed by Tukey test.
3 Result
3.1 Isolation of pathogenic fungi associated with post-harvest spoilage of sweet orange
The study collected 60 citrus samples from three supermarkets in Jimma Town to identify fungi responsible for spoilage in sweet oranges. Five major fungal species were identified: Penicillium spp., Aspergillus spp., Rhizopus spp., Mucor spp., and Fusarium spp. These pathogens are known for causing deterioration and decomposition of sweet oranges, leading to spoilage. Among the identified fungal species, Penicillium spp. was found to be the most prevalent, indicating its significant role in causing spoilage in sweet oranges. The study concluded that sweet oranges sold in Jimma markets are highly susceptible to contamination by pathogenic fungi. There is a suggested relationship between the prevalence of disease-causing fungi species and conditions during the pre-harvest period, highlighting the importance of pre-harvest practices in controlling fungal contamination. A field survey identified Penicillium spp., Aspergillus niger, and Rhizopus stolonifer as the highest post-harvest fungal pathogens. Penicillium spp. had the highest frequency rate at 20.63%, followed by Aspergillus niger at 18.25% and Rhizopus stolonifer at 17.46%. These findings underscore the significance of understanding and managing fungal pathogens in sweet oranges both before and after harvest. Effective pre-harvest practices and post-harvest management strategies are crucial for mitigating spoilage and ensuring the quality and safety of sweet oranges in markets and throughout the supply chain (Table 1).
The study focused on assessing the growth rates of sweet orange fungal pathogens under different temperature conditions and using various growth media. The study observed variations in the growth rates of sweet orange fungal pathogens across different growth media.
The best growth was recorded on Potato Dextrose Agar (PDA) and Malt Extract Agar, indicating these media provide optimal conditions for fungal growth. Moderate growth rates were observed on Sabouraud Dextrose Agar (SDA) and Yeast Peptone Agar (YPA), suggesting these media support fungal growth but to a lesser extent compared to PDA and Malt Extract. The study identified an optimal temperature range for the growth of sweet orange pathogenic fungi, which was found to be between 20 °C and 27 °C. This temperature range is significant as it aligns with previous research indicating that sweet oranges are more susceptible to fungal infections during wet or humid weather conditions.
The results highlight the influence of both temperature and growth media on fungal growth rates. Optimal growth on PDA and Malt Extract Agar suggests these media provide essential nutrients and conditions favorable for fungal proliferation. The observed variations in growth rates under different media and temperatures underscore the importance of environmental factors in fungal pathogenicity and infection dynamics. By understanding the optimal conditions for fungal growth and the influence of temperature and growth media, the study provides insights into the environmental factors that contribute to the proliferation of sweet orange fungal pathogens. These findings can inform strategies for managing fungal diseases in sweet oranges, such as adjusting environmental conditions and selecting appropriate growth media for fungal isolation and studies (Table 2).
Absolutely, sweet orange fruits are indeed vulnerable to fungal contamination and spoilage throughout multiple stages of their lifecycle, from cultivation to consumer consumption. By addressing these challenges at each stage, it’s possible to minimize fungal contamination and spoilage in sweet oranges, ensuring better quality and safety for consumers.
3.2 Evaluating antagonistic activity of plant extracts against fungal pathogens
The study investigated the antifungal potential of medicinal plants and solvents against pathogenic fungal species that commonly affect sweet orange fruits, namely Aspergillus flavus, Aspergillus fumigatus, and Aspergillus niger. Three medicinal plants; Pterolobium stalatam, Ginger oficealae, and Vernonia amygdalina, were selected for their potential bioactivity. The extracts from these plants, along with various solvent fractions, were tested against the fungal species to assess their inhibitory effects. The findings highlighted the effectiveness of ethanol crude extract, which exhibited the highest inhibition activity against certain fungal pathogens. However, the inhibitory effects of the plant extracts and solvent fractions were generally lower compared to positive controls, indicating the need for further optimization. Interestingly, the chloroform solvent fraction showed exceptional inhibitory activity, emphasizing the significance of solvent selection in extracting bioactive compounds.
Overall, while the study’s results provide insights into the antifungal potential of medicinal plant extracts and solvents for controlling fungal pathogens in sweet orange fruits, further research is needed to refine extraction methods and identify specific bioactive compounds responsible for antifungal activity. This knowledge can facilitate the development of targeted antifungal agents to enhance sweet orange fruit protection in agricultural settings. The study investigated the potential of medicinal plants and solvents against pathogenic fungal spp. (Apsegillus flavus, Aspergillus fumigates, and Aspergillus niger) using extracts from Pterolobium stalatam, Ginger oficealae, and Vernonia amygdalina. The results showed that the crude extracts of these plants and solvent fractions showed smaller inhibitions than their positive control, except for chloroform. The crude extracts of ethanol showed the highest inhibition activity against some fungal pathogens from sweet orange fruits (Table 3).
The study’s investigation into the antifungal activity of ethanol crude extracts from sweet orange fruits yielded significant findings. Specifically, the ethanol crude extracts exhibited the highest antifungal activity among all tested concentrations, particularly at a concentration of 800 mg/ml. This concentration level resulted in substantial inhibition of fungal growth across all fungal species studied, with the most notable inhibition zone observed against Aspergillus flavus, a common pathogen known for its impact on sweet orange fruits. The results suggest that ethanol, as a solvent, effectively extracted bioactive compounds from sweet orange fruits that possess potent antifungal properties. The higher concentration of 800 mg/ml demonstrated enhanced inhibitory effects, indicating a dose-dependent relationship between extract concentration and antifungal activity. This concentration likely provided sufficient levels of bioactive compounds to effectively target and inhibit the growth of fungal pathogens.
The significant inhibition observed against Aspergillus flavus is particularly noteworthy, as this fungal species is notorious for causing significant damage to sweet orange fruits and other agricultural products. The ability of the ethanol crude extracts to effectively combat Aspergillus flavus suggests their potential utility as natural antifungal agents for protecting sweet orange crops. Overall, these findings underscore the importance of solvent selection and concentration optimization in extracting bioactive compounds with strong antifungal properties from sweet orange fruits. Further research and exploration into the specific bioactive compounds responsible for the observed antifungal activity can pave the way for the development of targeted antifungal treatments to safeguard sweet orange fruits against fungal pathogens (Table 4).
The study delved into evaluating the antimicrobial activity of Ginger officinale root extracts against fungal species known to affect sweet orange fruits, notably focusing on Aspergillus niger. Interestingly, the effectiveness of Ginger officinale root extracts was enhanced when ethanol was utilized as the solvent. At a concentration of 800 mg/ml, ethanol demonstrated the most potent antagonistic activity, albeit resulting in the smallest inhibition zone among the tested concentrations. This finding indicates a concentration-dependent relationship, where higher concentrations of ethanol extract may yield increased antimicrobial effects against Aspergillus niger and other fungal species affecting sweet orange fruits.
Additionally, the study explored the inhibition activity of crude extracts and solvent fractions derived from Pterolobium stalatam root against fungal species prevalent in infected sweet orange fruits. Notably, the highest inhibition zone was observed against Aspergillus flavus, showcasing the potential of Pterolobium stalatam root extracts to combat this specific pathogen effectively. The results suggest that Pterolobium stalatam root extracts contain bioactive compounds capable of inhibiting the growth of Aspergillus flavus, which is significant considering the detrimental impact of this fungal species on sweet orange fruit quality and yield.
Overall, these findings highlight the importance of solvent selection and concentration optimization in maximizing the antimicrobial activity of medicinal plant extracts against fungal pathogens affecting sweet orange fruits. The study’s insights pave the way for further research aimed at identifying and characterizing the specific bioactive compounds responsible for the observed antimicrobial effects. Such knowledge can contribute to the development of targeted antimicrobial treatments to combat fungal infections and enhance the quality and safety of sweet orange fruits in agricultural settings.
Ginger oficealae root’s antimicrobial activity against sweet orange fungal species, particularly Aspergillus niger, was more effective when ethanol was used as the solvent. Ethanol showed the most effective antagonistic activity at 800 mg/ml concentration, with the least inhibition zone. The study investigated the inhibition activity of crude extracts and solvent fractionation of Pterolobium stalatam root against fungal species in infected sweet orange fruits, with the highest inhibition zone against Aspergillus flavus (Table 5).
3.3 Minimum inhibitory concentration of crude extract against fungal species
The study found that the crude extract of Vernonia amygdalina plant inhibited fungal species with MICs of 5.25 mg/ml for Aspergillus flavus and 12.5 mg/ml for the rest species. The aqueous solvent was less potent than the crude extracts and other solvents. The ethanol fraction inhibited fungal stains with lower concentrations than the aqueous fraction. Ginger oficealae root had MICs of 12.5 ± 0.00 mg/mL. Pterolobium stalatam root had MICs of 2.5 mg/mL for both species. Petroleum ether had fewer patents than crude extracts of ethanol and chloroform (Table 6). MFC extract and ether fraction against tested fungal strains were 25 mg/ml for A/flavus and 50 mg/ml for A/niger/fumigates, with ethanol fraction showing the smallest growth on subcultured media (Table 6).
4 Discusion
Pathogens pose a significant threat to the quality and quantity of harvested sweet orange fruits, contributing to substantial postharvest losses ranging from 25 to 30%. These losses are often exacerbated by suboptimal storage and transportation practices that create favorable conditions for fungal growth and fruit spoilage. The study conducted a comprehensive assessment and identified a total of 252 fungal species responsible for post-harvest sweet orange spoilage across 60 samples. These fungal species were categorized into five major genera: Aspergillus, Mucor, Penicillium, Rhizopus, and Fusarium, with Aspergillus species emerging as the most prevalent among them [3, 4].The study identified 252 post-harvest sweet orange spoilage fungal species from 60 samples, grouped into five major genera: Aspergillus, Mucor, Penicillium, Rhizopus, and Fusarium, with Aspergillus species being the most prevalent.
The findings of Bukar et al. [5] corroborate these results, highlighting that Aspergillus, Mucor, Penicillium, and Rhizopus are the predominant fungal species commonly associated with oranges. These fungal species play a crucial role in the degradation of sweet orange fruits, contributing to softening and rot-related issues that lead to spoilage. The prevalence of these fungi underscores the need for effective postharvest management strategies to mitigate their impact on sweet orange quality and shelf life.
Furthermore, the study’s identification of these major fungal genera provides valuable insights for develo** targeted interventions and control measures. By understanding the specific fungal species responsible for post-harvest spoilage, stakeholders in the sweet orange supply chain can implement targeted practices to reduce losses and ensure the delivery of high-quality fruits to consumers. This knowledge also underscores the importance of maintaining proper hygiene, storage conditions, and handling practices throughout the postharvest handling process to minimize fungal contamination and preserve fruit quality. A study found that Fusarium, Aspergillus, Rhizopus, Penicillium, and Mucor species cause orange diseases in orange samples from Na’ibawa yan Lemu Market in Kano [5].
The presence of pathogens such as Aspergillus, Penicillium, and Rhizopus in sweet orange fruits is primarily attributed to their ability to produce resilient spores that are highly resistant to environmental factors such as light and chemicals. These resilient spores enable the pathogens to persist and thrive in various conditions, including post-harvest environments where fruits are susceptible to spoilage. The study underscores that fungal species like Aspergillus, Penicillium, and Rhizopus play a significant role in sweet orange spoilage, particularly during the post-harvest period. These fungi have been identified as common culprits responsible for deteriorating the quality of sweet oranges, leading to issues such as rot and decay.
Akintobi et al. [5] research further supports these findings by highlighting specific fungal species, including Aspergillus flavus, A. niger, and Fusarium, as major threats to sweet oranges in markets. These fungi are known for releasing toxins or enzymes that contribute to fruit degradation. Moreover, the high population density in market areas can exacerbate the spread and impact of these fungal pathogens, posing challenges in maintaining fruit quality and minimizing spoilage during storage and distribution. The collective evidence from both studies emphasizes the importance of implementing effective strategies to mitigate fungal contamination and spoilage in sweet orange fruits, particularly during post-harvest handling and storage. These strategies may include improved sanitation practices, proper storage conditions, and targeted interventions to control fungal growth and minimize the impact of pathogenic species on fruit quality and marketability.
These pathogens are prevalent in sweet orange fruit due to their capacity to produce resistant spores, which exhibit heightened resilience against light and chemicals. The study underscores the association of fungal species such as Aspergillus, Penicillium, and Rhizopus with sweet orange spoilage, especially during the post-harvest phase. Akintobi et al.[5] corroborate these findings by identifying Aspergillus flavus, A. niger, and Fusarium species as key aggressors targeting major markets. These pathogens release toxins or enzymes via their spores, contributing to the deterioration of sweet oranges. The dense population in market areas exacerbates the impact of these pathogens, necessitating robust strategies to mitigate fungal contamination and ensure fruit quality.only found in sweet orange fruit due to their ability to produce resistant spores, which are more resistant to light and chemicals. The study reveals that fungal species like Aspergillus, Penicillium, and Rhizopus are associated with sweet orange spoilage, particularly during post-harvest time. Akintobi et al. [6], found Aspergillus flavus, A. niger, and Fusarium species attack major markets due to their spores releasing toxins or enzymes, and the high population density in the market area.
Effiuvwevwere [7] conducted a study shedding light on the factors contributing to fungal contamination in fruits, including sweet oranges. The study identified several key practices within the food supply chain that can lead to fungi contamination, such as poor handling during harvest, inadequate storage conditions, improper distribution, suboptimal marketing strategies, and inadequate transportation practices. These factors create conducive environments for fungal growth and survival, as fungi possess the ability to produce spores, toxins, and enzymes that aid in their survival and proliferation.
The findings of Effiuvwevwere’s study align with Bukar’s [5] earlier research, which also highlighted the occurrence of spoilage in apparently healthy oranges upon re-inoculation with pathogens. This phenomenon underscores the resilience and virulence of fungal species like Aspergillus, Penicillium, Mucor, and Rhizopus, which are capable of causing spoilage in sweet oranges post-harvest. The study suggests that these fungi may originate from farms or stores, leading to cross-contamination in markets and supermarkets and ultimately contributing to post-harvest contamination and spoilage.
Given the high prevalence of these fungi, the study emphasizes the urgent need for implementing appropriate control measures to combat infection and reduce spoilage rates. This includes adopting best practices in harvest handling, storage, distribution, and transportation to minimize opportunities for fungal contamination. Furthermore, the study highlights the importance of collaboration among farmers, marketers, and consumers in creating an environment that discourages microorganism growth and ensures the quality and safety of fruits during the entire supply chain process.
The study conducted a screening of three medicinal plants to assess their antifungal properties against fungal isolates obtained from sweet orange fruits. Among these plants, Vernonia amygdalina leaf extracts exhibited notable antifungal activity against three selected fungal pathogens. The study further evaluated different solvent fractions of V. amygdalina leaf extracts and found that the ethanol extract displayed the highest antifungal activity, followed by the P/ether extract. Interestingly, chloroform extracts demonstrated significant inhibition at 60.86%, indicating the solubility of active compounds in chloroform.
Additionally, the research involved the assessment of V. amygdalina leaf extracts and their solvent fractions against a range of fungal species, resulting in observed growth inhibition. While previous studies have documented the antifungal activity of V. amygdalina extracts against common pathogens, this study stands out as the first to report on the efficacy of V. amygdalina extracts against unique fungal species found on human cadaver surfaces.
These findings suggest that V. amygdalina leaf extracts possess promising antifungal properties that can effectively inhibit the growth of fungal pathogens, including those with unique characteristics. The study’s results hold significant implications for potential applications in combating fungal infections and promoting food safety, underscoring the importance of further research to explore the mechanisms and active compounds responsible for V. amygdalina’s antifungal activity [8,9,10,11].
The study conducted on V. amygdalina revealed that its ethanol, chloroform, and ether fractions exhibited activity against fungal pathogens when tested through agar dilution methods. However, these fractions did not induce growth inhibition when evaluated using agar well diffusion assays. The observed discrepancy is attributed to the slow diffusion of nonpolar compounds into polar culture media, leading to challenges in accurately quantifying their diffusion amounts. This phenomenon underscores the complexities associated with assessing the efficacy of plant extracts against fungal pathogens and highlights the need for careful consideration of experimental methodologies and factors affecting diffusion rates [12, 13].
The study identified that certain solvent fractions from V. amygdalina failed to inhibit fungal growth, potentially due to insoluble active components or variations in distribution across different organic solvents. This highlights the importance of understanding the solubility properties of bioactive compounds when assessing their efficacy against fungal pathogens.
Moreover, while plant extracts demonstrated effective activity against standard strains of fungi, their efficacy was limited against clinical isolates. This reduced activity could stem from factors such as decreased cell permeability or the development of resistance to drug receptors in clinical fungal strains. These findings underscore the challenges associated with combating fungal infections and emphasize the need for ongoing research to develop effective strategies for addressing resistance mechanisms in fungal pathogens [14]. Exactly, decreased cell permeability, active efflux of test substances, modification of drug receptor sites, and the synthesis of resistant metabolic pathways are all potential mechanisms that can contribute to reduced efficacy of plant extracts against fungal pathogens. These mechanisms can lead to resistance in clinical isolates and hinder the ability of bioactive compounds to effectively inhibit fungal growth. Understanding these mechanisms is crucial for develo** strategies to overcome fungal resistance and enhance the effectiveness of plant-based antifungal treatments.
The study’s findings regarding the susceptibility of certain fungal species to extracts from Pterolobium stalatam, Ginger officinale, and Vernonia amygdalina are significant, particularly in the context of modifying embalming solutions and preserving corpses in southern Ethiopia. However, the study did not explore the potential for biological control of sweet orange fruit diseases using medicinal plants. Therefore, further research is warranted to investigate the efficacy of yeasts in antagonizing fungi, especially in agricultural settings.
By conducting additional studies, researchers can assess the effectiveness of yeasts in combating fungal pathogens that cause spoilage in sweet orange fruits. This information can then be utilized to develop an integrated disease management schedule that incorporates biological control methods alongside traditional practices. Implementing such a comprehensive approach can help mitigate the impact of spoilage pathogens and improve fruit quality and postharvest outcomes in agricultural settings.
The conclusion drawn from the extensive study on fungal pathogens affecting sweet orange fruits underscores the critical need for effective postharvest management strategies. With the identification of 252 fungal species across various samples, it’s evident that Aspergillus, Mucor, Penicillium, Rhizopus, and Fusarium are major contributors to postharvest spoilage. These findings align with previous research highlighting the prevalence of these fungi in oranges and their detrimental effects on fruit quality.
Moving forward, implementing targeted interventions is crucial to mitigate fungal contamination and reduce spoilage rates. This includes adopting best practices in harvest handling, storage, distribution, and transportation to minimize opportunities for fungal growth. Collaboration among stakeholders, including farmers, marketers, and consumers, is essential to create an environment that discourages microorganism growth and ensures fruit quality throughout the supply chain.
Furthermore, exploring the antifungal potential of medicinal plants like Vernonia amygdalina and assessing the efficacy of yeasts in antagonizing fungal pathogens are promising avenues for future research. Develo** integrated disease management schedules that combine biological control methods with traditional practices can significantly improve fruit quality, reduce postharvest losses, and enhance food safety. In summary, the study emphasizes the importance of continuous research and the implementation of comprehensive strategies to combat fungal contamination, preserve fruit quality, and meet consumer demand for high-quality sweet oranges.
Data availability
The data used and analyzed during the current study are available within the manuscript.
References
Gorinstein S, Martin-Belloso O, Park Y, Haruenkit R, Lojek A, Milan I, Caspi A, Libman I, Trakhtenberg S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001;74(3):309–15.
Rojas TR, Sampayo CAF, Vázquez BI, Franco CM, Cepada A. Study of interferences by several metabolites from Aspergillus spp. in the detection of aflatoxigenic strains in media added with cyclodextrin. Food Control. 2005;16:445–50.
Droby S. Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Hort. 2006;709:45–51.
Zhu SJ. Non-chemical approaches to decay control in postharvest fruit. In: Noureddine B, Norio S, editors. Advances in postharvest technologies for horticultural crops. Trivandrum: Research Signpost; 2006. p. 297–313.
Bukar A, Mukhtar MD, Adamu S. Isolation and identification of postharvest spoilage fungi associated with sweet oranges (Citrus sinensis) traded in Kano metropolis. Bayero J Pure Appl Sci. 2009;2(1):122–4.
Akintobi AO, Okonko IO, Agunbiade SO, Akano OR, Onianwa O. Isolation and identification of fungi associated with the spoilage of some selected fruits in Ibadan, South-Western Nigeria. Acad Arena. 2011;3(11):1–10.
Effiuvwevwere BJO. Microbial spoilage agents of tropical and assorted fruits and vegetables (An illustrated references book). Port Harcourt: Paragraphics publishing company; 2000. p. 39.
Nwachukwu E, Umechuruba C. Antifungal activities of some leaf extracts on seedborne fungi of African yam bean seeds, seed germination and seedling emergence. J Appl Sci Environ Manage. 2001;5(1):29–32.
Teugwa M, Sonfack D, Fokom R, Penlap B, Amvam Z. Antifungal and antioxidant activity of crude extracts of three medicinal plants from Cameroon pharmacopea. J Med Plants Res. 2013;7(21):1537–42.
Obey J, von Wright A, Orjala J, Kauhanen J, Tikkanen-Kaukanen C. Antimicrobial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. J Pathog. 2016. https://doi.org/10.1155/2016/1453428.
Hailu G, Bitew M, Temesgen M. In vitro antifungal activity of Croton macrostachys and Allium sativum extracts against Candida albicans and Trichophyton mentagrophytes isolates. Int J Biotechnol Food Sci. 2017;5(3):42–7.
Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40(2):223–31.
Klancnik A, Piskernik S, Jersek B, Mozina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Meth. 2010;81(2):121–6.
Mammed, A.A. Mycoflora and mycotoxins of major cereal grains and antifungal effects of selected medicinal plants from Ethiopia. PhD thesis, Gottingen University, Germany. mango Mangifera indica through production practices and climatic factors; 2002.
Ngongu I, Ugese F, Liamngee K, Agatsa DT. Isolation and identification of fungi causing postharvest decay of oranges (Citrus sinensis) during storage in Makurdi. Mind Sourcing Bio. 2018;3(12):1–8.
Acknowledgements
The authors are would like to thanks, all individual and organization who were participate in supporting and facilitated data collecting, support and encouragement and data collectors for their support initiation and encouragement and assistant starting from the begging.
Funding
No funding agency for this research project.
Author information
Authors and Affiliations
Contributions
Desalegn Amenu, Rael Dessalegn, Chimdesa Tolera, Ayantu Nugusa and Temesgen Tafesse participating on Data collection, field work and Data conceptualization, in addition, they are participating 1st, 2nd and 3rd author. All authors were participating on data analysis, edition and conceptualization; furthermore, DA is the corresponding author. Copy editing, translation, paraphrasing and finally edition was supervised and processed by DA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amenu, D., Desalegn, R., Nugusa, A. et al. Evaluation of antifungal activity of plant extracts against plant pathogen associated with sweet orange (Citrus sinensis L.), in Jimma, Western Ethiopia. Discov Appl Sci 6, 357 (2024). https://doi.org/10.1007/s42452-024-06054-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06054-2