Abstract
The overuse of pesticides and fertilizers to grow high-quality roses unintentionally introduces heavy metals into the soil which is detrimental to the human health. Thus, the aim of the current study is to examine the concentrations of the essential (Ca, Mg, Zn, Fe, Cu, Fe, Mn and Ni) and toxic metals (Cd and Pb) in the soil samples from the flower farm using Flame atomic absorption spectrometry (FAAS) and to assess the risk that heavy metals pose to human health. To obtain an optimum condition for the wet digestion of soil samples, the volume ratio of reagents, reflux time and temperature were optimized. The concentrations of Ca, Fe, Mn, Mg, Zn, Cr, Ni, Cu were found to be 3468, 4926, 450, 255, 74, 70,48.5 and 25 and 2150, 4862,472, 241, 71, 58, 37 and 12.5 mg/kg for the soil samples collected from the flower farm and road side, respectively. Cadmium and lead were not detected in the studied soil samples. Statistical analysis of variance (ANOVA) showed the concentrations of Ca and Cu in the two soil samples are significantly different. Correlation analysis showed there was strong positive association for Fe with Zn, Cr and Ni, Mg with Ca, Cu, Ni and Cr, Zn with Cr and Cu and Ni with (Cu, Zn and Cr). Values of hazard quotient (HQ) and hazard index (HI) for the heavy metals under the study in the all exposure pathways are below 1.0 indicating that none of them could be harmful to human health.
Article Highlights
-
Determination of the levels of the essential and toxic metals in the soil samples.
-
Optimization of analytical method and validation of the optimized procedure.
-
Non‑carcinogenic and carcinogenic risk assessment of exposure to heavy metals in soil samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soil is an important natural resource. An excessive load of industrial and agricultural activity has the potential to be detrimental on the characteristics and quality of the soil [1, 2]. On the other side, sustainable growth depends on maintaining the quality of the soil and preventing its degradation.
Soil consists of organic materials, minerals, air, water and living organisms [3].The quality of the soil is extremely important because it provides vital nutrients to plants. Soil is one of the most significant sinks for different pollutants, including heavy metals, pesticides, herbicides, petroleum hydrocarbons, emerging pollutants and persistent organic pollutants [2, 4]. These pollutants enter the body via oral ingestion, dermal contact and inhalation exposure to soil. Furthermore, these contaminants have the potential to accumulate in the soil and have a detrimental impact on the quality and productivity of the soil [2, 4, 5]. As a result, they may endanger human health and other living organism [4,5,6,7]. Thus, soil pollution is among the most critical environmental issues facing the world.
Metals are classified as essential macronutrients (Na, K, Ca and Mg), essential micronutrients (Fe, Ni, Mn, Cu, Co, Mo and Zn) and non-essential metals (Pb, Hg, Ag, Sn, Al). Essential and trace essential metals are very crucial for plants and animals to grow and remain healthy. Even though, they are very beneficial, too much of them can be harmful to living organisms [8,9,10]. The non-essential metals have no physiological function and can impede biological processes in living things [5, 8, 11, 12].
Heavy metals are primarily characterized by their non-biodegradability, toxicity and accumulation patterns [3, 8, 13]. This can lead to an imbalance in the biochemical cycles of the food chains and ecosystem, which can result in the degradation of the water ecosystem and changes in soil characteristics [8, 13,14,15,23,24,25,26,27].
Floriculture is a branch of horticulture focused on the cultivation of decorative and flowering plants [28]. The floral industry is relatively new and growing rapidly in Ethiopia [29]. Although the floriculture sector in Ethiopia is relatively new, it has grown from five in 2002 to more than a hundred in 2011 [28]. Ethiopia is now the sixth-largest rose producer in the world and the second-largest producer in Africa [10, 29,30,31].
Despite the fact that the floriculture sector creates jobs opportunity and contributes for economic growth of the country, it is criticized for utilizing excessive amounts of fertilizers and pesticides that leak into the environment [10, 29, 31]. The regular application of chemical pesticides and fertilizers to grow high-quality roses has a negative effect on the environment [10, 28, 31]. The production employs over 300 chemicals as growth regulators and insecticides, which destroy beneficial soil organisms and disrupt biodiversity [10].
Fertilizers and pesticides contain nutrient elements that are required for plant growth. However, overuse of herbicides and fertilizers unintentionally introduces heavy metals into the soil [28, 32]. Therefore, to determine the origins of soil metal contamination and to develop strategies for soil remediation in industrial plants, investigations on the amounts of metals in the soil and evaluation of their potential hazards to human health are important. As far as we know, there has not been much information on the extent of metal pollution of soils and health problems associated with it in the floriculture industry in Ethiopia. Thus, the current investigation aims to examine the amounts of essential metals (Zn, Cu, Ni, Mg, Mn, Fe, Cr, and Ca) and toxic metals (Cd and Pb) in the soil from the flower farm and road side around the flower farm in Roshara roses Bishoftu and to assess their potential human health threat.
2 Material and methods
2.1 Description of the study area
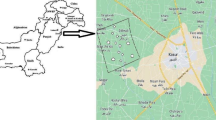
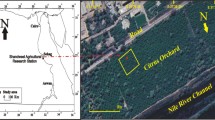
Bishoftu is located at a distance of nearly 47 km South east of Addis Ababa in East Shewa Zone, Oromia Regional State, Ethiopia. It has a latitude of 8˚45' N and longitude of 38.98˚ E and an elevation of 1920 m above sea level. The geographical location of the study site is displayed in Fig. 1.
2.2 Collection of samples
The soil samples were gathered from the flower farm and road side around the flower farm in Bishoftu, Ethiopia. Soil samples were taken with a soil auger, spade and spoon from topsoil at the depth of 0–20 cm. After removing all the foreign materials such as leaves, roots, wood and stones, the soil samples were thoroughly mixed to form representative sample.
2.3 Apparatuses and instruments
Polyethylene plastic bags were used for soil samples collection. Analytical balance (Mettler Toleado, Model AG204, Switzerland) was employed for weighing the soil samples. Drying oven (Digitheat, J.P.selecta, S.a, Spain) was employed for drying the soil samples. The samples were digested using Kjeldahl heating apparatus (Gallenhamp, England). The amounts of selected metals in the soil samples were determined using Flame Atomic Absorption Spectrophhotometer (FAAS) (Model ZEEnit 700P, Germany).
2.4 Chemicals and reagents
In this experiment, soil samples were digested using HNO3 (69–70%) and HCl (37%). Calibration and intermediate standards were prepared using stock standard solutions (1000 mg L−1) of the metals (BDH Chemicals, England). Soil samples were diluted and standard solutions were prepared using distilled water.
2.5 Preparation of soil samples
The soil samples were allowed to dry at room temperature for five days. After that, the samples were dried for four days at 70 °C in an oven. The dried soil samples were ground and the powdered samples were allowed to pass through a 2 mm sieve to remove big particles.
2.6 Optimization of digestion conditions for soil samples
To obtain best working procedure for the digestion of soil samples, volume of reagents, digestion temperature and reflux time were optimized. The best wet digestion procedures for the soil samples were selected by considering the clarity of the digests, less digestion duration, lower temperature and minimum acids volume needed to complete the digestion of soil samples. From the optimization procedures, 3.0 mL of HNO3 (69–72%) and 1.0 mL HCl (37%) at temperature of 210 °C and digestion time of 3 h were found to be the optimum working conditions to digest 0.5 g soil samples.
2.7 Wet digestion of soil samples
Optimum amount of HNO3 and HCl were added to a 100 mL round bottomed flask containing 0.5 g of soil sample. The mixture was then digested for 3 h at 210 °C. After cooling the digested sample, the solution was filtered in to 25 mL flask and then filled to the mark with distilled water. The levels of the selected metals in the soil sample were analyzed by FAAS. To assess the levels of metals in the reagent blank, the acid mixture was digested using similar digestion protocol as for the soil samples.
2.8 Preparation of calibration standards
1000 mg/L stock standard solutions of the metals were used for the preparation of intermediate standard solutions (10 mg/L) and working standard solutions of each metal. FAAS was calibrated using five sets of working standard solutions. Then after, FAAS was employed to assess the metal concentrations in the soil samples.
2.9 Method validation for metal determination
2.9.1 Limit of detection and limit of quantification
Limit of detection (LOD) is the lowest concentration of analyte that can be measured but not necessarily quantified with acceptable uncertainty. Six replicates of acid mixtures which were digested using the same protocol as for the actual samples were analyzed to determine LOD. In this investigation, the detection limits of each metals were computed as three times standard deviation of the blank (LOD = 3Sblank, n = 6, where Sblank is the standard deviation of the blank) [3, 33]. Limit of quantification (LOQ) is the minimum concentration of analyte in the sample that can be measured with acceptable uncertainty [34]. LOQ of each metal was determined from the analysis of six replicates of blank samples. The LOQ of each metal was computed as ten times the blank standard deviation (10Sblank, n = 6, where Sblank is the standard deviation of the blank) [3, 33, 34]
2.9.2 Precision
The precision of the developed procedure indicates the degree of closeness between a set of measurements. It is usually reported using percentage relative standard deviation (%RSD) or standard deviation (SD) of the replicate measurements.
2.9.3 Recovery test of the optimized procedure
In this study, soil samples were spiked with known standard concentrations of the metals to check the validity of the method. The digestion protocol used for the real samples was also applied for the spiked soil samples. The following formula was used to compute the percent recoveries (% R):
2.10 Determination of metals with flame atomic absorption spectrophotometer
In this study, calibration curve established from a plot of absorbance readings against concentration were used to find the levels of the investigated metals in the digested soil samples. The final concentrations of metals in the soil samples were determined using the following equation:
where V is the final volume of solution in liter, and W is the weight in kg of the soil sample taken.
2.11 Human health risk assessment of heavy metals
2.11.1 Exposure assessment of heavy metals via ingestion, dermal contact and inhalation
Exposure evaluation of heavy metals can be carried out via directly ingesting the soil through the mouth (EDIoral), absorbing the soil through the skin (EDIdermal), and inhaling suspended soil particulates (EDIinh) [8, 16]. The average daily intake (mg/kg/day) of heavy metal via oral intake, skin contact and inhalation exposure was calculated using Eqs. (3)–(5), respectively [8, 16, 35, 36].
where EDIoral is the daily dose of heavy metal through direct oral ingestion of the soil, C is the amount of the metal in the soil (mg/kg), OIR is the oral ingestion rate (100 mg/day for adults), ED is the exposure duration (25 years for adults), EF is the exposure frequency (365 days/year), ABSo is the oral absorption factor (1), BW is the average body weight (56.8 kg assumed for adults) and AT is the average exposure time for adults in their whole lifetime (365 days/year × 25 years), 10–6 is the conversion factor, EDIdermal is the daily intake of heavy metal via soils adhered to exposed skin, ESS is the exposed skin surface area to pollutants (6032 cm2 for adults), ARS is the adherence rate of soil on skin (0.2 mg·cm−2), DEF is the daily exposure frequency of the dermal contact event (1), ABSd is the dermal absorption factor (0.001), EDIinh is the daily dose of heavy metal intake of suspended soil particulates through inhalation, PM10 is the content of inhalable particulates in ambient air (0.15 mg·m−3), DAH is the daily air inhalation rate of adults (14.5 m3·days−1), RFIP is the retention fraction of inhaled particulates in the body (0.75), FSBPO is the fraction of soil-borne particulates in outdoor air (0.5), FSBPI is the fraction of soil-borne particulates in indoor air (0.8), OEF is the outdoor exposure frequency(62.5 day/year), and IEF is the indoor exposure frequency (187.5 day/year).
2.11.1.1 Non‑carcinogenic risk assessment
The possible non-carcinogenic hazard associated with heavy metal exposure to human was determined using the target hazard quotient (HQ). If HQ value is less than 1⋅0, no potential health risks result due to exposure to heavy metal contamination. But, when the HQ value is greater than or equal to one, exposure to heavy metal-contaminated soil may pose a non-cancer risk [37]. The HQ values via the three pathways (oral, dermal, and Inhalation) for each heavy metal in the soil samples were evaluated using Eqs. (6)-(8) [8, 38].
where HQoral is the hazard quotient of the individual heavy metal via oral ingestion, SAF is the soil allocation factor (0.2), RFDo is the oral reference dose of the heavy metal (mg/kg/day). Accepted RFDo values (mg/kg day) are for Mn: 0⋅1, Fe: 0⋅7, Ni: 0⋅02, Cr: 0.003, Cu: 0⋅04, Zn: 0⋅3, Cd: 0⋅001, Pb: 0.004 [22, 24, 25]. HQdermal is the hazard quotient of the individual heavy metal via the soils adhered to exposed skin, RFDd is the reference dose of the heavy metal via the soils adhered to exposed skin. Accepted RFDd values (mg/kg day) are for Cu: 0.012, Zn: 0.06, Ni: 0.0008, Cr: 0.003, Cd: 0.000025, Mn: 0.023, Pb: 0.000525 [37, 38]. HQinh is the hazard quotient of the individual heavy metal intake of suspended soil particulates via inhalation, RFDi is the reference dose of the heavy metal intake of suspended soil particulates via inhalation (mg/kg/day). Accepted RFDi values (mg/kg day) are for Cu: 0.0402, Zn: 0.3, Ni: 0.000023, Cr: 0.0000286, Cd: 0.000057, Mn: 0.0000143, Pb: 0.00352) [37, 38].
The overall non-carcinogenic health effect of multiple heavy metal exposure can be assessed using the hazard index (HI). HQ of each heavy metal is added up to give HI [37]. When the HI value is greater than 1, there may be a potential non-cancer health effect from exposure to soil polluted with heavy metals. The HI value was evaluated using the following equation:
If there are several exposure routes, a total exposure hazard index (THI) could be used to evaluate the non-cancer risks. The THI is expressed as follows:
2.11.1.2 Carcinogenic risk assessment
The cancer risk (CR) during a lifetime exposure to heavy metals having a carcinogenic property (Cd, Pb, Ni and Cr) [36] is evaluated using cancer slope factor (SF) [36]. The cancer risk associated with a lifetime exposure to heavy metals via ingestion (CRoral), dermal contact (CRdermal) and inhalation (CRinh) of the soil were calculated using Eqs. (11)—(13), respectively [16, 35,36,37].
where SFo, SFd, and SFi are the carcinogenic slope factors (mg/kg/day) of a heavy metal having a carcinogenic property via ingestion, dermal contact and inhalation, respectively. SFo, SFd and SFi values are 0.5, 20 and 32.9 for Cr and 0.84, 21 and 1.02 for Ni, respectively [8, 16, 35, 38].
CR, values of < 10−6 are regarded as having no carcinogenic health problem, while values of > 10−4 are considered to have substantial health impacts and CR values between 1 × 10−4 and 1 × 10−6 are considered as tolerable range [35,36,37].
2.12 Statistical analysis
In the current investigation, the metal levels of the soil samples were compared using one-way analysis of variance (ANOVA). ANOVA uses F-distribution to determine the existence of significant variation between the means at 95% confidence level [34]. Additionally, the extent of the linear association between the metals was compared using Pearson's correlation coefficient.
3 Results and discussion
3.1 Regression analysis
Procedures for standard preparation and calibration affect the quality of the results obtained for metals analysis using FAAS. As presented in Table S1 (Supplementary material), the calibration curves for all metals had correlation coefficients (R2) ≥ 0.9964 indicating that there was good correlation between absorbance and concentration. The analytical parameters, such as LOD, LOQ, calibration curve equations and correlation coefficients for metals determined by FAAS are shown in Table S1.
3.2 Recovery results of metal determination
As indicated in Table 1, the %R results for the metals varied from 90.7 to 105.3%. The recovery results indicate the accuracy of the proposed procedure for metals analysis in the soil samples.
3.3 Concentration of metals in soil samples
As illustrated in Table 2, the metal contents of the analyzed soil samples were reported as mean values ± SD. The precision of the proposed procedure was evaluated using %RSD of the replicate measurements. As shown in Table 2, the %RSD values were < 5.4% for the studied metals. These results show that the suggested analytical procedure was precise. Table 2 and Fig. 2 display the determined results of metals for each soil samples. As shown in Table 2 and Fig. 2, Ca, Mg, Zn, Cr, Fe, Cu, Fe, Mn, and Ni were present in both soil samples but the amounts of Pb and Cd were not detectable.
The metal contents revealed that the soil samples from the two sampling areas had high levels of calcium and iron. Except for Mn, the metals content of the flower farm is higher than that of the road side. Generally, the metal content profile in both samples were generally found to be Fe > Ca > Mn > Mg > Zn > Cr > Ni > Cu.
3.4 Comparison of the concentrations of metals in the soil samples with literature values and World Health Organization (WHO) standard values
Calcium (Ca) is the most essential macroelement for human health. It plays vital role in building strong bone and teeth, in muscle contraction and in maintaining regular heartbeat [39, 40]. On the other hand, overconsumption of Ca prevents the body from absorbing mineral. In the current investigation, the soil sample from the flower farm had a higher content of calcium. The concentrations of Ca were found to be above the WHO recommended limit of Ca in the soil (100 mg/kg) [41].
Magnesium (Mg) is a vital micronutrient for both plant and animals. The accessibility of Mg to plants is affected by a number of factors, including agricultural systems, human activities, and the location and composition of the rock material [42, 43]. The shortage of Mg affects the process of carbohydrate partitioning and photosynthesis in plants [44]. In the current study, the contents of Mg in the investigated soil samples were found to be above the WHO recommended value of Mg in the soil (60 mg/kg) [41].
Iron (Fe) is important for oxygen transport in the body as it is the most important component of hemoglobin [45,46,47]. Moreover, it involves in some enzymatic reactions and energy producing processes [45,46,47]. Even though iron is necessary, taking too much of it can lead to tachypnea, low blood pressure, seizures and siderosis in liver and pancreas thyroid [48]. Fe has a variety of significant biological activities in plants, including photosynthesis, chloroplast formation, and chlorophyll biosynthesis [49]. In this study, the levels of Fe in both soil samples are approximately equal. The amounts of Fe in the investigated samples were below the permissible threshold of 50,000 mg/kg in the soil [3, 8]. In comparison to results reported by Tefera [50], Gebeyehu and Bayissa [7] and Addis and Abebaw [3], Fe is present in lower concentration but it is higher than what was reported by Minase [51].
Zinc (Zn) is a vital mineral in the human diet [19]. It is important for normal brain activity, various enzymatic reactions, DNA synthesis, fetus growth and development, physiological processes and metabolism [46, 48]. In the present investigation, the concentrations of Zn in the soil samples from the flower farm and road side are approximately equal (Table 2, Fig. 2). The analysed soil samples had Zinc contents below the safe limit of 300 mg/kg Zn in the soil [3, 8, 51]. The amount of zinc obtained in this investigation is consistent with values reported by Kinfu [28] but is less than values reported by Gebeyehu and Bayissa [7] and more than that reported by Addis and Abebaw [3].
Copper (Cu) is an essential nutrient needed for living things [8, 47, 51]. In humans, it facilitates the synthesis of blood hemoglobin [19]. Cu is very important for plants to produce seeds, feed themselves, resist disease, and regulate their water intake [21]. Overconsumption of copper causes digestive problem, damages liver, kidneys, brain tissue and coronary vessels [10, 52]. Shortage of Cu in the diet may cause anemia, inadequate growth, problems with reproduction, abnormalities of the neurological system and diseases of the circulatory system [19]. High contents of Cu in the soil inhibit development of plants [21, 53]. In the present investigation, the soil samples from the flower farm had a higher concentration of Cu (25 mg/kg) than the road side sample (12.5 mg/kg). The levels of Cu in both soil samples were below the allowable value of 100 mg/kg in the soil [8, 20, 51]. The amounts of Cu found in the current investigation is lower than values reported by Tefera [50], Minase [51] and Addis and Abebaw [3] but it is consistent with the results of Gebeyehu and Bayissa [7].
Manganese (Mn) is an important trace micronutrient for human health. It is important for the catalytic activity of various enzymes, metabolism of several vitamins, regulation of immune system and metabolism of lipids, amino acids and glucose [46, 52]. However, accumulation of excess Mn in the brain results in cerebella dysfunctions, memory problems, persistent tremors, and motor incoordination [22, 45, 46]. In plants, Mn plays a crucial role in the structure of photosynthetic enzymes and proteins [54]. However, when taken in excess, it lowers the rate of photosynthesis in leaves [55]. In the current study, both the investigated soil samples had Mn levels below the recommended limit of 2000 mg/kg [3, 7, 51]. Compared with values reported by Minase [51] and Addis and Abebaw [3], the amount of Mn obtained in the current investigation is high.
Nickel (Ni) is found naturally in soils in trace amount. However, the use of fertilizers, mining, smelter emissions, and other human activities raise the concentration of Ni in soils [5, 19]. In plant, high concentration of Ni in the soil results in a variety of physiological changes [56]. In humans, excessive Ni exposure can harm the nervous system, induce cancer, and irritate the skin [19, 22]. In this investigation, the concentrations of Ni in the soil samples were above the acceptable amount of 50 mg/kg in soil [3, 51]. The amounts of Ni obtained in the soil samples in the current investigation were found to be higher than results reported by Gebeyehu and Bayissa [7] but lower than those reported by Addis and Abebaw [3].
Chromium (Cr) is found naturally in the earth, sea, rocks, soil and plants. It can be found in the soil as trivalent chromium (Cr3+) or hexavalent chromium (Cr6+) [19, 54, 57]. Cr3+ is an essential nutrient in the diet, but it is needed in very small amounts [58]. Cr6+ is toxic and carcinogenic [22, 52]. Long term exposure to Cr6+ can damage kidney, liver and nerve tissue [54, 58]. The recommended safe limit for Cr in soils used for agriculture is 100 mg/kg [59]. Both soil samples in the current study had Cr concentrations below the permissible value. In comparison to the findings of Tefera [50] and Gebeyehu and Bayissa [7], the amount of Cr reported in the present study is high.
Lead (Pb) has no biological importance and it is highly toxic to plants, animals, and microorganisms [8, 21, 60]. When Pb enters the body, it can cause neurological disorder, liver and kidney damage, cardiovascular dysfunction, gastrointestinal cramps, reproductive system and respiratory system damage [19, 20]. Pb has a number of negative effects on soil, including decreased soil fertility, microbial diversity, and soil nutrients. Pb buildup in plants disrupts enzymatic processes and results in physiological issues, such as DNA destruction and the damage of root and shoots systems [21]. The recommended safe limit of Pb in uncontaminated soil is 100 mg/kg [3, 8, 51]. In this investigation, the amount of Pb in the soil samples was not detectable.
Cadmium (Cd) is mainly released to the atmosphere from manufacturing of plastics, electroplating, mining, smelting, tobacco smoking, manufacturing of fertilizers, paint pigment and alloy preparation [5, 19, 61]. The application of fertilizer, sewage sludge, and emissions from industrial sources can contaminate soils, increasing the amount of Cd that crops and vegetables absorb. Human exposure to Cd is linked to increased apoptosis, oxidative stress, DNA methylation, and DNA damage [22]. The metal is also well-known for being a strong carcinogen that damages the pancreas, kidney, lung, and prostate [19, 22]. Accumulation of Cd in plants results in physiological and metabolic alterations [21, 22]. Moreover, high concentration of Cd in plants inhibits mineral transportation [19].The recommended safe limit of Cd in soils for agriculture is 3 mg/kg [3, 8, 51]. The experimental results of this investigation demonstrated that Cd was not detected in both soil samples.
3.5 Statistical analysis
According to the statistical analysis of ANOVA at 95% confidence interval, with the exception of Ca and Cu, the levels of all metals in the two soil samples are not significantly different (Table 3).
3.6 Pearson’s correlation coefficients of metals within soil samples
In the current investigation, the influence of the amount of one metal on the concentration of other metals was correlated using Pearson correlation coefficients (r). The correlation values are classified as high correlation (r > ± 0.71), moderate correlation (± 0.5 < r < ± 0.7) and low correlation (r < ± 0.5) [2].
As shown in Table 4, there was strong positive association for Fe with Zn, Cr and Ni, Mg with Ca, Cu, Ni and Cr, Zn with Cr and Cu and Ni with Cu, Zn and Cr indicating that the sources (anthropogenic or natural) by which they reach the soil at this location is similar [16]. A moderate positive association was found between Fe and Mg, Fe and Cu, Fe and Ca, Fe and Cu, Zn and Mn, and Zn and Mg. A low positive correlation was observed for Fe with Ca and Mn, Ca with Cr and Ni and Mn with Cr, Ni and Cu.
3.7 Human health risk assessment of heavy metals via exposure to soil
3.7.1 The estimated daily exposure dose of heavy metals
The daily doses (EDI) of heavy metals through the three exposure routes are given in Table 5. The result shown in Table 5 indicates direct oral ingestion exposure pathway is the predominant contributor to the EDI. The EDI of Fe is the highest whereas that of Cu is the lowest. The EDI results for all the studied metals are lower than the recommended limits.
3.7.2 Non-carcinogenic risk assessment
In this investigation, the impact of each exposure routes to non-carcinogenic health problem is indicated in Table 6. Exposure to heavy metal-contaminated soil may pose a non-carcinogenic hazard in the following sequence: Cr (HQ = 0.21) > Mn (HQ = 0.132) > Fe (HQ = 0.043) > Ni (HQ = 0.032) > Cu (HQ = 0.005) > Zn (HQ = 0.00222). Although all heavy metals have both HQ and HI values less than 1, the oral intake exposure route is the main contributor (73.3%) to non-cancer risk followed by inhalation pathway (24.3%).
3.7.3 Carcinogenic risk assessment
The CR results obtained for carcinogenic metals (Cr, Ni, Pb and Cd) for each of the exposure routes are shown in Table 7. As shown in Table 7, the health hazard resulting from oral intake of heavy metals accounts for the greatest portion of the overall CR. The calculated CR values of Cr and Ni are within the permissible range indicating these heavy metals do not pose potential carcinogenic health risks.
4 Conclusion
The gradual accumulation of metals in soils is overwhelmingly threatening to the ecosystem and life on earth. Since the majority of metals are necessary for life, it is imperative that the soil contains desirable concentrations of these elements. Heavy metal exposure via different routes, including direct oral consumption, inhalation and skin contact, is linked to various human health risks. Therefore, in the current investigation, the levels of some metals (Ca, Mg, Mn, Fe, Cu, Zn, Cr, Ni, Pb and Cd) were analyzed in the soil samples taken from the flower farm and road side by FAAS. The findings of the current investigation indicated the soil samples accumulated higher amount of Fe among the determined metals. The contents of the analyzed metals in both soil samples are generally found to be Fe > Ca > Mn > Mg > Zn > Cr > Ni > Cu. The levels of Pb and Cd in the soil samples were not detectable. Except for Ca and Mg, the amounts of the investigated metals are found within the recommended limit set by WHO. At 95% confidence level, there is a significant difference between the contents of Ca and Cu in the two soil samples. The use of insecticides and fertilizers containing calcium and copper for the soil at the flower farm could be the cause of this significant difference. From human risk assessment study, EDI is the highest for Fe and the lowest for Cu. Furthermore, exposure via direct oral intake is the predominant exposure routes for all heavy metals. The estimated HQ via direct soil exposure follows the order: HQing > HQinh > HQdermal. For the investigated metals, both the HQ and the HI were found to be below 1⋅0. Furthermore, the risks of cancer resulting from heavy metal exposure are within acceptable limits. However, due to rapid development of the floriculture industry in Ethiopia more investigation is required to determine other elements in all the flower farms.
Data availability
The data used to support the findings of the study are included within the article and supplementary material.
References
Soffianian A, Madani ES, Arabi M. Risk assessment of heavy metal soil pollution through principal components analysis and false color composition in Hamadan Province. Iran Environ Syst Res. 2014;3:1–14.
Saygın F. Determination of heavy metal concentrations in cultivated soils and prediction of pollution risk ındices using the ANN approach. Rendiconti Lincei Scienze Fisiche e Naturali. 2024. https://doi.org/10.1007/s12210-024-01240-1.
Addis W, Abebaw A. Determination of heavy metal concentration in soils used for cultivation of Allium sativum L.(garlic) in East Gojjam Zone, Amhara Region Ethiopia. Cogent Chem. 2017;3:1419422.
Qi H, Zhao B, Li L, Chen X, An J, Liu X. Heavy metal contamination and ecological risk assessment of the agricultural soil in Shanxi Province. China R Soc Open Sci. 2020;7: 200538.
Latosińska J, Kowalik R, Gawdzik J. Risk assessment of soil contamination with heavy metals from municipal sewage sludge. Appl Sci. 2021;11:548.
Li W, Niu N, Guo N, Zhou H, Bu J, Ding A. Comparative Study on the determination of heavy metals in soil by XRF and ICP-MS. JPCS. 2021;2009: 012075.
Gebeyehu HR, Bayissa LD. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area. Ethiopia PloS one. 2020;15: e0227883.
Antunović V, Blagojević D, Baošić R, Relić D, Lolić A. Health risk assessment of heavy metals in soil, plant, and water samples near “Gacko” power plant, in Bosnia and Herzegovina. Environ Monit Assess. 2023;195:596.
Alemu WD, Bulta AL, Doda MB, Kanido CK. Levels of selected essential and non-essential metals in wheat (Triticum aestivum) flour in Ethiopia. J Nutr Sci. 2022;11: e72.
Gemechu Y. Determination of heavy metals in the soils of flower farms at Zeway, Sebeta and Holeta, Ethiopia. M. Sc Thesis. Addis Ababa University. 2020.
Yang B, Zhang C, Zhang X, Wang G, Li L, Geng H, Nie C. Survey of aflatoxin B1 and heavy metal contamination in peanut and peanut soil in China during. Food Control. 2020;118: 107372.
Chen L, Ding M, Li Z, Li X, Deng L. Determination of macro, micro and toxic element concentrations in peanuts from main peanut producing areas of China by ICP-MS: a pilot study on the geographical characterization. RSC Adv. 2022;12:16790–7.
Taghavi M, Bakhshi K, Zarei A, Hoseinzadeh E, Gholizadeh A. Soil pollution indices and health risk assessment of metal (loid) s in the agricultural soil of pistachio orchards. Sci Rep. 2024;14:8971.
Wan Y, Liu J, Zhuang Z, Wang Q, Li H. Heavy metals in agricultural soils: sources, influencing factors, and remediation strategies. Toxics. 2024;12:63.
Haghighizadeh A, Rajabi O, Nezarat A, Hajyani Z, Haghmohammadi M, Hedayatikhah S, Asl SD, Beni AA. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring techniques, and remediation strategies. Arab J Chem. 2024;17:105777.
Yang D, Zhu H, Liu J, Zhang Y, Wu S, **ong J, Wang F. Risk assessment of heavy metals in soils from four different industrial plants in a medium-sized city in North China. Toxics. 2023;11:217.
Ma M, Fang L, Zhao N, Ma X. Detection of cadmium and lead heavy metals in soil Samples by portable laser-induced breakdown spectroscopy. Chemosensors. 2024;12:40.
Shahbazi K, Beheshti M. Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Appl Sci. 2019;1:1–19.
Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Not. 2011;2011: 402647.
Fosu-Mensah BY, Ofori A, Ofosuhene M, Ofori-Attah E, Nunoo FE, Darko G, Appiah-Opong R. Assessment of heavy metal contamination and distribution in surface soils and plants along the west coast of Ghana. West Afr J Appl Ecol. 2018;26:167–78.
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 2021;9:42.
Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6: e04691.
Štofejová L, Fazekaš J, Fazekašová D. Analysis of heavy metal content in soil and plants in the dum** ground of magnesite mining factory Jelšava-Lubeník (Slovakia). Sustainability. 2021;13:4508.
Alomar T, Al-Anazia R, Hassania T, Allahema A, Al-Warhia M, Al-Dkheela N, Al-Mutairia R, Al-Mutairia S, Al-Masouda N. Determination of heavy metals concentration in soil and its impact on humans and environment. J Environ Anal Chem. 2022; 9.
Nassar AM, Ahmed AS, Adnan AAA, Sahira KY. Determination of heavy metal concentration and physicochemical parameters of the soil samples from Al-Fajr. Thi-Qar Gov IJHSSR. 2024;4:478–88.
Adekiya AO, Oloruntoba A, Ewulo BS, Ogunbode T, Sangoyomi T. Heavy metal pollution in soils near Ilesha Gold Mining Area. Nigeria Open Agric J. 2024;2024:18.
Anggraeni D, Oginawati K, Fahimah N, Salami IRS, Absari HR, Mukhaiyar U, Adiyani L. Analysis of heavy metals (Pb and Cd) in soil layers of Indonesia: Spatial distribution, potential source, and groundwater effect. CSCEE. 2024;9:100652.
Kinfu A, Berhanu M, Gobena B, Hailu B. Determination of physicochemical and heavy metals concentration of selected flower farms in central Ethiopia. Ecol Conserv Sci. 2021;1: 555569.
Mebrat S, Degwale A, Mekonen T, Mebrat A. Flower production prospects and sustainability challenges in Ethiopia: a systematic review. Front Environ Sci. 2022;10:1026544.
Hawera T, Tefera B, Sahu O. Flower farms environmental performance evaluation in Ethiopia. Environ Earth Sci. 2021;3:48–58.
Gobena B, Kinfu A, Berhanu M. Social and environmental concerns of flower farms in central ethiopia. IJAER. 2020;6:70–8.
Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Not. 2011;2011:1–20.
Dado TB, Kastro C, Fikire Y, Gudishe K, Bojago E. ICP_OES determination of essential, trace essential, and non-essential elements in the anatomical parts of medicinal herb (Pentas Schimperiana) grown in Wolaita Zone. Ethiopia J Agric Res. 2023;12: 100547.
Tamirat F, Adane WD, Tessema M, Tesfaye E, Tesfaye G. Determination of major and trace metals in date palm fruit (Phoenix dactylifera) samples using flame atomic absorption spectrometry and assessment of the associated public health risks. Int J Anal Chem. 2024;2024:9914300.
Panqing Y, Abliz A, **aoli S, Aisaiduli H. Human health-risk assessment of heavy metal–contaminated soil based on Monte Carlo simulation. Sci Rep. 2023;13:7033.
Antoniadis V, Golia EE, LiuYT WSL, Shaheen SM, Rinklebe J. Soil and maize contamination by trace elements and associated health risk assessment in the industrial area of Volos. Greece Environ Int. 2019;124:79–88.
Cao S, Duan X, Zhao X, Ma J, Dong T, Huang N, Wei F. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci Total Environ. 2014;472(100):1–9.
Jolly YN, Rakib MRJ, Sakib MS, Shahadat MA, Rahman A, Akter S, Idris AM. Impact of industrially affected soil on humans: a soil-human and soil-plant-human exposure assessment. Toxics. 2022;10:347.
Dubale W, Hussen A, Rao VM. Concentrations of selected metals in coffee (Coffea arabica) beans, leaves and supportive soil of gedeo zone. Ethiopia AJST. 2017;8:6526–33.
Mekassa B, Chandravanshi BS. Levels of selected essential and non-essential metals in seeds of korarima (Aframomum corrorima) cultivated in Ethiopia. Braz J Food Technol. 2015;18(2):102–11.
Wanjala MP, Odokuma L, Etela I, Ramkat R. Assessment of soil metals status in parts of rivers state. Nigeria JASEM. 2019;23:545–50.
Gransee A, Führs H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil. 2013;368:5–21.
Essandoh PK, Takase M, Bryant IM. Impact of small-scale mining activities on physicochemical properties of soils in dunkwa east municipality of Ghana. Sci World J. 2021;2021:9915117.
Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. JNB. 2004;15:710–6.
Demir F, Kipcak AS, Ozdemir OD, Derun EM. Determination of essential and non-essential element concentrations and health risk assessment of some commercial fruit juices in Turkey. JFST. 2020;57:4432–42.
Azevedo R, Oliveira AR, Almeida A, Gomes LR. Determination by ICP-MS of essential and toxic trace elements in gums and carrageenans used as food additives commercially Available in the portuguese market. Foods. 2023;12:1408.
Besarab A, Hemmerich S. Iron-deficiency anemia”, Management of Anemia: A Comprehensive Guide for Clinicians. New York, USA: Springer; 2018. p. 11–29.
Kacholi DS, Sahu M. Levels and health risk assessment of heavy metals in soil, water, and vegetables of Dar es Salaam. Tanzania J Chem. 2018;2018:1402674.
Gill M. Heavy metal stress in plants: a review. J Adv Res. 2014;2:1043–55.
Tefera M, Gebreyohannes F, Saraswathi M. Heavy metal analysis in the soils of in and around Robe town, Bale zone, South Eastern. Ethiopia Eurasian J Soil Sci. 2018;7:251–6.
Minase NA, Masafu MM, Geda AE, Wolde AT. Heavy metals in agricultural soils of Central Ethiopia: the contribution of land use types and organic sources to their variability. Open J Soil Sci. 2016;6:99.
Tibebe D, Hussen M, Mulugeta M, Yenealem D, Moges Z, Gedefaw M, Kassa Y. Assessment of selected heavy metals in honey samples using flame atomic absorption spectroscopy (FAAS). Ethiopia BMC chem. 2022;16:87.
Lewis S, Donkin ME, Depledge MH. Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat Toxicol. 2001;51:277–91.
Emamverdian A, Ding Y, Mokhberdoran F, **e Y. Heavy metal stress and some mechanisms of plant defense response. Sci World J. 2015;2015: 756120.
Kitao M, Lei TT, Koike T. Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiol Plant. 1997;101:249–56.
Rahman H, Sabreen S, Alam S, Kawai S. Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr. 2005;28:393–404.
Osobamiro TM, Awolesi O, Alabi OM, Oshinowo AY, Idris MA, Busari FA. Heavy metal levels of soil samples collected from a major industrial area in Abeokuta, Southwestern Nigeria. IJSR. 2019;9:92125.
Achmad RT, Auerkari EI. Effects of chromium on human body. ARRB. 2017;13:1–8.
Chiroma TM, Ebewele RO, Hymore FK. Comparative assessment of heavy metal levels in soil, vegetables and urban grey waste water used for irrigation in Yola and Kano. IRJES. 2014;2014(3):01–9.
Dado TB, Kastro C, Fikire Y, Gudishe K, Bojago E. ICP_OES determination of essential, trace essential, and non-essential elements in the anatomical parts of medicinal herb (Pentas Schimperiana) grown in Wolaita Zone. Ethiopia J Agric Food Res. 2023;12: 100547.
Tunegova M, Toman R, Tančin V. Heavy metals-environmental contaminants and their occurrence in different types of milK. SJAS. 2016;49:122–31.
Acknowledgements
The authors gratefully acknowledge Department of Chemistry, Addis Ababa University for providing laboratory facilities.
Funding
This research did not receive any financial support from any funding agency.
Author information
Authors and Affiliations
Contributions
GT, ED, NG and MT proposed the study. ED collected the sample and carried out the experimental work. ED and NG drafted the manuscript. GT and ED performed the statistical analysis. NG and MT supervised the study and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desalegn, E., Getachew, N., Tesfaye, G. et al. Determination of the levels of selected essential and toxic metals in soil collected from the flower farm in Roshara Roses PLC (Bishoftu) of Ethiopia and assessment of associated human health risks. Discov Appl Sci 6, 362 (2024). https://doi.org/10.1007/s42452-024-06028-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06028-4