Abstract
The large tree species Terminalia catappa is a member of the Combretaceae family and is mainly found in tropical climates. They are commonly cultivated for shade because they have huge, dense foliage. Numerous polyphenols, including flavonoids, tannins, saponins, and phytosterols, are present in the leaves. In this study, the green chemical method was used to extract polyphenols from dried green almond leaves. They were employed in the synthesis of Cu2O nanoparticles as a reducing agent. FTIR and UV–Vis were used to describe the leaf extract of Terminalia catappa after the chlorophyll was removed. Copper salt was used to create Cu2O nanoparticles via a reduction process. The extract's potential for photocatalytic dye degradation has also been explored. The obtained Cu2O had a spherical shape with dimensions of 50–100 nm, and its band gap energy reached 1.945 eV to remove methylene blue from aqueous media under visible light irradiation conditions. At an initial MB concentration of 10 ppm, the decomposition efficiency reached 71.99% after only 2 h of exposure to simulated sunlight. The decomposition process occurred according to a pseudo-first-order kinetic model with a rate constant of 0.0084 min−1.
Article Highlights
-
Ultrasound-assisted polyphenol extraction from Terminalia catappa leaves for green synthesis of Cu2O nanoparticles.
-
Cu2O nanoparticles, synthesized using these polyphenols, exhibit efficient photocatalytic activity.
-
Photodegradation of methylene blue under simulated sunlight achieved 71.99% efficiency in 2 h.
-
Green chemistry approach with natural extracts offers eco-friendly synthesis methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polyphenols are aromatic compounds containing hydroxyl groups directly attached to benzene rings, and they are found in many plants, such as vegetables, fruits [1,2,3], flowers [4, 5], and some parts of plants [6,7,8]. Depending on the number and interaction position of OH groups with the chemical framework, the physicochemical properties or biological activity change. Polyphenols play a vital role in plant life, such as creating characteristic colors and protecting plants from the harmful effects of insects, oxidation, and ultraviolet rays [9]. In medicine, polyphenols are natural compounds with many effects, such as potent antioxidant [10, 11], anti-inflammatory [12,13,14], antibacterial [15,16,17], anti-aging [18, 19], and anti-cancer [20,21,22,23]. More than 10,000 polyphenol compounds have been found in various plant species. Polyphenols may be classified into different groups based on the number of phenol rings they contain and the structural elements that bind these rings to one another. The main classes include phenolic acids, flavonoids, stilbenes, and lignans.
Research on the extraction, enrichment, and purification of biologically active natural compounds is essential in modern pharmaceuticals and medicine. Recently, scientists have expanded the application range of natural compounds in many fields, such as environmental treatment and control, oil additives, and metal anti-corrosion additives. Natural compounds can be extracted from various sources, including plants, animals, bacteria, and fungi. Scientists separate substances, enrich compounds, or purify useful active substances from plant sources to effectively make the most of available natural sources. Many extraction methods have been used to obtain polyphenols from different plant sources [3, 4, 12, 24]. The efficiency of the extraction process depends on many factors, such as the nature of the plant, the solvent, the ratio of solvent and raw material, temperature, extraction time, etc. In addition, additional steps must be taken to remove other compounds and impurities. Polyphenol extraction techniques are diverse and can be combined, including thermal extraction [25, 26], microwaves [26, 27], ultrasound [28, 29] with ionic liquid [30, 31], supercritical CO2 [32, 33], deep eutectic solvents [28, 34, 35], etc.
Copper(I) oxide is an inorganic compound with the formula Cu2O and one of the principal oxides of copper. Cu2O is very stable with heat, insoluble in water, slowly soluble in concentrated alkali or ammoniac, and well soluble in acidic solutions. Depending on the particle size, cuprous oxide can have colors ranging from yellow to orange to red. In addition, particle size also affects the photochemical properties of Cu2O. Cu2O has a band gap energy of Eg = 2.14 eV at the micrometer scale. However, as the particle size decreases to the nanometer region, the size effect changes the band gap energy. Along with the development of nanotechnology, Cu2O nanoparticles are applied in many different fields, such as catalysis [36,37,38], sensors [39,40,41], semiconductors [42, 43], solar cells [44,45,46], etc. Methods Diverse methods of synthesizing Cu2O nanoparticle include pyrolysis [47, 48], hydrothermal [37, 49], thermal oxidation [50,51,52], electrochemical [53, 54], etc. In addition, the chemical reduction method with reducing agents is used to manufacture Cu2O nanoparticle [55,56,57,58].
Over the past 10 years, nanotechnology has been regarded as one of science's most important frontiers. Numerous industrial and technological sectors, including information technology, homeland security, healthcare, energy, transportation, food safety, and environmental research, are significantly improved by nanotechnology. Its many uses and rapidly increasing market demand have opened the door for creative approaches to producing higher-quality nanomaterials. Many research projects related to the synthesis, characterization, and applications of nanoparticles have been published [59,60,61,62,63,64]. Initially, nanomaterials were synthesized using conventional synthesis techniques that required high-energy input and carcinogenic chemicals. There is a demand for environmentally safer synthesis techniques because of the pollution that traditional synthesis methods produce. Natural agents are used in green nanomaterial synthesis methods to produce nanomaterials [65]. Green synthesis uses low-energy techniques and naturally available starting materials to produce nanomaterials with the same effectiveness as classic synthesis but more sustainably. Therefore, including green synthesis offers a possible way around the drawbacks of conventional synthesis techniques. Recently, scientists have been interested in using natural compounds as reactants in chemical reactions in green chemistry processes. For example, Cu2O-based nanocomposites have been synthesized using Commelina benghalensis leaf extract and oolong tea leaf extract [66,67,68]. These are some of the many raw material sources for extracting natural polyphenols as reducing agents in synthesizing nanomaterials [69,70,71]. To date, plant species containing many polyphenols have been exploited. However, the extraction of polyphenols from Terminalia catappa leaves as a synthetic agent for Cu2O nanoparticles has yet to be evaluated. In this study, polyphenols from Terminalia catappa leaf extract are considered green reducing agents to synthesize Cu2O nanoparticles for the photocatalytic decomposition of toxic organic compounds in water.
2 Experiments and methods
2.1 Chemical
Ethanol (C2H5OH, 95%), Chloroform (CHCl3, 98%), Sodium carbonate (Na2CO3, 99.5%), Copper (II) sulfate pentahydrate (CuSO4·5H2O, 98.0%), Potassium sodium tartrate tetrahydrate (KNaC4H4O6·4H2O, 99.0%), Ethylene Glycol (C2H6O2, 99.5%) and Sodium chloride (NaCl, 99.5%) were purchased from Macklin Company (China). Sodium hydroxide (NaOH, 97.0%), Gallic acid (C7H6O5, 98.5%) and Folin-Ciocalteu’s phenol reagent (2N) were purchased from Sigma-Aldrich (USA).
2.2 Preparation of the Terminalia catappa extract
The Terminalia catappa shoots were harvested in the summer. Leaves were cut to about 1 cm, dried under dry air for 72 h, and ground to 100 μm. Disperse the dry leaf powder in a water/ethanol mixture (v/v: 1/1) at a ratio of 50 g/L under ultrasound for 30 min. The suspension was heated at 60 °C for 2 h and filtered to remove the residue. Chloroform is used to remove chlorophyll in the extract. The infrared spectrum of polyphenols extracted from Terminalia catappa in the range of 4000–400 cm−1 demonstrates the presence of characteristic functional groups. Polyphenol content was determined using the Folin-Ciocalteu method by UV–Vis spectroscopy at 713 nm. The equation for determining polyphenol concentration is shown as follows: C = 25,302 * Abs − 0.3903 (R2 = 0.9916).
2.3 Synthesis of Cu2O nanoparticles
100 ml of the solution mixture containing 7.055 g KNaC4H4O6·4H2O and 2 g NaOH was slowly added (5 ml per minute) into 50 ml of 0.5 M CuSO4 solution under circular stirring conditions. Then, 50 ml of ethylene glycol was added to the mixture and stirred for 10 min. 50 ml of purified extract is gradually dropped into the stirring suspension, raising the temperature to 60 °C. After 60 min, the precipitate was collected and washed with distilled water three times (250 ml/time). The purified Cu2O nanoparticle was dried in Ar gas at 80 °C for 24 h.
2.4 Characterization of Cu2O nanoparticles
The material's morphology was observed using scanning electron microscopy (SEM) techniques with magnification capabilities up to 100,000 times. The crystal structure of the Cu2O nanoparticles was analyzed through an X-ray diffraction (XRD). At 1.54 Å in a copper X-ray tube (Cu-Kα), the device is operated at 44 mA and 40 kV in the range (2θ) from 10° to 80°. The optical absorption characteristics of the Cu2O nanoparticle were determined by ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis DRS) in the range of 200–800 nm.
2.5 Removing methylene blue with Cu2O photocatalyst
The photocatalytic activity of the prepared Cu2O nanoparticle: In each typical experiment, 25 mg Cu2O nanoparticle was exposed to 50 mL of 10 ppm MB solution in a transparent glass tube. The adsorption process took place in the dark after 30 min. Then, the glass tubes are put into the lighting cabinet, using the light source as a Xenon lamp under air circulation conditions. The MB solution was removed from the mixture after a predetermined amount of time, and the concentration was measured using UV–Vis photometry on UV–Vis DV-8200 equipment (Drawell). The equation to determine MB concentration was built as follows: C = 6.3746 * Abs − 0.2229 (R2 = 0.9938) where C is the concentration of MB solution, ppm; Abs is the light absorption intensity at λ = 662 nm.
The degradation efficiency of MB in the presence of a catalyst is calculated according to the formula:
The MB removal data by photocatalysis were fed into a pseudo-first-order kinetic model for kinetic analysis:
where Co and Ct are concentrations of MB (ppm) initially and at time t, and k1 is the constant reaction rate.
3 Results and discussion
3.1 Characterization of the Terminalia catappa extract
The polyphenols isolated from Terminalia catappa are shown in Fig. 1a, illustrating the existence of distinctive functional groups. A prominent obtuse peak is detected at 3219 cm−1 in the wavenumber range. The symmetric and asymmetric relaxation vibrations of the polymer hydroxyl group (O–H), or H-bond relaxation, are represented by the range 3400–3200 cm−1 and are typical of polyphenolic compounds [72]. A vibration that may be detected at 1713 cm−1 suggests that the six-carbon aromatic ring has a relationship with the –C=O group [73]. Further evidence for the presence of the –CH– group of methylene on aromatic rings stretching comes from deformation fluctuations at wave numbers 1440 cm−1 in the regions 1430–1470 cm−1, which is attributed to C–H linkage [74]. The area spanning from 1000 to 1100 cm−1 is commonly called the fingerprint zone due to the abundance of distinctive low-intensity single bands linked to particular functional groups within this range. A vibration at 1052 cm−1 is associated with the –C–O–C– stretching [75]. Ultimately, the phenyl radical's C–H bond can be attributed to the wavenumber at 745 cm−1 [76].
The UV–Vis photometric spectrum of Terminalia catappa leaf extract is shown in Fig. 1b. On the spectrum, two adjacent peaks at 220 nm and 259 nm are believed to be characteristic of polyphenols in Terminalia catappa leaves. In addition, a lower peak at 379 nm was also observed. Polyphenol content in Terminalia catappa extract was calculated through the equation to determine polyphenol concentration by UV–Vis photometric method. The result of polyphenol concentration in Terminalia catappa extract was 24,291 mg/L.
3.2 Characterization of Cu2O nanoparticle
Post-visual inspection and UV–Vis spectral analysis were utilized to confirm the synthesis of Cu2O nanoparticles. The creation of Cu2O nanoparticles is tentatively confirmed by the constant color shift to brick red after heating the Terminalia catappa leaf extract and copper sulfate solution. It also shows that during the manufacturing process, the phenolic compounds change the valency of copper from 2 to 1. Figure 2 shows the spectra of the leaf extract from Terminalia catappa and the Cu2O nanoparticles between 190 and 590 nm. The extract's inherent phenolic moieties underwent an electronic transition, either from π to π* or from n to π*, as shown by the absorption double peaks seen in both spectra at 234 nm/264 nm and 256/283 nm. The formation of Cu2O nanoparticles was recorded when a broad peak appeared at 414 nm. At the same time, the narrow peak at 370 nm in the spectrum of Terminalia catappa leaf extract disappeared. This confirms that the production of Cu2O nanoparticles is aided by reducing agents found in Terminalia catappa leaf extract. Monitoring the phenomenon during the formation of Cu2O is recorded in Fig. 2. The hypothesis about the formation process of Cu2O is explained [77, 78]. The predicted mechanism for the formation of Cu2O nanoparticles can be given as follows:
The particle size of Cu2O nanoparticles depends mainly on the synthesis mode, including initial concentration, surfactant content, stirring speed, etc. Meanwhile, the material particle shape depends on the above factors and the selected reducing agent. The SEM image of Cu2O nanoparticle obtained by the reduction method with Terminalia catappa leaf extract is shown in Fig. 3. The morphology of the created Cu2O at 60 °C has a uniform round sphere shape. Particle size distribution ranges from 50 to 150 nm. Cu2O nanoparticles synthesized at 40 °C have a uniform morphology, but their size ranges from a few dozen nanometers to more than 150 nm. Meanwhile, Cu2O nanoparticles obtained at higher temperatures (80 and 95 °C) tended to be heterogeneous in morphology and size. The sample synthesized at 95 °C also shows cubic and obtuse cubic crystals.
Most studies have used ethylene glycol as a stabilizer in the synthesis of nanoparticles. Some other studies use EG as a size control agent for nanoparticles [79,80,81,82]. The results of investigating the influence of EG on the state of Cu2O nanoparticles are shown on the SEM image in Fig. 4. For the sample without EG, the obtained Cu2O nanoparticles were uneven; the nanoparticle surface was rough and tended to mechanical instability. The presence of EG in the reaction mixture helps the formed Cu2O nanoparticles have a uniform size and smooth surface, which can create higher mechanical strength.
The Cu2O’s crystal structure was observed by examining the XRD pattern (Fig. 5a). The 2θ diffraction peaks at 29.6°, 36.5°, 42.4°, 61.5°, 73.7°, and 77.6°, respectively, are in excellent accord with the standard cards (JCPDS file no. 05-0667) of Cu2O [37, 47]. These correspond to the [110], [111], [200], [220], [311] and [222] crystal planes of Cu2O [37, 50, 53]. The absence of Cu, CuO, and Cu(OH)2 diffraction peaks suggests that the produced Cu2O nanoparticle are in their pure crystal phase. Furthermore, the sharp crystallinity of the products was demonstrated. The Cu2O nanoparticle’s chemical composition was determined using the energy-dispersive X-ray method. The results of determining the elemental content shown on the EDX spectrum (Fig. 5b) show the existence of two elements, Cu and O, with respective masses of 80.31% and 9.67% (elemental ratio is approximately 2:1), similar to the phase composition in the XRD data.
The optical properties of the material were also evaluated through the UV–Vis DRS spectrum. The results presented in Fig. 6 show that the band gap energy Eg of Cu2O nanoparticle, calculated according to the formula of Tauc, is 1.945 eV, respectively, with the highest adsorption intensity at wavelength 477 nm. The results on the band gap energy of Cu2O nanoparticle obtained are equivalent to previous publications [83,84,85]. Cu is a transition metal element. Although the outermost electron is distributed in the 4s1 layer, it belongs to the d subshell, so this single electron can easily change its energy level to achieve photocatalytic activity and is well represented in the visible region.
3.3 Removing methylene blue with nano Cu2O photocatalyst
The photocatalytic ability of Cu2O nanoparticle to oxidize dye (methylene blue) was evaluated under simulated sunlight conditions. Experimental conditions were set up, including an initial MB concentration of 10 ppm, catalyst content of 0.5 g/L, and Xenon lamp power of 300 W.
The results showed that MB decomposed quickly after 10 min of irradiation and the photodegradation efficiency reached ~ 72% after 120 min (Fig. 7a). It can be said that the photocatalytic efficiency of MB decomposition of biologically synthesized Cu2O nanoparticles using Terminalia catappa leaf extract is comparable to researched and published works as shown in Table 1.
Theoretically, adsorbed O2 and focused electrons on the Cu2O surface may react to form ·O2– under simulated sunlight. Following that, hydrogen peroxide (H2O2) and hydroxyl radical (·OH) are produced when ·O2– reacts with H2O. This explains why Cu2O nanoparticle has a high activity in breaking down MB. O2– and OH are the main reaction types of MB degradation, and the following reactions illustrate the photocatalytic process of MB by Cu2O nanoparticle:
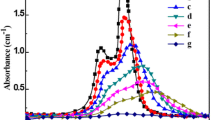
Methylene blue (MB) degradation was investigated for up to 4 h under visible light irradiation to determine the photocatalytic activity of Cu2O nanoparticle. The MB decomposition efficiency of Cu2O nanoparticle after 10, 30, and 120 min was 26.82, 44.31, and 71.99%, respectively, as shown in the graph in Fig. 7b. As illustrated in Fig. 8, the photocatalytic degradation data were computed using a pseudo-first-order reaction kinetic model. The MB decomposition rate constant (k1) of Cu2O nanoparticle is 0.0084 min−1.
4 Conclusions
To sum up, polyphenols from Terminalia catappa leaves were extracted using a green chemical method. With the use of indirect ultrasound and heat treatment at 60 °C, the ethanol/water solvent mixture was extracted. In Terminalia catappa leaf extract, the polyphenol level exceeded 24 g/L, or 48% by weight. The reduction technique was utilized to produce copper(I) oxide nanoparticles using polyphenol from Terminalia catappa leaf extract. The generated Cu2O nanoparticle was appropriately characterized using physicochemical techniques. The obtained Cu2O nanoparticle showed a spherical shape morphology with particle sizes between 50 and 150 nm. In the visible spectrum, the produced Cu2O nanoparticle exhibits strong photocatalytic activity. In 2 h, the Cu2O nanoparticle's MB breakdown efficiency at a concentration of 10 ppm in simulated sunlight was 71.99%. The earth's environment is affected when natural components are used as chemical agents in the synthesis of products. Thus, using Terminalia catappa leaf extract as a reducing agent shows promise for a green approach in real-world applications.
Data availability
All data analysed during this study included in this article are available from the corresponding author on reasonable request.
References
He X, Chen L, Pu Y, Wang H, Cao J, Jiang W. Fruit and vegetable polyphenols as natural bioactive inhibitors of pancreatic lipase and cholesterol esterase: inhibition mechanisms, polyphenol influences, application challenges. Food Biosci. 2023;55: 103054.
Chen L, Pu Y, Xu Y, He X, Cao J, Ma Y, Jiang W. Anti-diabetic and anti-obesity: efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res Int. 2022;157: 111202.
Xu K, Cui X, Ren X, Meng J, Fu X, **a Q. Discovery of natural polyphenols from the wild vegetable Suaeda salsa L. with potential cardioprotective functions. Food Chem. 2023;405(Pt B): 134968.
Liu J-Z, Wen L-L, Tian X-L, Fu Y-J, Cui Q. An efficient two-step approach for the preparative separation and purification of eight polyphenols from Hibiscus manihot L. flower with high-speed countercurrent chromatography. Arab J Chem. 2023;16(6):104791.
Sanjaya SS, Park MH, Karunarathne W, Lee KT, Choi YH, Kang CH, Lee MH, Jung MJ, Ryu HW, Kim GY. Inhibition of alpha-melanocyte-stimulating hormone-induced melanogenesis and molecular mechanisms by polyphenol-enriched fraction of Tagetes erecta L. flower. Phytomedicine. 2024;126:155442.
Soendjaja V, Girard AL. Effects of plant polyphenols on lipid oxidation in pea and soy protein solutions. Food Chem. 2024;433: 137340.
Niu C, Dong M, Niu Y. Lead toxicity and potential therapeutic effect of plant-derived polyphenols. Phytomedicine. 2023;114: 154789.
Wen Y, Wang J, Wang F, Wu H, Zhou J, Dai Z, Guo H. Recent advances in membranes modified with plant polyphenols in wastewater treatment: a review. Sep Purif Technol. 2024;334:125861.
Tuladhar P, Sasidharan S, Saudagar P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In: Biocontrol agents and secondary metabolites. p. 419–441; 2021.
Abd El-Hack ME, de Oliveira MC, Attia YA, Kamal M, Almohmadi NH, Youssef IM, Khalifa NE, Moustafa M, Al-Shehri M, Taha AE. The efficacy of polyphenols as an antioxidant agent: an updated review. Int J Biol Macromol. 2023;250: 126525.
Lang Y, Gao N, Zang Z, Meng X, Lin Y, Yang S, Yang Y, ** Z, Li B. Classification and antioxidant assays of polyphenols: a review. J Future Foods. 2024;4(3):193–204.
Sun Y, Qi Z, Xu Y, Li C, Zhao J, Liu T. Anti-inflammatory, analgesic, antitussive and antipyretic activities of polyphenol-enriched fraction from Nymphaea candida. J Ethnopharmacol. 2024;324: 117789.
Khatun S, Kim T, Mollah MMI. Heat shock increases the anti-inflammatory and anti-obesity activity of soybean by increasing polyphenol, antioxidant and aglycon form isoflavones. Heliyon. 2023;9(11): e21944.
Narayanankutty A, Kunnath K, Famurewa AC, Ramesh V, Rajagopal R, Alfarhan A. Variations in the composition, cytoprotective and anti-inflammatory effects of natural polyphenols of edible oils extracted from fresh and dried coconut testa. Physiol Mol Plant Pathol. 2022;117:101742.
Lin Y, Wang R, Li X, Addo KA, Fang M, Zhang Y, Yu Y. Antibacterial mechanism of kojic acid and tea polyphenols against Escherichia coli O157:H7 through transcriptomic analysis. Food Sci Human Wellness. 2024;13(2):736–47.
Liu C, Dong S, Wang X, Xu H, Liu C, Yang X, Wu S, Jiang X, Kan M, Xu C. Research progress of polyphenols in nanoformulations for antibacterial application. Mater Today Bio. 2023;21: 100729.
Bilal K, Mehboob F, Akhtar N, Mirza IA, Okla MK, Dar MJ, Saleh IA, Zomot N, Fatima H. Wound healing, antioxidant and antibacterial activities of polyphenols of Psidium guajava L. leaves. S Afr J Bot. 2024;165:538–51.
Maleki M, Khelghati N, Alemi F, Bazdar M, Asemi Z, Majidinia M, Sadeghpoor A, Mahmoodpoor A, Jadidi-Niaragh F, Targhazeh N, Yousefi B. Stabilization of telomere by the antioxidant property of polyphenols: anti-aging potential. Life Sci. 2020;259: 118341.
Bucciantini M, Leri M, Scuto M, Ontario M, Trovato Salinaro A, Calabrese EJ, Calabrese V, Stefani M. Xenohormesis underlyes the anti-aging and healthy properties of olive polyphenols. Mech Ageing Dev. 2022;202: 111620.
Khan H, Reale M, Ullah H, Sureda A, Tejada S, Wang Y, Zhang ZJ, **ao J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: updates and future directions. Biotechnol Adv. 2020;38: 107385.
Farghadani R, Naidu R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed Pharmacother. 2023;165: 115170.
Zeb F, Naqeeb H, Osaili T, Faris ME, Ismail LC, Obaid RS, Naja F, Radwan H, Hasan H, Hashim M, AlBlooshi S, Alam I. Molecular crosstalk between polyphenols and gut microbiota in cancer prevention. Nutr Res. 2024;124:21–42.
Liu X, Cui S, Li W, **e H, Shi L. Elucidation of the anti-colon cancer mechanism of Phellinus baumii polyphenol by an integrative approach of network pharmacology and experimental verification. Int J Biol Macromol. 2023;253(Pt 6): 127429.
Qi C, Liu G, ** Y, Yang K, Tan Q, Zhang Y, Chen G, Huang X, Xu D. A comprehensive review of nano-delivery system for tea polyphenols: construction, applications, and challenges. Food Chem X. 2023;17: 100571.
Martínez-Olivo AO, Carlos-Murillo MU, Sáyago-Ayerdi SG, Sánchez-Burgos JA, Zamora-Gasga VM. Optimization of ultrasonic extraction for enhanced polyphenol profile and antioxidant capacity in mango seeds: a comparative study with thermal extraction. Food Chem Adv. 2023;3:100480.
**aokang W, Lyng JG, Brunton NP, Cody L, Jacquier JC, Harrison SM, Papoutsis K. Monitoring the effect of different microwave extraction parameters on the recovery of polyphenols from shiitake mushrooms: comparison with hot-water and organic-solvent extractions. Biotechnol Rep (Amst). 2020;27: e00504.
Jovanović AA, Vajić U-JV, Mi** DZ, Zdunić GM, Šavikin KP, Branković S, Kitić D, Bugarski BM. Polyphenol extraction in microwave reactor using by-product of Thymus serpyllum L. and biological potential of the extract. J Appl Res Med Aromat Plants. 2022;31:100417.
Zhang XJ, Liu ZT, Chen XQ, Zhang TT, Zhang Y. Deep eutectic solvent combined with ultrasound technology: a promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023;422: 136224.
Santos LG, Martins VG. Optimization of the green extraction of polyphenols from the edible flower Clitoria ternatea by high-power ultrasound: a comparative study with conventional extraction techniques. J Appl Res Med Aromat Plants. 2023;34:100458.
Ettoumi FE, Zhang R, Belwal T, Javed M, Xu Y, Li L, Weide L, Luo Z. Generation and characterization of nanobubbles in ionic liquid for a green extraction of polyphenols from Carya cathayensis Sarg. Food Chem. 2022;369: 130932.
Barrulas RV, Paiva TG, Corvo MC. NMR methodology for a rational selection of ionic liquids: extracting polyphenols. Sep Purif Technol. 2019;221:29–37.
Fernández-Acosta K, Salmeron I, Chavez-Flores D, Perez-Reyes I, Ramos V, Ngadi M, Kwofie EM, Perez-Vega S. Evaluation of different variables on the supercritical CO2 extraction of oat (Avena sativa L.) oil; main fatty acids, polyphenols, and antioxidant content. J Cereal Sci. 2019;88:118–24.
Kuś PM, Jerković I, Aladić K, Jokić S. Supercritical CO2 and ultrasound extraction and characterization of lipids and polyphenols from Phacelia tanacetifolia Benth. pollen. Ind Crops Products. 2023;206:117.
Aktas H, Kurek MA. Deep eutectic solvents for the extraction of polyphenols from food plants. Food Chem. 2024;444: 138629.
Wang W, Pan Y, Zhao J, Wang Y, Yao Q, Li S. Development and optimization of green extraction of polyphenols in Michelia alba using natural deep eutectic solvents (NADES) and evaluation of bioactivity. Sustain Chem Pharm. 2024;37:101425.
Nguyen Thi L, Nguyen Thi Tuyet M, Dang Thi Minh H, Tran Thi Thu H, Nguyen Kim N, Trinh Xuan A, Ta Ngoc D, Huynh Dang C, Nguyen Cong T, Luu Thi Lan A. Synthesis of Cu2O and Cu2O-C3N4 nanomaterials for the photodegradation of organic dyes under visible light irradiation. Vietnam J Catal Adsorpt. 2021;10(3):65–70.
Chen X, Cui K, Hai Z, Kuang W, Wang L, Zhang J, Tian X. Hydrothermal synthesis of Cu2O with morphology evolution and its effect on visible-light photocatalysis. Mater Lett. 2021;297:129921.
Wang J, Fu T, Meng F, Zhao D, Chuang SSC, Li Z. Highly active catalysis of methanol oxidative carbonylation over nano Cu2O supported on micropore-rich mesoporous carbon. Appl Catal B Environ. 2022;303:120890.
Li N, Hu J, Li J, Cheng M, Wei T, Liu Q, Wang R, Li W, Ling Y, Zhang Y, Liu B. Cu-MOF-74-derived Cu2O/CuO-C nanocomposite as an effective chemiresistive sensor for detection of NO2 at room temperature. J Alloys Compd. 2024;976:173074.
Wang N, Tao W, Gong X, Zhao L, Wang T, Zhao L, Liu F, Liu X, Sun P, Lu G. Highly sensitive and selective NO2 gas sensor fabricated from Cu2O-CuO microflowers. Sens Actuators B Chem. 2022;362:131803.
Fujita Y, Yamasaki A, Nitta R, Kubota Y, Katayanagi Y, Matsushita N. Cu2O films without impurities fabricated via the mist spin spray technique using a mixed alkaline solution for flexible glucose sensors. Thin Solid Films. 2023;787:140123.
Ma J, Zhong W, You L, Pei Y, Lu C, **ao Z, Shen Z, Jiang X, Qian N, Liu X, Zhang S. Band bending caused by forming heterojunctions in Cu-Cu2O/rGO-NH2 semiconductor materials and surface coordination of N-methylimidazole, and the intrinsic nature of synergistic effect on the catalysis of selective aerobic oxidation of alcohols. Appl Surf Scince. 2022;605:154563.
Khakzad A, Ebrahimian Pirbazari A, Esmaeili Khalil Saraei F, Aroon MA. Combination of Cu2O semiconductor with reduced graphene oxide nanocomposites for boosting photocatalytic performance in degradation of organic pollutant. Phys B Condens Matter. 2021;603:412736.
Lakshmanan A, Alex ZC, Meher SR. Cu2O thin films grown by magnetron sputtering as solar cell absorber layers. Mater Sci Semicond Process. 2022;148:106818.
Sun B, Chen H, Yan K, Feng X-D. Numerical investigation of the Cu2O solar cell with double electron transport layers and a hole transport layer. Opt Mater. 2022;131:112642.
Ivashchenko MM, Diachenko OV, Opanasyuk AS, Buryk IP, Kuzmin DV, Čerškus A, Shapovalov O, Plotnikov SV, Gryshko IA. A numerical simulation of solar cells based on the CuO and Cu2O absorber layers with ZnMgO window layer. Mater Sci Eng B. 2024;300:117.
Ugalde-Reygadas M, Moreno-Regino VD, Torres-Castanedo CG, Bedzyk MJ, Castanedo-Pérez R, Torres-Delgado G. Cu2O thin films deposited by spray pyrolysis using diethanolamine and L-ascorbic acid as reducing agents. Mater Today Commun. 2022;32:103999.
Yang Q, Ma X, Li Y, Jiang L, Chen D, He J, Wang J. One-pot pyrolysis and enhanced efficient solar evaporation of Cu/Cu2O/biochar. Mater Today Sustain. 2023;22:100363.
Liu K, Song Q, **e H, Ning Z. Influence of pH on hydrothermal synthesis of photoactive Cu2O films in an acetate solution. Int J Electrochem Sci. 2022;17(6):220660.
Cheon M, Jung B, Kim SJ, Jang JI, Jeong SY. High-quality epitaxial Cu2O films with (111)-terminated plateau grains obtained from single-crystal Cu (111) thin films by rapid thermal oxidation. J Alloy Compd. 2019;801:536–41.
Behjati S, Sheibani S, Herritsch J, Gottfried JM. Photodegradation of dyes in batch and continuous reactors by Cu2O-CuO nano-photocatalyst on Cu foils prepared by chemical-thermal oxidation. Mater Res Bull. 2020;130:110920.
Guo D, Ju Y. Preparation of Cu2O/ZnO p-n junction by thermal oxidation method for solar cell application. Mater Today Proc. 2016;3(2):350–3.
Çetinel A. Characterization of octahedral Cu2O nanostructures grown on porous silicon by electrochemical deposition. Mater Chem Phys. 2022;277:125532.
Syrek K, Jażdżewska M, Kozieł M, Zaraska L. Photoelectrochemical activity of Cu2O electrochemically deposited at different temperatures. J Ind Eng Chem. 2022;115:561–9.
Madhuri Peddada L, Phyu Cho P, Dulgaj S, Annapragada R, Raja Kanuparthy P. Facile synthesis of green engineered CuO/Cu2O-C nano heterostructures with the controlled Cu2O content for the photodegradation of crystal violet. Results Opt. 2023;13:100537.
Wang Q, Dong Y, Huang H, Du G, Zhao P, **e S. Facile synthesis of porous Cu2O hollow nanospheres for accelerating electroreduction of CO2 towards C2 products. Mater Lett. 2023;351:135045.
Alp E. The facile synthesis of Cu2O-Cu hybrid cubes as efficient visible-light-driven photocatalysts for water remediation processes. Powder Technol. 2021;394:1111–20.
Mallik M, Monia S, Gupta M, Ghosh A, Toppo MP, Roy H. Synthesis and characterization of Cu2O nanoparticles. J Alloys Compd. 2020;829:154623.
Juturu R, Murty VR, Selvaraj R. Efficient adsorption of Cr (VI) onto hematite nanoparticles: ANN, ANFIS modelling, isotherm, kinetic, thermodynamic studies and mechanistic insights. Chemosphere. 2024;349:140731.
Zulfiqar Z, Khan RRM, Summer M, Saeed Z, Pervaiz M, Rasheed S, Shehzad B, Kabir F, Ishaq S. Plant-mediated green synthesis of silver nanoparticles: synthesis, characterization, biological applications, and toxicological considerations: a review. Biocatal Agric Biotechnol. 2024;57:103.
Raut S, Bhatavadekar A, Chougule R, Lekhak U. Silver nanoparticles synthesis from Crinum moorei: optimization, characterization, kinetics and catalytic application. S Afr J Bot. 2024;165:494–504.
Vinayagam R, Nagendran V, Goveas LC, Narasimhan MK, Varadavenkatesan T, Chandrasekar N, Selvaraj R. Structural characterization of marine macroalgae derived silver nanoparticles and their colorimetric sensing of hydrogen peroxide. Mater Chem Phys. 2024;313:128787.
Şengönül H, Demircan O. Utilization of Prunus serrulata leaf extract for the synthesis and characterization of ZnO nanoparticles. Nano-Struct Nano-Objects. 2024;37:101.
Vodyashkin A, Stoinova A, Kezimana P. Promising biomedical systems based on copper nanoparticles: synthesis, characterization, and applications. Colloids Surf B Biointerfaces. 2024;237:113861.
Nagaraj B, Divya T, Krishnamurthy N, Dinesh R, Negrila C, Predoi D. Phytosynthesis of gold nanoparticles using Caesalpinia pulcherrima (peacock flower) flower extract and evaluation of their antimicrobial activities. Digest J Nanomater Biostruct. 2012;7(3).
Kumar R, Kumar K, Thakur N. Biosynthesis of CuO/Cu2O-ZnO nanocomposites via Commelina benghalensis leaf extract and their antibacterial, photocatalytic and antioxidant assessment. Inorg Chem Commun. 2023;157:111400.
Dou L, Zhang X, Zangeneh MM, Zhang Y. Efficient biogenesis of Cu(2)O nanoparticles using extract of Camellia sinensis leaf: evaluation of catalytic, cytotoxicity, antioxidant, and anti-human ovarian cancer properties. Bioorg Chem. 2021;106: 104468.
Karakuş EE, Sert E, Erol A, Kalındemirtaş FD, Özbaş F, Karakuş S. Enhancement of cytotoxic and apoptotic activity through oolong tea extract-mediated CuO/Cu2O nanoparticles. J Drug Deliv Sci Technol. 2024.
Sneha N, Chandrayan VR, Srinivas M. Exploring bimetallic Au–Ag core shell nanoparticles reduced using leaf extract of Ocimum tenuiflorum as a potential antibacterial and nanocatalytic agent. Chem Pap. 2022;76:6487–97.
Nayak S, Goveas LC, Selvaraj R, Mutalik S, Sajankila SP. Use of Cyclea peltata mediated gold nanospheres for adsorptive degradation of methyl green dye. Bioresour Technol Rep. 2022;20:101.
Bhavana P, Priyanka H, Jagadeesh AK, Ramakakanavar CG, Sneha N, Rao CV. Biosynthesis of copper nanoparticles from areca nut extract and its antibacterial and antioxidant properties. Agric Nat Resour. 2019;53:386–94.
Le PH, Ho LTT, Le DHT, Nguyen V. Purification of coffee polyphenols extracted from coffee pulps (Coffee arabica L.) using aqueous two-phase system. Molecules. 2023;28(15):5922.
Navarro-Pardo F, Martinez-Barrera G, Martinez-Hernandez AL, Castano VM, Rivera-Armenta JL, Medellin-Rodriguez F, Velasco-Santos C. Effects on the thermo-mechanical and crystallinity properties of nylon 6,6 electrospun fibres reinforced with one dimensional (1D) and two dimensional (2D) carbon. Materials (Basel). 2013;6(8):3494–513.
Kirakosyan A, Lee D, Choi Y, Jung N, Choi J. Poly(styrene sulfonic acid)-grafted carbon black synthesized by surface-initiated atom transfer radical polymerization. Molecules. 2023;28(10):4168.
Muthulakshmi L, Prabakaran S, Ramalingam V, Rajulu AV, Rajan M, Ramakrishna S, Luo H. Sodium alginate nanofibers loaded Terminalia catappa scaffold regulates intrinsic apoptosis signaling in skin melanoma cancer. Process Biochem. 2022;118:92–102.
Wang X, He X, Wang X. FTIR analysis of the functional group composition of coal tar residue extracts and extractive residues. Appl Sci. 2023;13(8):5162.
Luo F, Wu D, Gao L, Lian S, Wang E, Kang Z, Lan Y, Xu L. Shape-controlled synthesis of Cu2O nanocrystals assisted by Triton X-100. J Cryst Growth. 2005;285(4):534–40.
<Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity.pdf>.
Chen Y, Liew KY, Li J. Size-controlled synthesis of Ru nanoparticles by ethylene glycol reduction. Mater Lett. 2008;62(6–7):1018–21.
Jacob JA, Kapoor S, Biswas N, Mukherjee T. Size tunable synthesis of silver nanoparticles in water–ethylene glycol mixtures. Colloids Surf A. 2007;301(1–3):329–34.
Vaka M, Walvekar R. An experimental study on thermal efficiency of hybrid GO/MWCNTs nanoparticles suspended in a binary mixture of ethylene glycol and water. Mater Today Proc. 2023;73:354–60.
Wang H, Li T, Li J, Tong W, Gao C. One-pot synthesis of poly(ethylene glycol) modified zeolitic imidazolate framework-8 nanoparticles: size control, surface modification, and drug encapsulation. Colloids Surf A. 2019;568:224–30.
Oudah MH, Hasan MH, Abd AN. Synthesis of copper oxide thin films by electrolysis method based on porous silicon for solar cell applications. IOP Conf Ser Mater Sci Eng. 2020;757(1):012051.
Güneri E, Göde F, Arı M, Saatçi B. The effect of Cu2O layer on characteristic properties of n-CdS/p-Cu2O heterojunction. J Mol Struct. 2021;1241:130679.
Zhang M, He X, Xue Y, Lin Z, Tong N-H, Lai W, Liang S. Improving thermoelectric properties of Cu2O powder via interface modification. Solid State Commun. 2022;357:114982.
Mrunal VK, Vishnu AK, Momin N, Manjanna J. Cu2O nanoparticles for adsorption and photocatalytic degradation of methylene blue dye from aqueous medium. Environ Nanotechnol Monitor Manag. 2019;12: 100265.
Muthukumaran M, Niranjani S, Barnabas KS, Narayanan V, Raju T, Venkatachalam K. Green route synthesis and characterization of cuprous oxide (Cu2O): visible light irradiation photocatalytic activity of MB dye. Mater Today Proc. 2019;14:563–8.
Dustgeer MR, Asma ST, Jilani A, Raza K, Hussain SZ, Shakoor MB, Iqbal J, Abdel-wahab MS, Darwesh R. Synthesis and characterization of a novel single-phase sputtered Cu2O thin films: structural, antibacterial activity and photocatalytic degradation of methylene blue. Inorg Chem Commun. 2021;128: 108606.
Xu L, Xu H, Wu S, Zhang X. Synergy effect over electrodeposited submicron Cu2O films in photocatalytic degradation of methylene blue. Appl Surf Sci. 2012;258(11):4934–8.
Funding
This work is conducted and funded by the Department of Inorganic Materials, Institute of Chemistry and Materials. This work was also supported in part by the Korea Environment Industry & Technology Institute (KEITI) through the project for develo** innovative drinking water and wastewater technologies, funded by the Korea Ministry of Environment (MOE) (2021002690003).
Author information
Authors and Affiliations
Contributions
Hoai Phuong Nguyen Thi: methodology, investigation, data curation, writing—original draft; Phuong Nguyen Thi: conceptualization, methodology, data curation; Anh Thu Nguyen Thi: visualization, writing—review and editing; Tien Dung Nguyen: data curation, writing—review and editing; Swoong Chang: resources, writing—review and editing; D. Duc Nguyen: conceptualization, supervision, resources, writing—review and editing; D. Duong La: conceptualization, supervision, investigation, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, T.H.P., Nguyen, T.P., Nguyen, T.A.T. et al. Terminalia catappa leaf extract as a bio-reducing agent to synthesize Cu2O nanoparticles for methylene blue photodegradation. Discov Appl Sci 6, 309 (2024). https://doi.org/10.1007/s42452-024-05990-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05990-3