Abstract
Microfluidic devices have become a vastly popular technology, particularly because of the advantages they offer over their traditional counterparts. They have such a wide range of uses and can make complex tasks quite efficient. One area of research or work that has benefited greatly from the use of microfluidics is biosensing, where microfluidic chips are integrated into biosensor setups. There are growing numbers of applications of microfluidics in this area as researchers look for efficient ways to tackle disease diagnostics and drug discovery, which are critical in this era of recurring pandemics. In this work, the authors review the integration of microfluidic chips with biosensors, as well as microfluidic applications in biosensing, food security, molecular biology, cell diagnostics, and disease diagnostics, and look at some of the most recent research work in these areas. The work covers a wide range of applications including cellular diagnostics, life science research, agro-food processing, immunological diagnostics, molecular diagnostics, and veterinarian diagnostics. Microfluidics is a field which combines fundamental laws of physics and chemistry to solve miniaturization problems involving fluids at the nanoscale and microscale, and as such, the authors also examine some fundamental mathematical concepts in microfluidics and their applications to biosensing. Microfluidics has relatively new technologies with great potential in terms of applications.
Article Highlights
-
This work highlights some key limitations of biosensors.
-
The work reviews the advantages of integrating biosensors with microfluidic devices.
-
The work also highlights different areas (applications) of biosensing that have benefited from the integration of biosensing devices with microfluidics and a future overview.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past few decades, there has been a surge in the emergence of new viral and bacterial strains around the world [1]. Most recently, we had the COVID-19 pandemic, which was greatly devastating, killing millions of people around the world. Viruses such as the Corona virus, such as SARS-COV, are constantly emerging and can lead to outbreaks, such as epidemics and pandemics. This highlights the ever-growing importance of having reliable diagnostic tools and the urgent need for fast development of treatments to reduce the spread of related illnesses. Biosensors coupled with microfluidic chips and/or devices offer a promising performance enhancement for the development of effective biosensing devices. Microfluidic chips were first introduced in the 1980 s by several researchers, including George Whitesides at Harvard University, Stephen Quake at Stanford University, and Andreas Manz at Twente University based in the Netherlands [2]. Microfluidics refers to an interdisciplinary branch of science that looks at the manipulation of and the behavior of fluids at the microliter and nanoliter volumes inside microsized channels. In the article by Yang et al. a microfluidic system or device is defined or desribed as, “A small, non-turbulent, highly ordered, portable fluid flow system made up of several microchannels molded or carved into a material” [3]. Microfluidic chips have found a considerable scope of applications since their inception, such as disease diagnostics [4,5,6,7,8,9], food security [10,11,12,13] and biological studies [14,15,16,17,18,20]. In this review, the authors look at the application of microfluidics in biosensing work. Biosensors are described / defined as a device that combines a biological recognition element (e.g., tissue, microbe, organelle, cell receptor, enzyme, or antibody) with a transducer to detect and convert specific biological interactions or reactions into measurable signals [21]. When the biological recognition element interacts with its target molecule in an introduced analyte, a physiological chemical change occurs as a result of the interaction of the target analyte with the target biological substance. The transducer then detects this change, which transforms it into an analog electronic signal for further processing and analysis [21]. The scale of the analog electronic signal generated by the transducer has a direct proportionality relation to the concentration of a particular analyte or the collection of analytes [22]. Biosensors consist of a biorecognition element and a mechanism for transforming biological signals into quantifiable electrical signals, such as color, current, voltage, capacitance, light intensity, wavelength, and phase. The critical factors to evaluate the performance of a biosensor include sensitivity, stability, repeatability, response time in real-time and diagnosis, sample volume, consumption, throughput, and user-friendliness [21].
Shows a diagram which shows key metrics to measure the performance of a biosensorranging from sensitivity, stability repeatability to throughput. The image was drawn using information from [23]
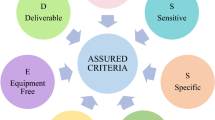
Fig. 1 shows the critical metrics to consider when designing or analyzing a biosensor. An ideal biosensor should exhibit high sensitivity, which is influenced by factors such as efficient capture of the analyte, accurate characterization of the analyte, the ability to convert biological signals into electronic signals, and the impact of both systematic and environmental interferences [23]. Stability and repeatability are crucial elements in evaluating biosensor performance. Stability refers to a biosensor’s consistent and reliable performance under specified conditions or environments. This aspect is particularly important for portable or wearable biosensors used in dynamic settings, where factors such as temperature, velocity, humidity, pressure, and lighting conditions may vary. Repeatability, on the other hand, concerns the consistency in the performance of a biosensor in the long run under identical conditions, which is crucial for ensuring its reliability. In commercial biosensors, repeatability is often assessed regularly for recalibration purposes [21]. High throughput is another important feature of biosensors, as research aims to develop devices capable of multitasking. Additional key evaluation metrics include low sample volume consumption, user-friendly operation, and rapid response or real-time analysis.
Shows a diagram which shows the key challenges encountered by researchers in designing biosensors. This image was drawn using information from [23]
Fig. 2 highlights several challenges encountered in the design of biosensors, such as specific and nonspecific binding, the design of the biosensor assay matrix, the properties of the bioreceptors and the detection of low concentration target molecules in samples. Specific binding poses a challenge due to the complexity of the sample components, which contain various molecules [24]. In such cases, ensuring the specificity of the biosensor is crucial to recognize the correct target in a solution containing other molecules. For example, a biosensor designed to identify a particular antibody in human blood must be highly specific to eliminate interference from other antibodies, cells, and electrolytes in the sample. Nonspecific binding, or biofouling, can sometimes cause significant signal noise, drift, or delay in biosensors [25]. To minimize nonspecific binding, it is essential to thoroughly clean the binding region or surface with a buffer after the binding process has been completed and before starting a new reaction. This step helps to reduce the weak binding caused by nonspecific interactions [25,26,27]. Variability in bioreceptor properties can also present challenges. Even when the same protocol is followed, bioreceptors can exhibit inconsistent coverage rates and repeatability [28,29,30,31,32]. Factors such as operation and environment can greatly influence surface activation, modification, and functionalization protocols. Thus, a thorough study of surface activation and comprehensive testing of surface treatment protocols are crucial before conducting binding experiments. Furthermore, the immobilization process involving multiple layers on the biosensor surface can be challenging to replicate. This is particularly true for stable sensor surfaces (metallic) such as gold, silver, and silicon [25]. The alignment of bioreceptors also plays a significant role in the results of the binding event. Connecting layer molecules are often randomly polarized and oriented, potentially causing destructive interference and reduced collective charge polarization. This may cause a decrease in the sensitivity of the detection. In recent work attempts to improve the sensitivity and limit of detection in biosensing work also include the use of quantum resources [33,34,35,36,37] and machine learning [38, 39]. This outside of the scope of this work however.
Some of the advantages of microfluidic chips are shown in Table 1.
The microfluidics field began with the miniaturization of analytical chemistry methods to separate and analyze sample mixtures. Miniaturization provides several advantages to microfluidic channels, such as reduced sample consumption, lower contamination risk, lower cost per analysis, streamlined operations, increased sensitivity and specificity, and greater reliability [25]. Microfluidics technology has become a popular research area due to its widespread use in science and technology for applications such as cancer diagnosis, metastasis, drug delivery, and tissue engineering. The long-term objective of microfluidic technologies is to create automation-based lab-on-chip systems, reducing time-consuming clinical tests in various laboratories [44]. Microfluidic chips, which fall under the umbrella of microfluidics science, offer the advantage of integration into experimental research laboratories for tasks that involve sample preparation, reaction, separation, and detection [45].
Schematic illustration (a) Microfluidics Flow, (b) Continuous flow of liquid and (c) Droplet generation at T Junction in discrete flow. Taken with permission from [46]
Microfluidic systems exhibit two primary types of flows, illustrated in Fig. 3 continuous and discrete, which are influenced by external factors [47]. Continuous flow microfluidics (Fig. 3b) enable the precise control of liquid flow through microchannels using integrated external pressure pumps or mechanical micropumps. Such continuous flow processes find application across various fields including bioanalytical, chemical, energy, and environmental studies [48]. In contrast, droplet microfluidic devices employ discrete water droplets within an immiscible oil phase, stabilized by surfactants, serving as microscale sample containers. This discrete flow can be further categorized into segmented-flow microfluidics and Digital Microfluidics (DMF) [48, 49]. The Micro-droplet approach, a high-throughput technology, minimizes reagent usage, offers user-friendly operation, and mitigates cross-contamination between reagents [50].
Pang et al. [51] investigated a droplet-based Microfluidic System (MFS) and its impact on flow dynamics using a continuous stream of two or more immiscible fluids at a T-junction, a commonly used geometric configuration (Fig. 3c). The T-junction microscale channel consists of two inlets and one outlet for droplet formation, with channel geometries significantly influencing the process. Under varied parameters, three key guidelines for drop formation emerge: drip**, squeezing, and parallel flowing streams. In the drip** regime, droplet breakup occurs when viscous shear stress surpasses interfacial tension, akin to the breakup of spherical droplets. Adjusting the capillary number appropriately ensures droplets are emitted without channel blockage; conversely, low capillary numbers can obstruct the continuous phase, leading to increased hydrodynamic pressure and subsequent droplet pinch-off. Flow-focusing techniques, widely utilized for droplet fabrication, involve coaxially aligning a nozzle with a downstream orifice. The fluid in the orifice applies shearing force, facilitating droplet production [52, 54]. For a classification of pressure measurement techniques in microchannels according to the kinds of sensor elements employed, see Fig 5. The mechanism at which fluid flows is of importance, especially considering biological species for passage they took towards the target area. In most cases, the solid microfluidic walls restrict the flow and, while the open walls with air interfaces provide the flow without restriction [55]. The flow of liquid can be laminar turbulent, and it is possible for flow to be both laminar and turbulent, depending on the force applied. The small dimensions of microchannels automate the microfluidic process to generate laminar flow rather than turbulent flow [56]. However, the best way to determine if the flow is turbulent or laminar is to apply the Reynolds number (See Eq. 1):
Re is the dimensionless Reynolds number, \(\rho \) is density, \(\eta \) is the dynamic viscosity of water, w is the linear flow velocity, h is the channel height, and w is the channel width [56]. When the numerical number above 2000 indicates the turbulent and when below 2000 it indicates laminar flow [57]. The velocity which the liquid accelerates upon entrance has effects on the flow; in all cases laminar flows are caused by small velocity upon entrance, while turbulent flows are caused by high velocity. The higher capillary force induces a higher flow velocity, which causes movement to be irregular instead of parallel, turbulent flows are characterized by irregular flows while laminar flows are characterized by parallel flow movement [58]. In microfluidics, other commonly used measures are shown in Fig. 4
The inertial force is a force that acts on an object due to its mass and acceleration. The inertial force is also known as the force of inertia. According to Newton’s first law of motion, “ An object in motion will remain in motion at a constant velocity unless acted upon by an external force" [67]. The inertial force arises from this law and represents the resistance of an object to change its velocity. The larger the mass of the object, the greater its inertial force. In fluid mechanics, inertial force is a term used to describe the force due to the velocity and acceleration of the fluid. Inertial forces in fluid flow are proportional to the product of the fluid’s density and the square of its velocity. Inertial forces play a crucial role in the flow of fluids, particularly in high-speed flows, where the fluid velocity is large. In microfluidics, the Reynolds number is used to characterize fluid flows on the basis of the relative magnitude of inertial forces to viscous forces. The Reynolds number is a useful tool for designing microfluidic devices and for controlling fluid flows in these devices. Viscous force is a frictional force that acts on an object due to friction within a fluid through which the object is moving. In fluid mechanics, a viscous force arises from friction between the fluid layers as they move past each other. This friction creates resistance to fluid flow and a force that acts to slow the fluid velocity. The magnitude of the viscous force is proportional to the viscosity of the fluid, the velocity gradient, and the area over which the force acts. In microfluidics, the viscous force plays a crucial role in the flow of fluids through small-scale channels and devices. Adjusting the Reynolds number allows researchers to produce laminar or turbulent flows, which can be useful for different types of microfluidic applications. Table 2 shows the dimensionless measures in fluid dynamics and transport phenomena.
Microfluidics is also described as a sub discipline of fluid mechanics that studies the fundamentals equations that describe the physics of fluid at larger length scale identical to the equations underlying microfluidics [68]. These equations are the Navier–Stokes equations. The Navier–Stokes equations are a set of partial differential equations named after the French mathematician and physicist Claude-Louis Navier and the Irish mathematician George Gabriel Stokes [69]. In microfluidics, the Navier-Stokes equations are used to study the flow of fluids in channels with dimensions on the order of micrometers. This is important for several applications, including the development of microfluidic devices for lab-on-a-chip systems, which can be used for a variety of applications, such as medical diagnostics, chemical synthesis, and environmental monitoring [70]. By solving the Navier–Stokes equations for specific microfluidic geometries and fluid properties, researchers can gain insights into the behavior of fluid flows in these systems. For example, they can study the flow of different fluids in microfluidic channels, including their velocity, pressure, and mixing behavior, which can be used to optimize the design of microfluidic devices for specific applications. Additionally, the Navier-Stokes equations can be used to study the behavior of complex fluid flows, such as the flow of non-Newtonian fluids or the flow of fluids with multiple phases, which are important for many applications in microfluidics [71]. Solving the Navier–Stokes equations for microfluidic systems can be challenging, especially when considering the small length scales and high Reynolds numbers involved. The equations are typically solved numerically using methods such as a finite difference or finite volume or using simulations using software such as OpenFOAM or COMSOL. Limit conditions must also be carefully defined and considered in simulations, as they can have a significant impact on flow behavior [72].
The Navier–Stokes equation is given as,
where \({v}\) is the velocity vector of the fluid, t is time, \(\rho \) is the density of the fluid, p is the pressure, \(\nu \) is the kinematic viscosity and \(\nabla \) is the del operator representing the gradient.
The Navier–Stokes equations are derived from the principles of mass, momentum, and energy conservation. These principles can be mathematically expressed [73]. The principle of mass conservation states that, “The principle of mass conservation states that the rate of change in mass in a control volume is equal to the rate of mass flow into the control volume" [74]. This can be expressed mathematically as shown in Eq. 3:
Where \(\rho \) is the fluid density and u is the fluid velocity. The principle of momentum conservation states that, “The rate of change in momentum in a control volume is equal to the net force acting on the control volume" [75]. This can be expressed as in Eq. 4:
Where p is the fluid pressure, v is the fluid viscosity, and F is the body force per unit volume. The principle of energy conservation states that, “The rate of change of energy in a control volume is equal to the net rate of energy flow into the control volume" [75]. This can be expressed as in Eq. 5,
Where E is the fluid internal energy per unit volume and \(\epsilon \) is the fluid dissipation per unit volume. By combining these three principles, we obtain the Navier–Stokes equations, which describe the motion of fluid substances and can be used to study fluid flows in a variety of systems, including microfluidic devices. The Navier-Stokes equations are a set of partial differential equations (PDEs) that describe the motion of fluids and are widely used in microfluidics applications. They are used to predict the flow behavior of fluids in small devices and channels, which can have a significant impact on the behavior of the fluid and its transport properties [76].
Categorizing methods for measuring pressure in microchannels based on the types of sensor elements used [77]
3 Materials
This image shows the range of fabrication techniques used to develop microfluidic chips [78]
The success of fabricating microfluidic devices depends on the materials used, as they have an impact on the properties of devices [79]. The different fabrication techniques which can be used to develop microfluidic chips are shown in Fig 6 (These are described more indepth in Table 3). Recently, different materials such as silicon, quartz, glass, ceramics, polymers, and metals have been used to fabricate microfluidics, and each material shows difference in functionalities per device [80]. Silicon has been used to fabricate microfluidic chips since the origin of microfluidics because of its resistance to organic solvents, ease in metal deposition, high thermoconductivity, and electro-osmotic mobility [79]. The wide surface area of silicon allows for modifications on its surface and that made it flexible for the manufacture of thin membranes, which decreases thermal mass and allows high temperature ramp rates [81]. Silicon has a high conductivity, which is important for a large temperature distribution when used [81]. Although silicon is a good material, its opacity properties have limitations and make it incompatible for optical detection in the visible and ultraviolet regions [79, 82]. Glass microfluidics have good optical transparency, inertness, thermostable, electrical insulation properties, and are compatible with biological samples [79,80,81]. Glass microfluidics also have good detection performance and allow for high-pressure and high-temperature experiments due to glass property [79]. The disadvantage of glass is the cost of the instruments for fabrication and the bonding of the etched devices to form microfluidics requires a high temperature or pressure [80, 81]. However, chemical etching is impractical for prototy** applications since it requires the use of a clean room environment and generates toxic by-products [79,80,81]. For prototype purposes, however, chemical etching is not feasible due to the need for a clean environment and the hazardous byproducts it creates. [81]. Poly dimethyl siloxane (PDMS) is one of the most widely used plastics to fabricate microfluidic. The inert surface of pdms is reactive with many reagents and the surface can be modified, the material is not toxic to biological samples [83, 84]. A PDMS-made microdevice can be used to detect bacteria, protein, and DNA, this advantage is good for disease detection.
4 Areas of biosensing making use of microfluidics
The figure shows the range of microfluidic systems (a) continuous; (b) drop-based; and (c) digital. It was taken with permission from [93]
In this section, we look at the applications of microfluidics in different areas of biosensing research. Because of the convenience which microfluidic chips bring they are very attractive in many areas of research. Microfluidic biosensors are currently widely used in a variety of domains, including both practical application and basic research. We will not present an exhaustive list of application fields in this section. Rather, we will highlight the most recent advancements and developments in research, particularly as they relate to the use of microfluidic biosensing. Figure 8 shows the list of areas in which microfluidic chips are used and the areas which will be discussed in this section. Microfluidic devices can be classified on the basis of the microfluidic system they use (see Fig. 7).
The figure shows the range of applications of microfluidics in biosensing and diagnostics. It was drawn from information taken from [23]
4.1 Microfluidics in cellular diagnostics
Microfluidic devices have been used extensively in cellular diagnostics for a diverse range of applications which include cell sorting, cell culture, and genetic analysis [46, 94, 95]. A hypothetical device for cell extraction is shown in Fig 9. Microfluidic systems offer high-level precision and accuracy in performing measurements on single cells or large populations [96]. With the average cell size being a few micrometers, it is necessary to build devices that interact with cells using the characteristics of microengineered devices on a similar size scale [97]. The use of microfluidic devices allows the position of cells and the environment to be controlled using a variety of cellular microfluidic building blocks and measurements can be taken in situ or after processing with little to no operator intervention. This allows high-throughput cell culture, labeling, and imaging while also allowing selection, lysing, and analysis of the contents of a single cell [98,99,100,101]. Recently, a microfluidic flow-free automated immunoassay method for CD4 cell counting was developed. The developed microfluidic device uses magnetic beads coated with antibodies to generate a flow-free magnetic actuation platform that efficiently gathers CD4+ T cells from a 30\(\mu \)L drop of whole blood [102]. The innovative tool extracts CD4+ T cells from a complete blood drop using highly specific antibody-conjugated magnetic beads; debris and other unattached entities are subsequently removed by washing [103]. This device uses a disposable microfluidic chip in place of manual sample preparation, washing, and lysis procedures. Furthermore, advancements in micro/nanotechnologies and consumer electronics have made it possible to build mobile healthcare technologies, including POC smartphone microfluidic-based diagnostic assays for illness detection and therapy monitoring [104]. A disposable microfluidic chip that has been functionalized and a mobile phone make up the microfluidic-based smartphone gadget. Table 4 shows example applications of microfluidics in cellular diagnostics.
4.2 Microfluidics in molecular diagnostics
This digram shows the design of a hypothetical device for simultaneous examination of circulating tumor cells (CTCs) and circulating biomolecules like proteins and DNA from whole blood. The device channels whole blood through various small chambers and channels, utilizing multiple microscale separation methods to segregate different elements, including immune cells, CTCs, circulating tumor DNA (ctDNA), proteins, and exosomes. Subsequently, these cells and substances are either collected for further analysis or examined directly within the device. It was taken with permission from [111]
Most molecular diagnostic assays are performed in laboratories by trained personnel and require manual manipulation of tiny-volume reagents and specialized tools [112]. This can be a very centralized and time-consuming process which can be made to operate in a more portably, at high-speed, and low-cost way by using a microfluidic device. For example, both the pre- and post-PCR test procedures can be integrated into the cartridge format using microfluidic devices, which may also result in a reduction in the amount of reagent needed [113,114,115,116]. The goal in using microfluidic devices here is to ensure that the user experience be as easy as inserting the cartridge into the instrument after loading the specimen, thus opening up a wider range of users and use cases. In this context, microfluidic chips can be used for a variety of applications, including genetic testing, disease diagnosis, and drug discovery [117, 118]. An example application of microfluidic molecular diagnostics is the use of microfluidic laboratory-on-a-chip devices for the detection of genetic mutations associated with diseases such as cancer [119,120,121]. These devices use microfabricated channels and sensors to isolate and analyze individual DNA molecules, allowing the detection of specific mutations associated with the disease [122]. Microfluidic devices that detect viral particles can rapidly isolate and analyze viral particles, allowing for the early diagnosis of the disease. Figure 10 shows example steps which are necessary for nucleic acid testing, which is useful for molecular diagnostics. The first step is preprocessing off the chip, which is followed by on-chip sample processing and nucleic acid amplification. This is followed by an off-chip signal read-out.
This digram shows the main steps when using microfluidics-based strategies for molecular diagnostics of infectious diseases. This image is taken from [123] which has given right to the work under an open license, the article is licensed under a Creative Commons Attribution 4.0 International License
There are several steps that can be replaced with microfluidic chips, such as nucleic acid isolation. After the sample (either liquid or solid) is applied directly to a chamber on the microfluidic cartridge, automated sample preparation procedures carried out on board a microfluidic device can be used to isolate and purify nucleic acid [122]. Amplification in microfluidic cartridges and many amplification techniques, including PCR (end point, LAMP), qPCR, and RT-PCR, have been performed effectively [122, 124, 125].Detection fluorescence, optical absorption, and electrochemical detection are just a few examples of the various detection techniques that can be used. When immobilized on a surface or in a carefully regulated compartment, the target amplicon can be quantified (e.g. microarray) [126]. Using a twofold filtration microfluidic system, a molecular diagnostic tool was created that can quickly separate exosomes in POC environments by applying size-exclusion principles. The apparatus can successfully extract exosomes from 50 to 100 \(\upmu \)L of plasma in 50 min. The apparatus was contrasted with a widely used exosome isolation method, polyethene glycol (PEG)-based precipitation [127, 128]. Because the microchip is meant to be used just once, it may be readily disposed of after use. The main goal is to use the developed apparatus to concentrated exosomes found in trace levels in human biological samples. After that, the concentrated exosome sample can be applied to goals related to diagnosis and treatment [129]. Table 5 shows example applications of microfluidic devices in molecular diagnostics.
4.3 Microfluidics in immunological diagnostics
Immunological diagnostic devices are used to detect the presence of specific antigens or antibodies in a patient’s sample, such as blood or urine [136,137,138,139]. These devices can be used to diagnose a wide range of conditions, including infectious diseases, autoimmune disorders, and cancers [140, 141]. The earliest disposable point-of-care diagnostics were immunological tests, which are still the most common microfluidic diagnostic today [142].
This digram shows an illustrative depiction of the fluorescent lateral flow assay (LFA) utilizing magnetic quantum dot nanobeads (MQBs). This assay enables swift enrichment and remarkably sensitive identification of the influenza A virus within a human specimen. This image is used with permission and has been taken from [143]
Microfluidic immunological diagnostics often allow the assay to be reduced to only a few steps in the procedure, providing quick findings with minimal user involvement [144]. This may lead to extremely affordable and user-friendly biosensors, e.g. lateral flow assays. Strict controls are required to ensure that the antibodies utilized remain in a stable condition and that the reagents are added in a repeatable and regulated manner. An example application where microfluidics could be integrated in immunology studies is shown in Fig. 11, microfluidic chips can be integrated with lateral flow assays for development of better biosensing devices. If there are multiple reaction sites in a experiment and controls when multiple targets are required require a more sophisticated implementation, microfluidics have the ability to reduce this complexity. In traditional sandwich immunoassay, a capture antibody is used to immobilize the target analyte in the samples. The target must be supplied over the capture surface of the prepared specimen on a cartridge at a flow rate compatible with the kinetics of protein binding [145]. Table 6 applications of microfluidics in immunological diagnostic device development and manufacture.
There are several different types of immunological diagnostic devices, including:
-
Enzyme-linked immunosorbent assay (ELISA): This is a widely used assay to detect specific proteins in a sample. ELISAs can be used to detect antibodies or antigens in a sample and are often used for diagnostic purposes [146,147,148,149].
-
Rapid diagnostic tests (RDT): RDTs are simple point-of-care diagnostic tests that can provide results in a matter of minutes. These tests are often used in resource-limited settings and can be used to diagnose a wide range of conditions, including infectious diseases such as malaria, HIV, and dengue fever [150,151,152].
-
Western blot: This is a laboratory-based assay that is used to detect specific proteins in a sample. Western blots are often used to confirm the results of ELISAs and are considered a gold standard assay for protein detection [153, 154].
4.4 Microfluidics in life-science research
a Microfluidic devices show promise for assessing individual patient responses to treatments. These devices use surgical or biopsy specimens for both histopathological examination and molecular analysis. The tissue is further processed to extract patient-specific cells, which are then cultivated outside the body. Various cell types, including tumor, stromal, endothelial, and lymphatic cells, are grown within the microfluidic system. The device then monitors their functional response, offering insights into tumor progression and patient outcomes. b Microfluidic systems can also integrate with other in vitro culture advancements, such as 3D bioprinting. This combination allows for the creation of intricate structures using biologically sourced extracellular matrices, enhancing the complexity and relevance of the models. c The ability of microfluidic devices to handle numerous samples simultaneously positions them as effective tools for extensive drug screening. This high-throughput capacity is crucial for identifying the most suitable drug for each patient, tailoring treatment on an individual basis. This image is used with permission and has been taken from [111]
The study of life and organisms, including humans, is the focus of a wide range of applications in the subject of life sciences research [160]. Figure 12 shows applications of microfluidics in life sciences research. In comparison to conventional benchtop methods, microfluidic life-science devices can provide several benefits, including high throughput and repeatability as well as decreased cost and complexity. Lab-on-chip devices provide a highly regulated environment onboard that offers additional practical, physical, and technological benefits for researching biological systems in addition to speed and cost advantages. Microfluidic chips small-scale properties let scientists do experiments that would be impractical on a bench top. For instance, precise manipulation of individual cells in vitro is made possible by designed features at the cellular scale [156, 161]. Additionally, because microfluidic life-science devices are frequently made to operate with other tools and apparatus, they are simple to integrate into existing infrastructure and automated or computerized workflows. Microfluidics have become increasingly popular in the life sciences, as it allows for precise control over the environment of cells and other biological samples and enables the development of new types of assays and techniques. There are a wide range of microfluidic products (Table 7) that have been developed for life science research, including cell culture platforms, chip-based PCR systems, and lab-on-a-chip devices for genetic analysis [162, 163]. Some examples of these products include:
-
The C1 Single-Cell Auto Prep system, developed by Fluidigm, is a microfluidic device that allows for the isolation and analysis of individual cells from a sample [164]. It can be used for a variety of applications, including single-cell RNA sequencing and CRISPR-based genome editing [165].
-
The RainDrop Digital PCR system, developed by RainDance Technologies, is a microfluidic device that enables highly sensitive and specific detection of DNA and RNA [166, 167]. It can be used for applications such as cancer research, genetic testing, and pathogen detection.
-
The Cytosort Single-Cell Platform, developed by BD Biosciences, is a microfluidic device that allows for the isolation and analysis of specific cell populations from a sample [168, 169]. It can be used for a variety of applications, including stem cell research and immunology.
4.5 Microfluidics in veterinarian diagnostics
A veterinarian microfluidic diagnostic device is a device that is used to diagnose various diseases and conditions in animals [176,177,178]. Testing for companion animals (pets) and cattle makes up most of the veterinary diagnostic market. Pet diagnostics are moving toward automation in centralized labs with an emphasis on high throughput and quick turnaround [179]. Regular biochemistry, immunodiagnostics, hematology, urinalysis, molecular diagnostics, and urinalysis are examples of common companion animal diagnostics that are like human pathological tests [180, 181]. One example of a veterinarian microfluidic diagnostic device is a portable device for the detection of tick-borne diseases in dogs [182]. A small blood sample from the dog and runs it through a microfluidic chip that is capable of detecting the presence of multiple tick-borne diseases, such as Lyme disease, in a single test [183]. Another example is a point-of-care diagnostic device for the detection of Canine parvovirus (CPV) in dogs [184]. Microfluidic devices can detect CPV with high accuracy and sensitivity using a small blood sample from the dog. Table 8 shows applications of microfluidics in veterinarian diagnostic device development and manufacturing.
4.6 Microfluidics in agro-food testing
Microfluidic technology is increasingly being used in the agro-food industry for testing and analysis of food products [189, 190]. Microfluidic devices are small, portable, and cost-effective, and can be used to perform a wide range of tests, including food safety, authenticity, and quality control [191]. When microfluidic devices are compared with traditional benchtop techniques, the agro-food industry can benefit from a number of these benefits. These benefits include affordability, speed of execution, great sensitivity, small size, high throughput, and minimal consumption of samples and reagents. In addition, many lab-on-chip technologies significantly lower the level of testing expertise needed. Small amounts of complex fluids can be measured effectively and quickly using microfluidic devices. Therefore, they are well adapted for the rapid and accurate detection of infections, poisons, chemical traces, and heavy metals in food [192,193,194,195]. An example of a microfluidic agro-food testing device is a portable device for the detection of food-borne pathogens, such as E. coli and Salmonella [196,197,198,199]. These devices utilize microfluidic technology to isolate and amplify DNA from food samples, allowing for rapid and accurate detection of pathogens. Another example application is in the detection of genetically modified organisms (GMOs) in food products [200,201,202,203]. The microfluidic devices isolate and analyze DNA from food samples, allowing for the detection of GMOs with high accuracy and sensitivity. In addition to food safety and authenticity, microfluidic technology can also be used for the analysis of food quality and composition [204, 205]. For example, a device that utilizes microfluidic technology for the analysis of sugar content in fruits [206, 207]. The device can be used to detect the sugar content of fruits and to check its quality. Figure 13 shows areas in agro-food tesing which could benefit from the integration of microfluidic devices by improving the detection of toxic materials. Table 9 shows the diverse range of applications of microfluidics in agro-food testing.
This digram shows an illustrative depiction of the agroprocessing areas where microfluidic devices can have an impact. This image is used with permission and has been taken from [208]
5 Microfluidcis for virus capture, quantification, and detection
This picture shows the use of air sampling devices to capture and concentrate viruses. This image is used with permission and has been taken from [214]
The application of microfluidics in virus capture, quantification, and detection represents a significant advancement in biomedical and diagnostic technology [214,215,216]. Early and accurate detection of viral infections is critical for effective disease management and control. Accurate quantification of viral particles is essential for various applications, including disease diagnosis, monitoring viral load in patients, and assessing the efficacy of antiviral therapies (Fig. 14). One approach to virus quantification involves leveraging the optical, fluidic properties of microfluidic devices by combining fluorescence or luminescence-based detection methods with microfluidic channels, researchers can achieve real-time monitoring of virus concentrations in biological samples [217, 218]. This enables rapid and quantitative assessment of viral load with high sensitivity and specificity. Furthermore, the integration of computational analysis software allows for automated processing of imaging data obtained from microfluidic-based virus quantification assays. Advanced algorithms can analyze the spatial distribution and intensity of fluorescent signals associated with captured viruses, enabling precise quantification even at low concentrations. The combination of microfluidics with advanced imaging technologies, such as lensless microscopy or smartphone-based imaging, enables real-time visualization and analysis of captured viruses [219]. These compact and portable imaging systems eliminate the need for bulky and expensive microscopy equipment, making virus detection more accessible and scalable. Machine learning algorithms can be trained to recognize patterns associated with specific viruses, further enhancing the sensitivity and specificity of detection assays [220]. These miniaturized and integrated systems offer rapid, sensitive, and cost-effective solutions for a wide range of applications in biomedical research, clinical diagnostics, and public health surveillance. An innovative, reasonably priced tool for quantifying HIV-1 viral particles was developed which uses a portable lensless imaging device, magnetic beads coated with antibodies, a surface-functionalized microprocessor, and computational analysis software [221]. The instrument measures the HIV-1 viral load in biological samples using function-alized microfluidic chip surfaces and high refractive index magnetic microbeads [222]. Microfluidics holds great promise for revolutionizing virus capture, quantification, and detection technologies. Table 10 shows the diverse range of applications of microfluidics in virus capture, quantification, and detection..
6 Comparison of different microfluidic devices
Microfluidic devices are versatile tools used in various fields, including biology, chemistry, engineering, and medicine. Here we compare different types of microfluidic devices based on their design, applications, advantages, and limitations:
-
1.
Continuous Flow Microfluidic Devices [226]:
-
Design: These devices operate by continuously pum** fluids through microchannels.
-
Applications: They are commonly used for chemical synthesis, cell culture, drug delivery, and DNA analysis.
-
Advantages: Offers high throughput, precise control over flow rates, and compatibility with standard laboratory equipment.
-
Limitations: Limited ability to handle discrete samples, and can suffer from clogging issues due to small channel dimensions.
-
-
2.
Digital Microfluidic Devices [227]:
-
Design: Utilizes electric fields to manipulate discrete droplets of fluids on a substrate.
-
Applications: Widely used in biochemical assays, point-of-care diagnostics, and sample preparation for sequencing.
-
Advantages: Enables precise handling of individual droplets, reduces sample volume requirements, and allows for automation of complex protocols.
-
Limitations: Limited scalability for high-throughput applications, and challenges in achieving uniform droplet manipulation at high speeds.
-
-
3.
Paper-Based Microfluidic Devices [228]:
-
Design: Utilizes paper as a substrate for fluid manipulation via capillary action.
-
Applications: Suitable for low-cost diagnostics, environmental monitoring, and resource-limited settings.
-
Advantages: Low-cost fabrication, simplicity, and portability.
-
Limitations: Limited precision and sensitivity compared to other microfluidic platforms, restricted to relatively simple fluid handling tasks.
-
-
4.
Organ-on-a-Chip Devices [229]:
-
Design: Mimics the structure and function of human organs using microfluidic channels and living cells.
-
Applications: Used in drug screening, disease modeling, and personalized medicine.
-
Advantages: Provides a physiologically relevant environment for studying organ-level responses, reduces the need for animal testing, and enables tailored drug testing.
-
Limitations: Complexity in design and fabrication, challenges in recapitulating the complexity of native tissue environments, and limitations in long-term culture stability.
-
-
5.
Lab-on-a-Chip Devices [230]:
-
Design: Integrates multiple laboratory functions onto a single chip, typically including sample preparation, analysis, and detection.
-
Applications: Widely used in clinical diagnostics, environmental monitoring, and food safety testing.
-
Advantages: Reduced sample and reagent consumption, rapid analysis, portability, and potential for automation.
-
Limitations: Initial setup costs can be high, complexity in chip design and integration, and challenges in achieving standardization and mass production.
-
Each type of microfluidic device offers distinct advantages and limitations, making them suitable for different applications and research needs. The choice of device depends on factors such as desired throughput, sample volume, complexity of the experiment, and available resources.
7 Challenges and limitations
One of the primary challenges in microfluidic biosensing is the complexity of sample preparation. Real-world samples often contain a myriad of interfering substances, requiring intricate pre-processing steps to isolate target analytes effectively. Integrating these steps seamlessly into microfluidic platforms while maintaining simplicity and efficiency remains a significant hurdle [231]. Achieving high sensitivity and specificity in detection is crucial for accurate biosensing. However, microfluidic systems may encounter limitations in detecting low-concentration analytes or distinguishing between closely related analytes, especially in complex biological matrices [232]. Enhancing the signal-to-noise ratio and improving selectivity are ongoing challenges. While miniaturization is a hallmark of microfluidics, scaling up these devices for practical applications without compromising performance remains a challenge. Issues such as uniformity of flow, reproducibility, and integration with other components become more pronounced as device size decreases. Develo** scalable fabrication techniques and robust manufacturing processes is essential for widespread adoption. The materials used in microfluidic devices must be biocompatible to ensure minimal interference with biological samples and compatibility with downstream analysis techniques [233]. However, many conventional materials pose challenges in terms of biocompatibility, chemical inertness, or optical transparency. Exploring novel materials and surface modifications to address these concerns is an active area of research. Scaling up production is also another problem [231]. Despite advancements, the cost of microfluidic devices and associated instrumentation remains a barrier to widespread adoption, particularly in resource-limited settings. Develo** low-cost fabrication methods and user-friendly platforms is essential to democratize access to microfluidic biosensing technology [234].
8 Future outlook
Combining multiple detection modalities, such as optical, electrochemical, and mechanical sensing, can enhance sensitivity and specificity while enabling multiplexed analysis. Future microfluidic biosensing platforms may leverage these multimodal approaches to overcome limitations associated with individual detection techniques [235,236,237,238]. Integration of nanomaterials and nanotechnology holds promise for enhancing the performance of microfluidic biosensors [239,240,241,242]. Nanomaterials exhibit unique properties such as high surface-to-volume ratio, tunable surface chemistry, and enhanced signal amplification, making them attractive candidates for improving detection sensitivity and selectivity. Continued efforts in develo** on-chip sample preparation and analysis techniques can streamline workflows, reduce sample loss, and improve overall assay performance [243,244,245]. Integration of techniques such as droplet microfluidics, acoustic manipulation, and digital microfluidics can enable precise control over sample handling and processing steps. Another key area of application for microfluidic devices is in the development of point-of-care devices. The development of portable, easy-to-use microfluidic devices for point-of-care applications holds immense potential for revolutionizing healthcare delivery. Future research efforts should focus on designing robust, user-friendly platforms capable of delivering rapid and accurate diagnostic results outside of traditional laboratory settings. With the advent of big data analytics and machine learning algorithms, there’s an opportunity to extract valuable insights from complex biosensing data generated by microfluidic devices. Integrating advanced data analytics tools into microfluidic platforms can improve decision-making processes and facilitate personalized healthcare solutions [246,247,248,249]. Numerous research teams have developed cutting-edge methods for creating and characterizing microfluidics devices. Nonetheless, it is imperative to evaluate scholarly findings in the context of commercialization. It is important to work on accelerating this shift and closing the knowledge gap between business and academics. One of the top priorities for the commercialization of microfluidics systems should be the development of appropriate and generally recognized standardized methodologies. Microfluidics technology is a rapidly develo** discipline that holds out endless possibilities [250]. Its growth curve is gradually gaining the necessary capacity to propel it out of the research lab and into practical applications in POC diagnostics and healthcare.
Given the current developments in immunosensors, microelectronic devices, and biomarker identification, microfluidics holds great promise for the creation of practical POC diagnostic instruments. Microfluidics and microarray-based tailored diagnostic tools may become commonplace in critical care, genetic screening, and disease screening applications. One special application of integrated microfluidic microarray chip technology that has been demonstrated is the parallel and quick screening for the simultaneous detection of many bioanalytes [109, 251,252,253]. One could argue that the shift in society’s reliance from traditional biochemical assays to clinical diagnostic equipment based on microfluidics represents a fundamental advancement in point-of-care and efficient illness management. The intended expansion of POC diagnostics requires research and development in the design of innovative devices, clinical assessment, and eventual commercialization. Moreover, prompt action is necessary to address the difficulties posed by the thorough gathering and examination of the enormous volume of data derived from a single patient. Additive manufacturing, i.e., 3D printing, is another tool that has great potential to develop future microfluidic devices [254,255,256].
9 Conclusion
The world was hit by a major shock in the form of the COVID-19 pandemic and it became quickly clear that from a diagnostic perspective, the world was not ready to effectively handle such pandemics. Therefore, it is essential that new diesease detection technologies continue to be developed in preparation for another pandemic. Microfluidic devices offer many advantages, particularly to biosensing-related work, and have the potential to make our biosensors far more rapid and effective than they are in their current state. This work tries to highlight the advantages that may be possible from working with microfluidic devices in the biosensing space and food/agroprocessing space. Microfluidic chips have a wide range of applications and are proving to be very effective devices that can enhance and automate many complex experiments in the biosensing space. This work reviewed some fundamentals of biosensing and also reviewed some fundamentals of microfluidic devices along with their integration with the biosensors and potential applications of thesemicrofluidic-biosensor hybrid devices. In this paper, the authors reviewed the integration of microfluidic devices with biosensors and their application as biosensing devices. In conclusion, the ever-expanding application of microfluidic devices in various fields, specifically biosensing, shows the potential of these technologies to revolutionize disease diagnostics, drug discovery, food security, and other critical areas. By synthesizing fundamental mathematical concepts with the intricate physical and chemical properties of fluids on a nanoscale, microfluidic devices allow for the miniaturization and enhancement of complex processes. As this review has detailed, from cellular diagnostics to veterinarian diagnostics, the practicality of microfluidics continues to expand and redefine boundaries. In the future, the continued integration of microfluidic technologies with various biosensing methods could pave the way for unprecedented advances in the treatment of recurring pandemics and other major challenges. The novel capabilities of microfluidics hold immense promise for a future marked by more efficient, precise, and accessible solutions. The future outlook for microfluidics-based biosensing technology appears promising, driven by advancements in biomaterials, electronics, cloud computing, and the production of cost-effective smart devices. Microfluidics offers several advantages for pathogen detection, including rapid processing, reduced sample consumption, and compact, portable formats. However, challenges persist in integrating multiple functions into a single automated unit. Ensuring the reliability of assay results, simplifying external components, and enhancing user accessibility are critical considerations in device development. Early-stage efforts in technology and product development are essential to address these challenges effectively. Moving forward, the design of point-of-care diagnostic devices will necessitate a deeper understanding of the intended adoption settings and end-user requirements. Affordability remains a crucial factor, particularly for users in low-resource environments. As such, future innovations in microfluidics for biosensing should prioritize accessibility and usability while maintaining robustness and accuracy in diagnostic performance. The future of microfluidics in biosensing holds significant potential, particularly in addressing global healthcare challenges such as infectious disease monitoring and diagnosis. By leveraging emerging technologies and focusing on user-centric design principles, microfluidic devices have the opportunity to revolutionize healthcare delivery, especially in resource-limited settings where traditional laboratory infrastructure may be lacking. However, realizing this potential will require concerted efforts from researchers, engineers, and healthcare professionals to overcome existing technical and logistical barriers. Overall, the trajectory of microfluidics in biosensing appears promising, with the potential to make a meaningful impact on healthcare accessibility and outcomes worldwide.
Data availability
This declaration is not applicable.
Abbreviations
- LAMP:
-
Loop-mediated isothermal amplification
- LFA:
-
Lateral flow assay
- LOAD:
-
Lab on a disc
- LOCC:
-
Lab on a cartridge chip
- PCR:
-
Polymerase chain reaction
- POCT:
-
Point-of-care testing
- qPCR:
-
Quantitative real-time polymerase chain reaction
- RPA:
-
Recombinase polymerase amplification
- RT-LAMP:
-
Reverse-transcription loop-mediated isothermal amplification
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- PDMS:
-
Polydimethylsiloxane
References
Piret J, Boivin G. Pandemics throughout history. Front Microbiol. 2021;11: 631736.
Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–73.
Yang S-M, Lv S, Zhang W, Cui Y. Microfluidic point-of-care (poc) devices in early diagnosis: a review of opportunities and challenges. Sensors. 2022;22(4):1620.
Lee WG, Kim Y-G, Chung BG, Demirci U, Khademhosseini A. Nano/microfluidics for diagnosis of infectious diseases in develo** countries. Adv Drug Deliv Rev. 2010;62(4–5):449–57.
Sharma S, Zapatero-Rodríguez J, Estrela P, O’Kennedy R. Point-of-care diagnostics in low resource settings: present status and future role of microfluidics. Biosensors. 2015;5(3):577–601.
Tay A, Pavesi A, Yazdi SR, Lim CT, Warkiani ME. Advances in microfluidics in combating infectious diseases. Biotechnol Adv. 2016;34(4):404–21.
Sanjay ST, Fu G, Dou M, Xu F, Liu R, Qi H, Li X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst. 2015;140(21):7062–81.
Park S, Zhang Y, Lin S, Wang T-H, Yang S. Advances in microfluidic pcr for point-of-care infectious disease diagnostics. Biotechnol Adv. 2011;29(6):830–9.
Zhang Z, Nagrath S. Microfluidics and cancer: are we there yet? Biomed Microdevice. 2013;15:595–609.
Giri Nandagopal M, Krishnamurthy S, Venkatesh T. Food-on-a-chip: Relevance of microfluidics in food processing. In: Nonthermal processing in agri-food-bio sciences: sustainability and future goals, pp. 655–668. Springer, 2022.
Neethirajan S, Kobayashi I, Nakajima M, Wu D, Nandagopal S, Lin F. Microfluidics for food, agriculture and biosystems industries. Lab Chip. 2011;11(9):1574–86.
Xu B, Guo J, Fu Y, Chen X, Guo J. A review on microfluidics in the detection of food pesticide residues. Electrophoresis. 2020;41(10–11):821–32.
Atalay YT, Vermeir S, Witters D, Vergauwe N, Verbruggen B, Verboven P, Nicolaï BM, Lammertyn J. Microfluidic analytical systems for food analysis. Trends Food Sci Technol. 2011;22(7):386–404.
Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002;4(1):261–86.
Ren K, Chen Y, Wu H. New materials for microfluidics in biology. Curr Opin Biotechnol. 2014;25:78–85.
Ho CMB, Ng SH, Li KHH, Yoon Y-J. 3d printed microfluidics for biological applications. Lab Chip. 2015;15(18):3627–37.
Tian W-C, Finehout E. Microfluidics for biological applications vol. 16. Springer, 2009.
Weibel DB, Whitesides GM. Applications of microfluidics in chemical biology. Curr Opin Chem Biol. 2006;10(6):584–91.
**ong B, Ren K, Shu Y, Chen Y, Shen B, Wu H. Recent developments in microfluidics for cell studies. Adv Mater. 2014;26(31):5525–32.
Thwala LN, Ndlovu SC, Mpofu KT, Lugongolo MY, Mthunzi-Kufa P. Nanotechnology-based diagnostics for diseases prevalent in develo** countries: current advances in point-of-care tests. Nanomaterials. 2023;13(7):1247.
Schasfoort R, Tudos A. Handbook of surface plasmon resonance. Royal Society of Chemistry. London. 2008.
Bhalla N, Jolly P, Formisano N, Estrela P. Essays biochem. Introduction to biosensors. 2016;60(1):1–8.
Wang J, Ren Y, Zhang B. Application of microfluidics in biosensors. Advances in Microfluidic Technologies for Energy and Environmental Applications. 2020.
Xu P, Ghosh S, Gul AR, Bhamore JR, Park JP, Park TJ. Screening of specific binding peptides using phage-display techniques and their biosensing applications. TrAC, Trends Anal Chem. 2021;137: 116229.
Wang J, Smith RJ, Light RA, Richens JL, Zhang J, O’Shea P, See C, Somekh MG. Highly sensitive multipoint real-time kinetic detection of surface plasmon bioanalytes with custom cmos cameras. Biosens Bioelectron. 2014;58:157–64.
Schneider CS, Bhargav AG, Perez JG, Wadajkar AS, Winkles JA, Woodworth GF, Kim AJ. Surface plasmon resonance as a high throughput method to evaluate specific and non-specific binding of nanotherapeutics. J Control Release. 2015;219:331–44.
Wuethrich A, Howard CB, Trau M. Geometric optimisation of electrohydrodynamic fluid flows for enhanced biosensing. Microchem J. 2018;137:231–7.
Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JH. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015;33(11):692–705.
Ciampi S, Harper JB, Gooding JJ. Wet chemical routes to the assembly of organic monolayers on silicon surfaces via the formation of si-c bonds: surface preparation, passivation and functionalization. Chem Soc Rev. 2010;39(6):2158–83.
Liang G, Luo Z, Liu K, Wang Y, Dai J, Duan Y. Fiber optic surface plasmon resonance-based biosensor technique: fabrication, advancement, and application. Crit Rev Anal Chem. 2016;46(3):213–23.
Mok J, Mindrinos MN, Davis RW, Javanmard M. Digital microfluidic assay for protein detection. Proc Natl Acad Sci. 2014;111(6):2110–5.
Sun J, Wang S, Gao F. Covalent surface functionalization of semiconducting polymer dots with \(\beta \)-cyclodextrin for fluorescent ratiometric assay of cholesterol through host-guest inclusion and fret. Langmuir. 2016;32(48):12725–31.
Mpofu K, Mthunzi-Kufa P. Enhanced signal-to-noise ratio in quantum plasmonic image sensing including loss and varying photon number. Physica Scripta. 2023.
Mpofu K, Lee C, Maguire G, Kruger H, Tame M. Experimental measurement of kinetic parameters using quantum plasmonic sensing. J Appl Phys. 2022;131(8).
Mpofu K, Lee C, Maguire G, Kruger H, Tame M. Measuring kinetic parameters using quantum plasmonic sensing. Phys Rev A. 2022;105(3): 032619.
Mpofu KT. Quantum plasmonic sensing with application to hiv research. PhD thesis, University of KwaZulu-Natal. 2020.
Mpofu K, Ombinda-Lemboumba S, Mthunzi-Kufa P, et al. Classical and quantum surface plasmon resonance biosensing. Int J Optics. 2023;2023.
Cui F, Yue Y, Zhang Y, Zhang Z, Zhou HS. Advancing biosensors with machine learning. ACS Sensors. 2020;5(11):3346–64.
Schackart KE III, Yoon J-Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors. 2021;21(16):5519.
Alam MK, Koomson E, Zou H, Yi C, Li C-W, Xu T, Yang M. Recent advances in microfluidic technology for manipulation and analysis of biological cells (2007–2017). Anal Chim Acta. 2018;1044:29–65.
Hung TQ, Chin WH, Sun Y, Wolff A, Bang DD. A novel lab-on-chip platform with integrated solid phase pcr and supercritical angle fluorescence (saf) microlens array for highly sensitive and multiplexed pathogen detection. Biosens Bioelectron. 2017;90:217–23.
Huang H, Densmore D. Integration of microfluidics into the synthetic biology design flow. Lab Chip. 2014;14(18):3459–74.
Bange A, Halsall HB, Heineman WR. Microfluidic immunosensor systems. Biosens Bioelectron. 2005;20(12):2488–503.
Bhattacharjee N, Urrios A, Kang S, Folch A. The upcoming 3d-printing revolution in microfluidics. Lab Chip. 2016;16(10):1720–42.
Figeys D, Pinto D. Lab-on-a-chip: a revolution in biological and medical sciences. ACS Publications. 2000.
Kumari S, Saha U, Bose M, Murugan D, Pachauri V, Sai VR, Madaboosi N. Microfluidic platforms for single cell analysis: applications in cellular manipulation and optical biosensing. Chemosensors. 2023;11(2):107.
Abbas Y, Miwa J, Zengerle R, Von Stetten F. Active continuous-flow micromixer using an external braille pin actuator array. Micromachines. 2013;4(1):80–9.
Nguyen N-T, Hejazian M, Ooi CH, Kashaninejad N. Recent advances and future perspectives on microfluidic liquid handling. Micromachines. 2017;8(6):186.
Sattari A, Hanafizadeh P, Hoorfar M. Multiphase flow in microfluidics: from droplets and bubbles to the encapsulated structures. Adv Coll Interface Sci. 2020;282: 102208.
Cai H, Zhou C, Zhang Y, Yang Y, Ren T, Guo C, Liu J. Saw based mass-loading biosensor for dna detection. In: 2013 IEEE International Conference of Electron Devices and Solid-state Circuits, 2013;pp. 1–2. IEEE.
Pang Y, Lu Y, Wang X, Liu Z. Droplet behavior and its effects on flow characteristics in t-junction microchannels. Phys Fluids. 2021;33(6).
Zhang JM, Ji Q, Duan H. Three-dimensional printed devices in droplet microfluidics. Micromachines. 2019;10(11):754.
Ai Y, **e R, **ong J, Liang Q. Microfluidics for biosynthesizing: from droplets and vesicles to artificial cells. Small. 2020;16(9):1903940.
Mark D, Haeberle S, Roth G, Von Stetten F, Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Microfluidics based microsystems: fundamentals and applications, 2010;305–376.
Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, Maoz BM, Jeanty SS, Somayaji MR, Burt M, et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng. 2020;4(4):407–20.
Vangunten MT, Walker UJ, Do HG, Knust KN. 3d-printed microfluidics for hands-on undergraduate laboratory experiments. J Chem Educ. 2019;97(1):178–83.
Wang G, Yang F, Zhao W. There can be turbulence in microfluidics at low Reynolds number. Lab Chip. 2014;14(8):1452–8.
Campbell LA, Kandlikar S. Effect of entrance condition on frictional losses and transition to turbulence in minichannel flows. In: International Conference on Nanochannels, Microchannels, and Minichannels, 2004;vol. 41642, pp. 229–235.
Kumar S, Bhushan P, Bhattacharya S. Fluid transport mechanisms in paper-based microfluidic devices. Paper Microfluidics: Theory and Applications, 2019;7–28.
Oyama ST, Stagg-Williams SM. Inorganic polymeric and composite membranes: structure, function and other correlations vol. 14. Elsevier, 2011.
Brody JP, Yager P, Goldstein RE, Austin RH. Biotechnology at low Reynolds numbers. Biophys J. 1996;71(6):3430–41.
Zhang C, Oostrom M, Wietsma TW, Grate JW, Warner MG. Influence of viscous and capillary forces on immiscible fluid displacement: pore-scale experimental study in a water-wet micromodel demonstrating viscous and capillary fingering. Energy Fuels. 2011;25(8):3493–505.
Birovljev A, Furuberg L, Feder J, Jssang T, Mly K, Aharony A. Gravity invasion percolation in two dimensions: experiment and simulation. Phys Rev Lett. 1991;67(5):584.
Chen Q, Srebric J. A procedure for verification, validation, and reporting of indoor environment cfd analyses. Hvac &R Res. 2002;8(2):201–16.
Peakall J, Warburton J. Surface tension in small hydraulic river models-the significance of the weber number. J Hydrol (New Zealand). 1996;199–212.
Castrejón-Pita AA, Castrejón-Pita J, Hutchings I. Breakup of liquid filaments. Phys Rev Lett. 2012;108(7): 074506.
Thornton ST, Marion JB. Classical dynamics of particles and systems. Cengage Learning; 2021.
Rapp BE. Microfluidics: modeling, mechanics and mathematics. Elsevier; 2022.
Beam RM, Warming R. An implicit factored scheme for the compressible Navier-stokes equations. AIAA J. 1978;16(4):393–402.
Takken M, Wille R. Simulation of pressure-driven and channel-based microfluidics on different abstract levels: a case study. Sensors. 2022;22(14):5392.
Stone HA. Introduction to fluid dynamics for microfluidic flows. CMOS biotechnol. 2007;5–30.
Hu G, Li D. Multiscale modeling and numerical simulations. Springer; 2008.
Elliott JR, Lira CT, Lira CT. Introductory Chemical Engineering Thermodynamics vol. 668. Prentice Hall Upper Saddle River, NJ, 2012.
Azimi AH. An introduction to hydraulic structures. In: Water engineering modeling and mathematic tools, pp. 297–342. Elsevier, 2021.
Bairaktarova D, Eodice M. Thermodynamics in high rhythms and rhymes: creative ways of knowing in engineering. AEE J. 2017;6(2).
Gergely A, Néda Z. Computational fluid dynamics approach for oscillating and interacting convective flows. Fluids. 2022;7(11):339.
Shen F, Ai M, Li Z, Lu X, Pang Y, Liu Z. Pressure measurement methods in microchannels: advances and applications. Microfluid Nanofluid. 2021;25:1–31.
Kulkarni MB, Ayachit NH, Aminabhavi TM. Biosensors and microfluidic biosensors: from fabrication to application. Biosensors. 2022;12(7):543.
Ren K, Zhou J, Wu H. Materials for microfluidic chip fabrication. Acc Chem Res. 2013;46(11):2396–406.
Liu X, Lin B. Materials used in microfluidic devices. Encyclopedia of Microfluidics and Nanofluidics. New York: Springer; 2008. p. 1065–8.
Fu L-M, Ju W-J, Yang R-J, Wang Y-N. Rapid prototy** of glass-based microfluidic chips utilizing two-pass defocused co 2 laser beam method. Microfluid Nanofluid. 2013;14:479–87.
Iliescu C, Taylor H, Avram M, Miao J, Franssila S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics. 2012;6(1): 016505.
Sainiemi L, Nissilä T, Kostiainen R, Ketola RA, Franssila S. A microfabricated silicon platform with 60 microfluidic chips for rapid mass spectrometric analysis. Lab Chip. 2011;11(17):3011–4.
Chen C, Mehl BT, Munshi AS, Townsend AD, Spence DM, Martin RS. 3d-printed microfluidic devices: fabrication, advantages and limitations-a mini review. Anal Methods. 2016;8(31):6005–12.
Annabestani M, Shaegh AM, Esmaeili-Dokht P, Fardmanesh M. An intelligent machine learning-based sheath-free microfluidic impedance flow cytometer. In: 2020 10th International Conference on Computer and Knowledge Engineering (ICCKE), 2020;pp. 284–288. IEEE.
Ali S, Hassan A, Hassan G, Eun C-H, Bae J, Lee CH, Kim I-J. Disposable all-printed electronic biosensor for instantaneous detection and classification of pathogens. Sci Rep. 2018;8(1):5920.
Kulkarni MB, Goel S. Miniaturized dna amplification platform with soft-lithographically fabricated continuous-flow pcr microfluidic device on a portable temperature controller. Microfluid Nanofluid. 2021;25(8):69.
Ballacchino G, Weaver E, Mathew E, Dorati R, Genta I, Conti B, Lamprou DA. Manufacturing of 3d-printed microfluidic devices for the synthesis of drug-loaded liposomal formulations. Int J Mol Sci. 2021;22(15):8064.
Kulkarni MB, Goel S. Recent advancements in integrated microthermofluidic systems for biochemical and biomedical applications-a review. Sens Actuators, A. 2022;341: 113590.
Lee D-S, Park SH, Chung KH, Pyo H-B. A disposable plastic-silicon micro pcr chip using flexible printed circuit board protocols and its application to genomic dna amplification. IEEE Sens J. 2008;8(5):558–64.
Felix FS, Baccaro AL, Angnes L. Disposable voltametric immunosensors integrated with microfluidic platforms for biomedical, agricultural and food analyses: A review. Sensors. 2018;18(12):4124.
Das S, Srivastava VC. Microfluidic-based photocatalytic microreactor for environmental application: a review of fabrication substrates and techniques, and operating parameters. Photochem Photobiol Sci. 2016;15(6):714–30.
Luka G, Ahmadi A, Najjaran H, Alocilja E, DeRosa M, Wolthers K, Malki A, Aziz H, Althani A, Hoorfar M. Microfluidics integrated biosensors: a leading technology towards lab-on-a-chip and sensing applications. Sensors. 2015;15(12):30011–31.
Tavakoli H, Zhou W, Ma L, Perez S, Ibarra A, Xu F, Zhan S, Li X. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. TrAC, Trends Anal Chem. 2019;117:13–26.
Zhang R, Duan X, Zhang S, Guo W, Sun C, Han Z. Tunable microfluidic chip for single-cell deformation study. Nanotechnol Precision Eng. 2023;6(2): 023003.
Fernandes AC, Semenova D, Grundtvig IP. Microfluidic devices and their applicability to cell studies. In: Microfluidics for Cellular Applications, 2023;pp. 27–118. Elsevier.
Mehling M, Tay S. Microfluidic cell culture. Curr Opin Biotechnol. 2014;25:95–102.
Kellogg RA, Gómez-Sjöberg R, Leyrat AA, Tay S. High-throughput microfluidic single-cell analysis pipeline for studies of signaling dynamics. Nat Protoc. 2014;9(7):1713–26.
Rakszewska A, Tel J, Chokkalingam V, Huck WT. One drop at a time: toward droplet microfluidics as a versatile tool for single-cell analysis. NPG Asia Mater. 2014;6(10):133–133.
Joensson HN, Andersson Svahn H. Droplet microfluidics-a tool for single-cell analysis. Angew Chem Int Ed. 2012;51(49):12176–92.
Chen P, Chen D, Li S, Ou X, Liu B-F. Microfluidics towards single cell resolution protein analysis. TrAC, Trends Anal Chem. 2019;117:2–12.
Sher M, Asghar W. Development of a multiplex fully automated assay for rapid quantification of cd4+ t cells from whole blood. Biosens Bioelectron. 2019;142: 111490.
Fennell RD, Sher M, Asghar W. Development of a microfluidic device for cd4+ t cell isolation and automated enumeration from whole blood. Biosensors. 2021;12(1):12.
Kanakasabapathy MK, Pandya HJ, Draz MS, Chug MK, Sadasivam M, Kumar S, Etemad B, Yogesh V, Safavieh M, Asghar W, et al. Rapid, label-free cd4 testing using a smartphone compatible device. Lab Chip. 2017;17(17):2910–9.
Luo T, Fan L, Zhu R, Sun D. Microfluidic single-cell manipulation and analysis: methods and applications. Micromachines. 2019;10(2):104.
Gascoyne PR, Vykoukal JV. Dielectrophoresis-based sample handling in general-purpose programmable diagnostic instruments. Proc IEEE. 2004;92(1):22–42.
Gossett DR, Weaver WM, Mach AJ, Hur SC, Tse HTK, Lee W, Amini H, Di Carlo D. Label-free cell separation and sorting in microfluidic systems. Anal Bioanal Chem. 2010;397:3249–67.
Rozand C. based analytical devices for point-of-care infectious disease testing. Eur J Clin Microbiol Infect Dis. 2014;33:147–56.
Cui P, Wang S. Application of microfluidic chip technology in pharmaceutical analysis: a review. J Pharm Anal. 2019;9(4):238–47.
Ma C, Peng Y, Li H, Chen W. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol Sci. 2021;42(2):119–33.
Ayuso JM, Virumbrales-Muñoz M, Lang JM, Beebe DJ. A role for microfluidic systems in precision medicine. Nat Commun. 2022;13(1):3086.
Zeng Y, Wang L, Wu S-Y, He J, Qu J, Li X, Ho H-P, Gu D, Gao BZ, Shao Y. Wavelength-scanning spr imaging sensors based on an acousto-optic tunable filter and a white light laser. Sensors. 2017;17(1):90.
Chen L, Manz A, Day PJ. Total nucleic acid analysis integrated on microfluidic devices. Lab Chip. 2007;7(11):1413–23.
Yeo LY, Chang H-C, Chan PP, Friend JR. Microfluidic devices for bioapplications. Small. 2011;7(1):12–48.
Hua Z, Rouse JL, Eckhardt AE, Srinivasan V, Pamula VK, Schell WA, Benton JL, Mitchell TG, Pollack MG. Multiplexed real-time polymerase chain reaction on a digital microfluidic platform. Anal Chem. 2010;82(6):2310–6.
Zhu H, Zhang H, Ni S, Korabečná M, Yobas L, Neuzil P. The vision of point-of-care pcr tests for the covid-19 pandemic and beyond. TrAC, Trends Anal Chem. 2020;130: 115984.
Jain KK. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn. 2003;3(2):153–61.
Jain KK. The role of nanobiotechnology in drug discovery. Drug Discovery Today. 2005;10(21):1435–42.
Ziober BL, Mauk MG, Falls EM, Chen Z, Ziober AF, Bau HH. Lab-on-a-chip for oral cancer screening and diagnosis. Head Neck J Sci Specialties Head Neck. 2008;30(1):111–21.
Jayamohan H, Romanov V, Li H, Son J, Samuel R, Nelson J, Gale BK. Advances in microfluidics and lab-on-a-chip technologies. Mol Diagn, 2017;197–217.
Nahavandi S, Baratchi S, Soffe R, Tang S-Y, Nahavandi S, Mitchell A, Khoshmanesh K. Microfluidic platforms for biomarker analysis. Lab Chip. 2014;14(9):1496–514.
Kadimisetty K, Song J, Doto AM, Hwang Y, Peng J, Mauk MG, Bushman FD, Gross R, Jarvis JN, Liu C. Fully 3d printed integrated reactor array for point-of-care molecular diagnostics. Biosens Bioelectron. 2018;109:156–63.
Wang X, Hong X-Z, Li Y-W, Li Y, Wang J, Chen P, Liu B-F. Microfluidics-based strategies for molecular diagnostics of infectious diseases. Mil Med Res. 2022;9(1):1–27.
Ganguli A, Mostafa A, Berger J, Aydin MY, Sun F, Ramirez SAS, Valera E, Cunningham BT, King WP, Bashir R. Rapid isothermal amplification and portable detection system for sars-cov-2. Proc Natl Acad Sci. 2020;117(37):22727–35.
Zhang H, Xu Y, Fohlerova Z, Chang H, Iliescu C, Neuzil P. Lamp-on-a-chip: revising microfluidic platforms for loop-mediated dna amplification. TrAC, Trends Anal Chem. 2019;113:44–53.
Yu H, Alkhamis O, Canoura J, Liu Y, **ao Y. Advances and challenges in small-molecule dna aptamer isolation, characterization, and sensor development. Angew Chem Int Ed. 2021;60(31):16800–23.
Ramnauth N, Neubarth E, Makler-Disatham A, Sher M, Soini S, Merk V, Asghar W. Development of a microfluidic device for exosome isolation in point-of-care settings. Sensors. 2023;23(19):8292.
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9: 811971.
Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A, Hsu E-C, Gowrishankar G, Kanada M, Jokerst JV, et al. The exosome total isolation chip. ACS Nano. 2017;11(11):10712–23.
Burns MA, Johnson BN, Brahmasandra SN, Handique K, Webster JR, Krishnan M, Sammarco TS, Man PM, Jones D, Heldsinger D, et al. An integrated nanoliter dna analysis device. Science. 1998;282(5388):484–7.
Shang Y, Sun J, Ye Y, Zhang J, Zhang Y, Sun X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit Rev Food Sci Nutr. 2020;60(2):201–24.
Streets AM, Zhang X, Cao C, Pang Y, Wu X, **ong L, Yang L, Fu Y, Zhao L, Tang F, et al. Microfluidic single-cell whole-transcriptome sequencing. Proc Natl Acad Sci. 2014;111(19):7048–53.
Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary dna microarray. Science. 1995;270(5235):467–70.
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, et al. Nucleic acid detection with crispr-cas13a/c2c2. Science. 2017;356(6336):438–42.
**ang Y, Lu Y. Using personal glucose meters and functional dna sensors to quantify a variety of analytical targets. Nat Chem. 2011;3(9):697–703.
Corstjens PL, De Dood CJ, Kornelis D, Fat EMTK, Wilson RA, Kariuki TM, Nyakundi RK, Loverde PT, Abrams WR, Tanke HJ, et al. Tools for diagnosis, monitoring and screening of schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141(14):1841–55.
Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for covid-19. Am J Infect Control. 2021;49(1):21–9.
Dorward DW, Schwan T, Garon CF. Immune capture and detection of borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J Clin Microbiol. 1991;29(6):1162–70.
Domanguez J, Gal N, Blanco S, Pedroso P, Prat C, Matas L, Ausina V. Detection of streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest. 2001;119(1):243–9.
Hunter P. Novel diagnostic technologies for clinical and frontline use: advanced diagnostics based on molecular markers and analysis technologies has been improving diagnosis across a wide range of diseases. EMBO Rep. 2017;18(6):881–4.
Streckfus C, Bigler L. Saliva as a diagnostic fluid. Oral Dis. 2002;8(2):69–76.
St John A, Price CP. Existing and emerging technologies for point-of-care testing. Clin Biochemist Rev. 2014;35(3):155.
Bai Z, Wei H, Yang X, Zhu Y, Peng Y, Yang J, Wang C, Rong Z, Wang S. Rapid enrichment and ultrasensitive detection of influenza a virus in human specimen using magnetic quantum dot nanobeads based test strips. Sens Actuators, B Chem. 2020;325: 128780.
Kumar A, Parihar A, Panda U, Parihar DS. Microfluidics-based point-of-care testing (poct) devices in dealing with waves of covid-19 pandemic: the emerging solution. ACS Appl Bio Mater. 2022;5(5):2046–68.
Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE. Point of care diagnostics: status and future. Anal Chem. 2012;84(2):487–515.
Lequin RM. Enzyme immunoassay (eia)/enzyme-linked immunosorbent assay (elisa). Clin Chem. 2005;51(12):2415–8.
Reen DJ. Enzyme-linked immunosorbent assay (elisa). Basic Protein and Peptide Protocols, 1994;461–466.
Gan SD, Patel KR, et al. Enzyme immunoassay and enzyme-linked immunosorbent assay. J Invest Dermatol. 2013;133(9):12.
Konstantinou GN. Enzyme-linked immunosorbent assay (elisa). Food Allergens: Methods and Protocols, 2017;79–94.
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (rdt). Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77 (6) of American Journal of Tropical Medicine and Hygiene. 2007.
Mouatcho JC, Goldring JD. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62(10):1491–505.
Drain PK. Rapid diagnostic testing for sars-cov-2. N Engl J Med. 2022;386(3):264–72.
Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza a (h5n1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37(4):937–43.
Patra A, Herrera M, Gutiérrez JM, Mukherjee AK. The application of laboratory-based analytical tools and techniques for the quality assessment and improvement of commercial antivenoms used in the treatment of snakebite envenomation. Drug Test Anal. 2021;13(8):1471–89.
Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, et al. Microfluidics-based diagnostics of infectious diseases in the develo** world. Nat Med. 2011;17(8):1015–9.
Dittrich PS, Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discovery. 2006;5(3):210–8.
Homola J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem Rev. 2008;108(2):462–93.
Weeks BL, Camarero J, Noy A, Miller A, De Yoreo J, Stanker L. A microcantilever-based pathogen detector. Scanning J Scanning Microscopies. 2003;25(6):297–9.
Bakker E, Telting-Diaz M. Electrochemical sensors. Anal Chem. 2002;74(12):2781–800.
McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci. 2013;110(9):3229–36.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20(2):101–24.
Wimbles R, Melling LM, Cain B, Davies N, Doherty J, Johnson B, Shaw KJ. On-site genetic analysis for species identification using lab-on-a-chip. Ecol Evol. 2021;11(4):1535–43.
Kaigala G, Behnam M, Bidulock A, Bargen C, Johnstone R, Elliott D, Backhouse C. A scalable and modular lab-on-a-chip genetic analysis instrument. Analyst. 2010;135(7):1606–17.
Mora-Castilla S, To C, Vaezeslami S, Morey R, Srinivasan S, Dumdie JN, Cook-Andersen H, Jenkins J, Laurent LC. Miniaturization technologies for efficient single-cell library preparation for next-generation sequencing. J Lab Autom. 2016;21(4):557–67.
Brennecke P, Anders S, Kim JK, Kołodziejczyk AA, Zhang X, Proserpio V, Baying B, Benes V, Teichmann SA, Marioni JC, et al. Accounting for technical noise in single-cell rna-seq experiments. Nat Methods. 2013;10(11):1093–5.
Ahrberg CD, Manz A, Chung BG. Polymerase chain reaction in microfluidic devices. Lab Chip. 2016;16(20):3866–84.
Perkins G, Lu H, Garlan F, Taly V. Droplet-based digital pcr: application in cancer research. Adv Clin Chem. 2017;79:43–91.
Fankhauser R, Chang M, Garrison Z, Berryman R, Lucero OM, Fuiten A, DePatie N, Seifert H, Kulkarni RP. Single-cell identification of melanoma biomarkers in circulating tumor cells. Cancers. 2022;14(19):4921.
Bonadio RS, Alessio E, Cagnin S. Long non-coding rnas in a single-cell type: function and subcellular localization. Chem Biol Long Noncoding RNAs. 2020;103–129.
Di Carlo D, Irimia D, Tompkins RG, Toner M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc Natl Acad Sci. 2007;104(48):18892–7.
Huh D, Hamilton GA, Ingber DE. From 3d cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745–54.
Ranga A, Lutolf MP. High-throughput approaches for the analysis of extrinsic regulators of stem cell fate. Curr Opin Cell Biol. 2012;24(2):236–44.
Dittrich PS, Tachikawa K, Manz A. Micro total analysis systems latest advancements and trends. Anal Chem. 2006;78(12):3887–908.
Gach PC, Iwai K, Kim PW, Hillson NJ, Singh AK. Droplet microfluidics for synthetic biology. Lab Chip. 2017;17(20):3388–400.
Maeki M, Yamaguchi H, Tokeshi M, Miyazaki M. Microfluidic approaches for protein crystal structure analysis. Anal Sci. 2016;32(1):3–9.
Busin V, Wells B, Kersaudy-Kerhoas M, Shu W, Burgess ST. Opportunities and challenges for the application of microfluidic technologies in point-of-care veterinary diagnostics. Mol Cell Probes. 2016;30(5):331–41.
Verma N, Kulkarni R, Pandya A. Microfluidic tools for veterinary and zoonotic disease diagnostics. Prog Mol Biol Transl Sci. 2022;187(1):281–93.
Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32(7):347–50.
Everitt ML, Tillery A, David MG, Singh N, Borison A, White IM. A critical review of point-of-care diagnostic technologies to combat viral pandemics. Anal Chim Acta. 2021;1146:184–99.
Thrall MA, Weiser G, Allison RW, Campbell TW. Veterinary Hematology and Clinical Chemistry. John Wiley & Sons; 2012.
Wang L, Xu W, Wang B, Si X, Li S. Methods and advances in the design, testing and development of in vitro diagnostic instruments. Processes. 2023;11(2):403.
Upadhyay A, Waleed RM, Wang J, Zhao J, Guan Q, Liao C, Han Q. An innovative and user-friendly smartphone-assisted molecular diagnostic approach for rapid detection of canine vector-borne diseases. Parasitol Res. 2021;120:1799–809.
Oeschger TM, McCloskey DS, Buchmann RM, Choubal AM, Boza JM, Mehta S, Erickson D. Early warning diagnostics for emerging infectious diseases in develo** into late-stage pandemics. Acc Chem Res. 2021;54(19):3656–66.
Velayudhan B, Naikare H. Point-of-care testing in companion and food animal disease diagnostics. Front Vet Sci. 2022;9.
Ramdzan AN, Almeida MIG, McCullough MJ, Kolev SD. Development of a microfluidic paper-based analytical device for the determination of salivary aldehydes. Anal Chim Acta. 2016;919:47–54.
Cromwell EF, Leung M, Hammer M, Thai A, Rajendra R, Sirenko O. Disease modeling with 3d cell-based assays using a novel flowchip system and high-content imaging. SLAS TECHNOLOGY: Transl Life Sci Innov. 2021;26(3):237–48.
Wu W, Liu L, Dai Z, Liu J, Yang S, Zhou L, **ao X, Jiang C, Roy VA. Low-cost, disposable, flexible and highly reproducible screen printed sers substrates for the detection of various chemicals. Sci Rep. 2015;5(1):1–10.
Tran DH, Phung HTT. Detecting fasciola hepatica and fasciola gigantica micrornas with loop-mediated isothermal amplification (lamp). J Parasit Dis. 2020;44(2):364–73.
Neethirajan S, Ragavan V, Weng X, Chand R. Biosensors for sustainable food engineering: challenges and perspectives. Biosensors. 2018;8(1):23.
Abdulla H, Zamorano M, Rodríguez ML, El Shahawy A, Hosny S, Martín-Pascual J, El-Shatoury S. An overview of agro-food industry wastewater treatment: a bibliometric analysis and literature review. Appl Water Sci. 2023;13(2):47.
Weng X, Neethirajan S. Ensuring food safety: quality monitoring using microfluidics. Trends Food Sci Technol. 2017;65:10–22.
Fung F, Wang H-S, Menon S. Food safety in the 21st century. Biomed J. 2018;41(2):88–95.
Wu MY-C, Hsu M-Y, Chen S-J, Hwang D-K, Yen T-H, Cheng C-M. Point-of-care detection devices for food safety monitoring: proactive disease prevention. Trends Biotechnol. 2017;35(4):288–300.
Sohrabi H, Hemmati A, Majidi MR, Eyvazi S, Jahanban-Esfahlan A, Baradaran B, Adlpour-Azar R, Mokhtarzadeh A, Guardia M. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. TrAC, Trends Anal Chem. 2021;143: 116344.
Shenashen MA, Emran MY, El Sabagh A, Selim MM, Elmarakbi A, El-Safty SA. Progress in sensory devices of pesticides, pathogens, coronavirus, and chemical additives and hazards in food assessment: Food safety concerns. Prog Mater Sci. 2022;124: 100866.
Martzy R, Kolm C, Krska R, Mach RL, Farnleitner AH, Reischer GH. Challenges and perspectives in the application of isothermal dna amplification methods for food and water analysis. Anal Bioanal Chem. 2019;411:1695–702.
Geissler M, Brassard D, Clime L, Pilar AVC, Malic L, Daoud J, Barrère V, Luebbert C, Blais BW, Corneau N, et al. Centrifugal microfluidic lab-on-a-chip system with automated sample lysis, dna amplification and microarray hybridization for identification of enterohemorrhagic escherichia coli culture isolates. Analyst. 2020;145(21):6831–45.
Giuffrida MC, Spoto G. Integration of isothermal amplification methods in microfluidic devices: recent advances. Biosens Bioelectron. 2017;90:174–86.
Zhang C, Xu J, Ma W, Zheng W. Pcr microfluidic devices for dna amplification. Biotechnol Adv. 2006;24(3):243–84.
Qian C, Wang R, Wu H, ** J, Wu J. Recent advances in emerging dna-based methods for genetically modified organisms (gmos) rapid detection. TrAC, Trends Anal Chem. 2018;109:19–31.
Sun Y, Kwok Y-C, Foo-Peng Lee P, Nguyen N-T. Rapid amplification of genetically modified organisms using a circular ferrofluid-driven pcr microchip. Anal Bioanal Chem. 2009;394:1505–8.
Kaygusuz D, Vural S, Aytekin AÖ, Lucas SJ, Elitas M. Daimondna: a portable, low-cost loop-mediated isothermal amplification platform for naked-eye detection of genetically modified organisms in resource-limited settings. Biosens Bioelectron. 2019;141: 111409.
Li J, Li J, Lin S, Zhu L, Li X, Xu W. Advanced technologies in on-site detection of genetically modified products. Agriculture. 2022;12(6):888.
Scognamiglio V, Arduini F, Palleschi G, Rea G. Biosensing technology for sustainable food safety. TrAC, Trends Anal Chem. 2014;62:1–10.
Yu Z, Jung D, Park S, Hu Y, Huang K, Rasco BA, Wang S, Ronholm J, Lu X, Chen J. Smart traceability for food safety. Crit Rev Food Sci Nutr. 2022;62(4):905–16.
Nilghaz A, Mousavi SM, Li M, Tian J, Cao R, Wang X. based microfluidics for food safety and quality analysis. Trends Food Sci Technol. 2021;118:273–84.
Weston M, Geng S, Chandrawati R. Food sensors: challenges and opportunities. Adv Mater Technol. 2021;6(5):2001242.
Sabui P, Mallick S, Singh KR, Natarajan A, Verma R, Singh J, Singh RP. Potentialities of fluorescent carbon nanomaterials as sensor for food analysis. Luminescence. 2023;38(7):1047–63.
Jayawardane BM, Wei S, McKelvie ID, Kolev SD. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal Chem. 2014;86(15):7274–9.
Nakhlband A, Kholafazad-Kordasht H, Rahimi M, Mokhtarzadeh A, Soleymani J. Applications of magnetic materials in the fabrication of microfluidic-based sensing systems: recent advances. Microchem J. 2022;173: 107042.
Curulli A. Recent advances in electrochemical sensing strategies for food allergen detection. Biosensors. 2022;12(7):503.
Shi L, Li Y, Jia C, Shan J, Wang S, Liu S, Sun J, Zhang D, Ji Y, Wang J. An overview of fluorescent microfluidics into revealing the mystery of food safety analysis: mechanisms and recent applications. Trends Food Sci Technol. 2023.
Luo T, Li L, Wang S, Cheng N. Research progress of nucleic acid detection technology for genetically modified maize. Int J Mol Sci. 2023;24(15):12247.
Krokhine S, Torabi H, Doostmohammadi A, Rezai P. Conventional and microfluidic methods for airborne virus isolation and detection. Colloids Surf B. 2021;206: 111962.
Yang Y, Chen Y, Tang H, Zong N, Jiang X. Microfluidics for biomedical analysis. Small Methods. 2020;4(4):1900451.
Kaushik AM, Hsieh K, Wang T-H. Droplet microfluidics for high-sensitivity and high-throughput detection and screening of disease biomarkers. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(6):1522.
Hu X, Abbasi R, Wachsmann-Hogiu S. Microfluidics on lensless, semiconductor optical image sensors: challenges and opportunities for democratization of biosensing at the micro-and nano-scale. Nanophotonics. 2023;12(21):3977–4008.
Burger R, Amato L, Boisen A. Detection methods for centrifugal microfluidic platforms. Biosens Bioelectron. 2016;76:54–67.
Nath P, Kabir A, Khoubafarin Doust S, Kreais ZJ, Ray A. Detection of bacterial and viral pathogens using photonic point-of-care devices. Diagnostics. 2020;10(10):841.
Metsky HC, Welch NL, Pillai PP, Haradhvala NJ, Rumker L, Mantena S, Zhang YB, Yang DK, Ackerman CM, Weller J, et al. Designing sensitive viral diagnostics with machine learning. Nat Biotechnol. 2022;40(7):1123–31.
Sher M, Coleman B, Caputi M, Asghar W. Development of a point-of-care assay for hiv-1 viral load using higher refractive index antibody-coated microbeads. Sensors. 2021;21(5):1819.
Seok Y, Yin Q, Bai H, Bau HH. Sensitive, single-pot, two-stage assay for hepatitis viruses. Anal Chem. 2022;94(3):1778–86.
GhaderiShekhiAbadi P, Irani M, Noorisepehr M, Maleki A. Magnetic biosensors for identification of sars-cov-2, influenza, hiv, and ebola viruses: a review. Nanotechnology. 2023;34(27): 272001.
Khanthaphixay B, Wu L, Yoon J-Y. Microparticle-based detection of viruses. Biosensors. 2023;13(8):820.
Bates KE, Lu H. Optics-integrated microfluidic platforms for biomolecular analyses. Biophys J. 2016;110(8):1684–97.
Pamme N. Continuous flow separations in microfluidic devices. Lab Chip. 2007;7(12):1644–59.
Fair RB, Khlystov A, Tailor TD, Ivanov V, Evans RD, Srinivasan V, Pamula VK, Pollack MG, Griffin PB, Zhou J. Chemical and biological applications of digital-microfluidic devices. IEEE Design Test Comput. 2007;24(1):10–24.
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent developments in paper-based microfluidic devices. Anal Chem. 2015;87(1):19–41.
Zhang B, Radisic M. Organ-on-a-chip devices advance to market. Lab Chip. 2017;17(14):2395–420.
Lim YC, Kouzani AZ, Duan W. Lab-on-a-chip: a component view. Microsyst Technol. 2010;16:1995–2015.
Battat S, Weitz DA, Whitesides GM. An outlook on microfluidics: the promise and the challenge. Lab Chip. 2022;22(3):530–6.
Hitzbleck M, Delamarche E. Reagents in microfluidics: an ‘in’and ‘out’challenge. Chem Soc Rev. 2013;42(21):8494–516.
Kaminski TS, Scheler O, Garstecki P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip. 2016;16(12):2168–87.
Belotti Y, Lim CT. Microfluidics for liquid biopsies: recent advances, current challenges, and future directions. Anal Chem. 2021;93(11):4727–38.
Ashley BK, Hassan U. Point-of-critical-care diagnostics for sepsis enabled by multiplexed micro and nano sensing technologies. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(5):1701.
Munawar A, Ong Y, Schirhagl R, Tahir MA, Khan WS, Bajwa SZ. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019;9(12):6793–803.
Coskun AF, Topkaya SN, Yetisen AK, Cetin AE. Portable multiplex optical assays. Adv Opt Mater. 2019;7(4):1801109.
Liao Z, Zhang Y, Li Y, Miao Y, Gao S, Lin F, Deng Y, Geng L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens Bioelectron. 2019;126:697–706.
Chen T, Yin S, Wu J. Nanomaterials meet microfluidics: improved analytical methods and high-throughput synthetic approaches. TrAC, Trends Anal Chem. 2021;142: 116309.
Fattahi Z, Hasanzadeh M. Nanotechnology-assisted microfluidic systems for chemical sensing, biosensing, and bioanalysis. TrAC, Trends Anal Chem. 2022;152: 116637.
Welch EC, Powell JM, Clevinger TB, Fairman AE, Shukla A. Advances in biosensors and diagnostic technologies using nanostructures and nanomaterials. Adv Func Mater. 2021;31(44):2104126.
Barbosa AI, Rebelo R, Reis RL, Bhattacharya M, Correlo VM. Current nanotechnology advances in diagnostic biosensors. Med Devices Sens. 2021;4(1):10156.
Hosic S, Murthy SK, Koppes AN. Microfluidic sample preparation for single cell analysis. Anal Chem. 2016;88(1):354–80.
Ríos Á, Zougagh M, Avila M. Miniaturization through lab-on-a-chip: utopia or reality for routine laboratories? A review. Anal Chim Acta. 2012;740:1–11.
Li X, Pak HS, Huber F, Michaux J, Taillandier-Coindard M, Altimiras ER, Bassani-Sternberg M. A microfluidics-enabled automated workflow of sample preparation for ms-based immunopeptidomics. Cell Reports Methods. 2023;3(6).
Raji H, Tayyab M, Sui J, Mahmoodi SR, Javanmard M. Biosensors and machine learning for enhanced detection, stratification, and classification of cells: a review. Biomed Microdevice. 2022;24(3):26.
Galan EA, Zhao H, Wang X, Dai Q, Huck WT, Ma S. Intelligent microfluidics: the convergence of machine learning and microfluidics in materials science and biomedicine. Matter. 2020;3(6):1893–922.
Ma X, Guo G, Wu X, Wu Q, Liu F, Zhang H, Shi N, Guan Y. Advances in integration, wearable applications, and artificial intelligence of biomedical microfluidics systems. Micromachines. 2023;14(5):972.
Rodrigues JF, Florea L, Oliveira MC, Diamond D, Oliveira ON. Big data and machine learning for materials science. Discover Mater. 2021;1:1–27.
Zarei M. Portable biosensing devices for point-of-care diagnostics: recent developments and applications. TrAC, Trends Anal Chem. 2017;91:26–41.
Khandurina J, Guttman A. Bioanalysis in microfluidic devices. J Chromatogr A. 2002;943(2):159–83.
Araz MK, Tentori AM, Herr AE. Microfluidic multiplexing in bioanalyses. J Lab Autom. 2013;18(5):350–66.
Berlanda SF, Breitfeld M, Dietsche CL, Dittrich PS. Recent advances in microfluidic technology for bioanalysis and diagnostics. Anal Chem. 2020;93(1):311–31.
He Y, Wu Y, Fu J-Z, Gao Q, Qiu J-J. Developments of 3d printing microfluidics and applications in chemistry and biology: a review. Electroanalysis. 2016;28(8):1658–78.
Au AK, Huynh W, Horowitz LF, Folch A. 3d-printed microfluidics. Angew Chem Int Ed. 2016;55(12):3862–81.
Sekhwama M, Mpofu K, Mthunzi-Kufa P. Using 3d printing to fabricate microfluidic chips for biosensing applications. In: MATEC Web of Conferences, 2023;vol. 388, p. 05001. EDP Sciences.
Funding
The authors acknowledge the Council for Scientific and Industrial Research (CSIR) and the Department of Science and Innovation (DSI) for funding granted for this research. K.M was also supported by the CSIR’s Young Researchers Establishment Fund (YREFKM1).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval
This declaration is not applicable.
Competing interests
The authors declare that they have no Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sekhwama, M., Mpofu, K., Sivarasu, S. et al. Applications of microfluidics in biosensing. Discov Appl Sci 6, 303 (2024). https://doi.org/10.1007/s42452-024-05981-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05981-4