Abstract
Mint essential oil has several applications in pharmacy and food industries. As mint species multiplied, active chemicals evolved, resulting in changes in their biological effects. Mint growth in Egypt’s sandy soils is hampered by abiotic stress. Phenols like turmeric curcumin reduce abiotic stress which plant suffers. Finding active chemicals in various aromatic plant species to substitute synthetic ones is an enticing approach to develo** a new pharmaceutical regimen; these plants need to be studied to locate active components. Therefore, this study aimed to reduce the potential negative effects of abiotic stress in sand soil on horsemint (Mentha longifolia) and spearmint (Mentha spicata L) by applying turmeric curcumin (as organic elicitor), to assess if growing them in the new reclamation zones is a viable option in order to obtain essential oil naturally. Both species received 0, 30, 60, 90, and 120 mg L−1 turmeric curcumin. Horsemint exposed to 30 mg L−1 turmeric curcumin produced the maximum values of plant length (93 cm), fresh herb (114.73 g plant−1) and dry herb (33.81 g plant−1); spearmint × 30 mg L−1 turmeric curcumin resulted in the greatest amounts of chlorophyll a, chlorophyll b and carotenoids with values of 5.38, 1.83 and 1.45 mg g−1, respectively. The maximum values of eucalyptol (65.44%), camphor (23.81%), carvone (65.95%) and limonene (15.9%) were recorded under 90 mg L−1 turmeric curcumin. Horsemint plants subjected to 60 mg L−1 turmeric curcumin gave the highest essential oil yield (1.52 g plant−1), sesquiterpenes (31.4%), soluble sugars (102.19 mg g−1), free amino acids (8.73 mg g−1), phenols (3.71 mg g−1), flavonoids (5.28 mg g−1), free radical's inhibition (64.35%), protein (17.09%) and nitrogen (1.27 g plant−1); spearmint × 60 mg L−1 turmeric curcumin resulted in the greatest values of monoterpenes (98.76%), nitrogen (2.74%), phosphorous (0.54% or 0.17 g plant−1) and potassium (0.56% or 0.18 g plant−1). Control × spearmint gave the maximum value of sodium (0.17% or 0.04 g plant−1). To mitigate the detrimental effects of stress-related factors on sandy soil, turmeric curcumin can be applied to mint species; additionally, it could broaden the sources of natural products. On the other hand, this work provides as a guide for choosing mint species for usage in industries connected to essential oils based on pertinent components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Economic benefits of herb cultivation have been proven for the production of food, cosmetics, drugs and colognes. They include a variety of secondary compounds with a range of biological functions; studies have demonstrated that, in contrast to chemically produced components, these natural compounds have no unfavorable health consequences on people. So, it is vital to increase the cultivation of medicinal and aromatic plant species in order to increase their output for a variety of uses [1]. Mint (Mentha sp.) is a member of the Lamiaceae family; its essential oil has been described as anti-inflammatory, carminative, antiemetic, diaphoretic, antispasmodic, analgesic, stimulating, emmenagogue, anticatarrhal, fungicide and antioxidant [2].
Despite the fact that there are several species of mint in Egypt, breeders need to monitor them carefully to expand the use of plants as a source of natural products and to prevent extinction. That is possible through plant breeding programmers and the development of gene banks [3]. Previous investigations have shown that different aromatic plant species in the same genus generate essential oils with a range of constituents; it therefore has an impact on their biological activity [4, 5]. The same genus of aromatic plant has several species present, which results in different essential oil provenances to flourish and expand as a naturally occurring substance used in many different contexts [6].
In recent years, natural elicitors will keep expanding owing to the requirement to use safe practices to increase the production of aromatic plants by employing ecologically friendly agricultural techniques [7]. One type of natural elicitor is phenolic compounds that occur as a result of plants' stress. It improves a plant's inbuilt defenses to boost nutrient uptake, efficiency and the plant’s ability to withstand stress [7]. It has a specific function in promoting physiological plant activity during critical development phases, such as bud development, root emergence, flowering and maturity; consequently, it has an impact on the production of essential oil and other components [8]. Turmeric curcumin is the main component of turmeric and a type of natural orange-yellow pigment, belongs to the natural phenol family and gives some plants their yellow color. Turmeric curcumin is a potent antioxidant; it has been shown to effectively limit the cellular environment’s formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). On the other hand, turmeric curcumin controls two first line enzymes of defense against oxygen free radicals (superoxide dismutase and glutathione peroxidase) [9]. There are a very few investigations have been done on the effect of turmeric curcumin on plant productivity. Turmeric curcumin substantially increased vegetative growth characteristics, photosynthetic pigments, secondary metabolites, and antioxidant enzyme activity in sunflowers followed by substantial reductions in proline concentration, MDA and H2O2 [10].
Egyptian mint develo** in sandy areas which stands out for having certain difficulties, such as soil and water salinity or water shortage, which influence production, including yield, essential oil and numerous chemical components. In an attempt to create a new medication regimen, one of the most alluring paths is to look for active ingredients in different species of aromatic plants to replace synthetic ones; these plants must be examined to find active components. Therefore, this experiment was carried out to ascertain the production of horsemint (Mentha longifolia) and spearmint (Mentha spicata L) as a source of natural products, which were all given different dosages of turmeric curcumin (biological elicitor) to assess the possibilities for expanding their cultivation in the new sandy reclamation zone, which is characterized by exposure to environmental stress factors.
2 Materials and methods
2.1 Experimental descriptions
The experiment was successfully carried out over two seasons in the greenhouse of the National Research Centre in the Egyptian Giza Governorate (Fig. 1). Both species of mint {horsemint (Mentha longifolia), and spearmint (Mentha spicata L)} had uniform seedlings, that were obtained from central administration for seed and seedling production, Egypt’s Ministry of Agriculture, located at Giza governorate. Throughout the first week of March in both seasons, both species’ seedlings were put into clay pots, with a height of 50 cm and a diameter of 30 cm; thereafter, pots were moved to a greenhouse with natural atmosphere. Each pot was filled with 10 kg of air-dried sandy soil (Table 1). Weak plants were taken out after 30 days, leaving three healthy plants per pot. Pots separated into two parts, each part of single specie was made. Horsemint and spearmint were treated with 0, 30, 60, 90, and 120 mg L−1 turmeric curcumin through foliar application. Turmeric curcumin was sprayed on the leaves twice with equal amounts of run-off as a foliar application. 45 days after the transplant, the first one happened, and the second one followed the first by 21 days. Turmeric curcumin was created in a 1L volumetric flask by blending a few drops of ethanol (C2H5OH) with 0.03, 0.06, 0.09, and 0.12 g of pure turmeric curcumin, then distilled water was added to make the solution the 1L volume. During the plants’ growing stage, the following changes were made to the surrounding atmosphere: Temp. (36/25 °C, max./min.), RH (70/37%, day/night), and light intensity ~ 3700 µmol m−2 s−1. It is important to note that all pots received spout water irrigation every two days. All agricultural procedures were carried out in accordance with the advice provided by the Egyptian Ministry of Agriculture. Each pot received the following fertilizers: 2 g of organic compost, phosphorus (2 g pot−1) as calcium super phosphate (15.5% P2O5), nitrogen (2 g pot−1) as ammonium sulphate (20.5% N) and potassium (1.5 g pot−1) as potassium sulphate (48% K2O). All plant diseases were treated with organic pesticides and weeds were plucked by hand.
2.2 Harvesting
Plant aerial parts were twice clipped off at a height of 5 cm above the soil surface in the growing seasons. After 75 days following the transplant, the first one occurred; whereas the second occurred 60 days after the first. Fresh and dry weights of aerial parts (g plant−1) were listed.
2.3 Assessment of photosynthetic pigments
Fresh leaves of various mint species were examined for photosynthetic pigments (Chlorophyll a, Chlorophyll b and total carotenoids) by using the AOAC's methodology [11]. Using 80% acetone, fresh leaf tissues were pulverized in a mortar and pestle. For chlorophyll a and b, the optical density of the solution was measured at 662 nm and 645 nm, respectively, whereas for carotenoids, it was measured at 470 nm by means of a spectrophotometer (Shimadzu UV–1700, Tokyo, Japan). Photosynthetic pigment levels were given as mg g−1 of fresh weight.
2.4 Separation of essential oils
From each treatment, air-dried aerial parts were collected; by using of a Clevenger-style apparatus, 120 g from every replicate (three replicates were used) of various treatments were hydro-distilled for 3 h [12]. Considering the hydro-distillation, in a round-bottomed flask of 2 L, one L of water was added to samples of air-dried aerial parts. In order to get essential oil, it was anticipated to be 100 °C. Clevenger-style equipment conducted a three-hour procedure (a time when the production of essential oils might end); steam caused the essential oil to evaporate. A condenser received the vapors from the steam-essential oil combination, Condensation occurred. Following that, the essential oil and water condensate were separated. Because essential oil was lighter, it was collected and is buoyant. Using weight/weight, essential oil (%) was estimated, whereas the dry weight of the aerial pieces was used to compute the essential oil production (g plant−1).
2.5 Investigation of essential oils
Gas chromatography (GC) (Fig. 2) was used to examine essential oils derived from various treatments using a dimethylsiloxane, 5% phenyl column (DB5). However, studies use GC/MS (gas chromatography/mass spectrometry) utilised the same GC-like parameters. Different essential oil components were assessed using the mass spectra of various essential oil constituents in comparison to an electronic library of NIST/NBS and Wiley 275; or by using real compounds, and confirmed by comparing their retention indices (RI) with those of the real compounds or information from the literature [13]. The essential oil's ingredients, presented as percentages.
2.6 Total soluble sugars determination
Total soluble sugars in dried mint leaves were determined using the colorimetric technique developed by Dubois [14]. The extract was created by homogenizing foliar tissues with 80% ethanol and evaluated using measurements of absorbance at 490 nm with a standard D-glucose solution.
2.7 Total free amino acids
Free amino acid concentration was calculated as mg g−1 dry weight [15]. With 10 ml of 80% ethanol, 500 mg of dried leaves were extracted. Each test tube received 0.5 ml of ethanol extract and 0.5 ml of distilled water; following that, 1 ml of ninhydrin reagent was added. The tubes were boiled in a water bath for 30 min before being cooled. 5 mL of 50% isopropylalchol was added to test tubes and the absorbance was measured in a UV-Spectrophotometer (JASCO V750) at 570 nm. To create a calibration curve, glutamic acid was employed as a standard amino acid.
2.8 Total phenols analysis
To assess the total phenols content of leaf samples, the Folin-Ciocalteu reagent was utilised [16]. A known weight of dry samples was obtained and extracted three times with 80% cold methanol (v/v). The homogenate was filtered using filter paper (Whatman No.1). The filtrate was then made up to a known volume with cold methanol. A known volume of extract (0.5 mL) was mixed with 0.5 mL Folin-Ciocalteu reagent. The mixture was let to stand for 3 min. 1 mL solution of saturated sodium carbonate (At 70–80 °C, 25 g Na2CO3 was dissolved in 1000 ml distilled water, which was then cooled and filtered) was incorporated into the mixture. The mixture was left to stand for one hour. The optical density was measured at 725 nm with a UV-spectrophotometer (BUCHI MODEL B 169, SWITZERLAND). Total phenols were estimated using the gallic acid standard curve (99.5%) and reported as mg (gallic acid) g−1 dry weight.
2.9 Total flavonoids analysis
Total flavonoids in leaves were assessed by using the colorimetric technique of aluminum chloride [17]. In summary, 50 µL of crude extract (1 mg mL−1 ethanol) were filled to a volume of 1 mL with methanol, combined with 4 mL distilled water combined with 4 mL distilled water; After 5 min, 0.3 mL of 10% AlCl3 solution was added., then the mixture was let to stand for 6 min. After that, 2 mL of 1 mol L−1 NaOH solution was added, with double-distilled water, the mixture’s ultimate volume was reduced to 10 mL. The mixture was left to stand for 15 min; the wavelength was then determined to be 510 nm. Total flavonoids content was calculated using a calibration curve; this was then stated as mg rutin equivalent g−1 dry weight.
2.10 Analysis of antioxidant activity (free radical scavenging)
The antioxidant activity of leaf extract was evaluated using the 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) test established by Middha [18]. In brief, 200 µL of each extract (100–500 µg/mL) was combined with 3.8 mL DPPH solution and incubated at room temperature for 1 h in the dark. The absorbance of the combination was measured at 517 nm. Ascorbic acid was employed as a positive control. The ability of the sample to scavenge DPPH radicals was tested.
2.11 Evaluation of crude protein
The micro Kjeldahl technique was used to define the crude protein levels in leaves [11]. A heat block holding 15 mL of concentrated sulfuric acid (H2SO4) and two copper catalyst tablets was used to hydrolyze around 1 g of dry plant material at 420 °C for 2 h. Following cooling, hydrolysis was given H2O before being neutralized and titrated. The concentrations of nitrogen in the raw materials were multiplied with both the traditional conversion factor of 6.25.
2.12 Identification of elements
For plant sample digestion, a 0.5 g sample of the plants’ powdered leaves was weighed into Teflon PFA containers, where it was digested for 3 h at 85 °C with a conc. HNO3: HCl (3:1) combination. To speed up the oxidation process during digestion, conc. HClO4 (1.0 ml) was then added. The resultant solutions were filtered and diluted to a volume of 50 ml with distilled water. Without adding the sample, the blank solution was treated the same way [19]. According to AOAC [11], nitrogen (N) was measured using the micro Kjeldahl technique; According to Snell [20] description, phosphorous (P) was measured using a spectrophotometer; the flame photometer technique developed by Chapman [21] was used to quantify potassium (K) and sodium (Na).
2.13 Experimental design
Two variables in this experiment were mint (2 species) and turmeric curcumin (5 rates). Three replicates were used for each treatment; there were 10 pots in each. Each pot had three plants. In this experiment, 300 pots were utilized. The experiment was conducted using a complete random block design.
2.14 Data analysis
Two-way analysis of variance was used to statistically examine the data based on Snedecor [22]. The effects of each factor individually (mean or total) as well as interactions between turmeric curcumin and mint species were looked at in the results. P values are used to assess significance; P values below 0.05 are deemed significant (*), P values below 0.01 are deemed moderately significant (**), and P values below 0.001 are deemed highly significant (***). The STAT-ITCF software states that this approach was used in certain ways [23].
3 Results
3.1 Growth criteria’s reaction to the turmeric curcumin and different mint species
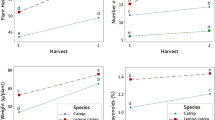
Different growth factors (plant length, fresh and dried aerial parts) were impacted by mint species and/or turmeric curcumin treatments (Table 2). Compared to the untreated control, different turmeric curcumin levels improved growth criteria of both mint species (spearmint and horsemint). The maximum values of plant length (93 cm), fresh weight (114.73 g plant−1) and dry weight (33.81 g plant−1) were recorded at horsemint plants that were treated with turmeric curcumin at 30 mg L−1. Horsemint growth factors had higher values than those observed with spearmint. The effects on various growth factors were highly significant in response to turmeric curcumin, mint species and their interactions, except the effects of turmeric curcumin × mint species on aerial parts dry weight, were significant.
3.2 Various mint species and turmeric curcumin’s impact on photosynthetic pigments
An increase of photosynthetic pigments (chlorophyll a, b and total carotenoids) resulted from the progressive increase in turmeric curcumin level; however, they were higher with spearmint leaves than horsemint (Table 2). Under the treatment of 30 mg L−1 turmeric curcumin, spearmint leaves gave the greatest value of chlorophyll a (5.38 mg g−1), chlorophyll b (1.83 mg g−1) and total carotenoids (1.45 mg g−1). The increases in chlorophyll a were highly significant for turmeric curcumin, mint species or their interactions. The increments of chlorophyll b were highly significant for turmeric curcumin or mint species, while they were significant for turmeric curcumin × mint species. The increases in total carotenoids were non significant for turmeric curcumin, mint species or their interactions.
3.3 Effects of mint species and turmeric curcumin on the compositions of essential oils
The essential oils (%) or outputs (g plant−1) of horsemint and spearmint were grown at different turmeric curcumin rates (Table 2). Horsemint essential oil (%) and outputs were found to have higher values than those seen in spearmint. Horsemint plants treated with turmeric curcumin at a concentration of 120 g L−1 yielded the highest amount of essential oil (2.95%); while horsemint plants that were subjected to turmeric curcumin at 60 g L−1 resulted in the highest value of essential oil output (1.52 g plant−1). For the turmeric curcumin, mint species, and turmeric curcumin x mint species, the increases in essential oil (%) or g plant−1 were highly significant.
Thirty-six compounds were discovered in both mint species' essential oils after turmeric curcumin treatments (Tables 3 and 4). Eucalyptol and camphor were the main constituents of spearmint essential oil; whereas limonene and carvone were the major ingredients of horsemint essential oil. Both essential oils’ major section was monoterpenes; while the minor part comprised sesquiterpenes. According to the interaction between turmeric curcumin and both mint species (Table 3), essential oil obtained from both mint species that were exposed to 90 mg L−1 turmeric curcumin produced the maximum amount of eucalyptol (65.44%), camphor (23.81%), carvone (65.95%) and limonene (15.9%); the highest amount of monoterpenes (98.76%) was recorded in spearmint essential under 60 mg L−1 turmeric curcumin, while, essential oil obtained from horsemint plants that were subjected to 60 mg L−1 turmeric curcumin produced the maximum amount of sesquiterpenes (31.4%). The changes in various detected components and their groups were highly significant for turmeric curcumin x mint species, except in α-phellandrene, α-terpinolene, camphor, menthone and terpinen-4-0l, they were non significant. According to mint species (Table 4), essential oils of spearmint gave higher values in eucalyptol, camphor and monoterpenes than those obtained from horsemint essential oils; while essential oils produced from horsemint produced higher amounts of carvone, limonene and sesquiterpenes than those obtained from spearmint essential oils. The variations in different constituent and sections were highly significant for mint species, except in α-terpinolene and menthone were non significant. Regarding to turmeric curcumin factor (Table 4), in response to turmeric curcumin applications, different changes were seen in both essential oils' constituents and their sections; plants treated with 90 mg L−1 turmeric curcumin resulted in the highest values of eucalyptol, camphor, carvone, limonene and sesquiterpenes; while those subjected to 60 mg L−1 turmeric curcumin gave the maximum amount of monoterpenes. The variations in all detected components and their chemical groups were highly significant for turmeric curcumin doses, except in α-phellandrene, α-terpinolene and menthone were non significant.
3.4 Turmeric curcumin and different species of mint's effects on the total soluble sugars
Various turmeric curcumin rates resulted in an increase in the total soluble sugar content in both mint species (Table 5). However, horsemint x turmeric curcumin at a concentration of 60 mg L−1 produced the largest amount of total soluble sugars (102.19 mg g−1). Horsemint plants had lower total soluble sugar levels than spearmint plants. The changes in total soluble sugars were highly significantly for different dosages of turmeric curcumin, even though they had no significant on the species of mint or on the relationship between the amounts of turmeric curcumin and the species of mint (Table 5).
3.5 Effects of turmeric curcumin and mint species on the total amount of free amino acids
When turmeric curcumin was applied at different rates, both mint species accumulated more free amino acids (Table 5). The maximum amount of total free amino acids (8.73 mg g−1) was found with the treatment of 60 mg L−1 turmeric curcumin × horsemint. The total free amino acid levels were lower in horsemint plants than in spearmint plants. For varied turmeric curcumin doses, the changes in total free amino acids were highly significant, but they have no significant on the species of mint or on the interaction between turmeric curcumin levels and mint species (Table 5).
3.6 Effects of turmeric curcumin and mint species on the total phenols
Table 5 displays how exposure to turmeric curcumin affected the amount of total phenols that accumulated. Various dosages of turmeric curcumin enhanced the phenol buildup in the tissues of mint species. Compared to horsemint plants, spearmint plants exposed to turmeric curcumin produced greater total phenols values. Horsemint plants treated with 60 mg L−1 turmeric curcumin had the largest concentration of phenols (3.71 mg g−1). For various turmeric curcumin dosages, the variations in total phenols were highly significant, however they have no significant on the species of mint or on the interaction between turmeric curcumin rates and mint species (Table 5).
3.7 Turmeric curcumin and mint species' effects on the total flavonoids
Different turmeric curcumin concentrations led to increase in total flavonoids (Table 5). Under the influence of turmeric curcumin rates, horsemint plants generated more flavonoids than spearmint. Horsemint plants that were given 60 mg L−1 of turmeric curcumin generated the greatest level of total flavonoids (5.28 mg g−1). Regarding different turmeric curcumin doses, changes in total phenols were highly significant, however they have no significant on the species of mint or on the interaction between turmeric curcumin rates and mint species (Table 5).
3.8 The effects of mint species and turmeric curcumin on the antioxidant activity
Exposure to turmeric curcumin improved the inhibition of free radical scavenging in both species of mint plants (Table 5). Turmeric curcumin-treated horsemint plants had greater levels of free radical scavenging inhibition than spearmint plants. Horsemint plants that were given 60 mg L−1 turmeric curcumin generated the greatest level of the inhibition of free radical scavenging (64.35%). Regarding various turmeric curcumin dosages, changes in the inhibition of free radical scavenging were highly significant, however they have no significant on the species of mint or on the interaction between turmeric curcumin rates and mint species (Table 5).
3.9 The effects of mint species and turmeric curcumin on the crude protein
Turmeric curcumin levels caused an increase in the protein accumulation rate of both mint species, the maximum value of protein concentration (17.09%) was obtained from spearmint plants that were exposed to 60 mg L−1 turmeric curcumin. Under turmeric curcumin levels, spearmint plants gave higher protein accumulation than those of horsemint plants. The variations in crude protein were highly significant for turmeric curcumin levels, mint species and their interactions (Table 5).
3.10 Impacts of mint species and turmeric curcumin on the nutrient contents and their uptakes
According to turmeric curcumin treatments, mint species, and their interactions, different variations were seen in the contents and uptakes of nitrogen, phosphorous, potassium and sodium (Table 6). The contents and uptakes of nitrogen, phosphorous and potassium were raised by turmeric curcumin application of both mint species, while, the opposite trend was found in sodium ion. Spearmint plants gave higher contents (%) of nitrogen, phosphorous, potassium and sodium than those of horsemint plants. Spearmint plants that were exposed to 60 mg L−1 turmeric curcumin resulted in the greatest contents of nitrogen (2.74%), phosphorous (0.54%) and potassium (0.56%); while untreated control treatment × spearmint plants gave the maximum value (0.17%) of sodium content and its uptake (0.04 g plant−1). The nitrogen and potassium uptakes were higher in horsemint plants than those of spearmint plants; while sodium uptake was higher in spearmint plants that of horsemint plants; on the other, hand no changes were found in potassium uptake of both mint species. The maximum value of nitrogen uptake (1.27 g plant−1) was recorded with the treatment of 60 mg L−1 turmeric curcumin × horsemint; while the maximum uptakes of phosphorous (0.17 g plant−1) and potassium (0.18 g plant−1) were obtained from the treatment of 60 mg L−1 turmeric curcumin × spearmint. The variation of various nutrient contents and potassium uptake were highly significant for turmeric curcumin levels, mint species and their interactions (Table 6). The variables of nitrogen uptakes were highly significant for mint species and turmeric curcumin levels, while they were non significant for mint species × turmeric curcumin doses. The changes in phosphorous uptake were non significant for mint species and they were highly significant (P < 0.001) for turmeric curcumin rates or the interactions between mint species and turmeric curcumin treatments. The changes in sodium uptake were non significant for mint species and they were significant for turmeric curcumin rates or the interactions between mint species and turmeric curcumin treatments.
4 Discussion
In this study, different increases in growth characteristics, photosynthetic pigments, essential oil yield and its major constituents, total soluble sugars, total free amino acids, total phenols, total flavonoids, antioxidant activity, crude protein, contents and uptakes of nitrogen, phosphorous, and potassium, as a result of turmeric curcumin spraying, mint species and their interactions under various stress conditions of sandy soil were noted. On the other hand, sodium ion and its uptake have decreased. As a result of using turmeric curcumin, growth characteristics and photosynthetic pigments have improved; this could be as a result of turmeric curcumin's phenolic component impacts on several physiological plant processes. The use of phenolic components may be linked to higher levels of naturally occurring growth hormones, in particular, indole acetic acid (IAA); this could then encourage cell division and/or expansion, as seen in the development of plant organs [24]. On the other hand, cell division, cellular differentiation, water and ion movement within cells, metabolism and respiration, phytohormones, photosynthesis, enzymatic processes, signal transmission, and gene expression are all significantly influenced by phenolic components [25]. Addition of phenolic compounds was shown to significantly increase plant photosynthetic activity and CO2 substomatal content [26]. In this study, various elevations were observed in essential oils (% or yield) and their main constituents in relation to turmeric curcumin. The presence of phenolic components triggers the activation of a number of enzymes necessary for the production of essential oil, resulting in an increase in essential oil concentration and its major components [27]; however phenolic compounds stimulate the formation of a number of other molecules (e.g., phenylpyranone, phenylphenol, phenylquinolone, phenylcynolone, etc.), which in turn form glycosides (or esters) that act as stress hormones [28]. Glycosides and esters make CoA thioesters, which are the building blocks for making other compounds like essential oils [29]. Phenolic compounds can help make essential oils by increasing the number of cells that contain them (called glands), as well as the enzymes that make essential oils from aromatic plants [30]. Previous investigators confirmed an increase in the total soluble sugars following turmeric curcumin use; they reported that different increments were found in total soluble sugars contents under phenolic substances rates through parsley plant growth stages [31]. Mansour [32] confirmed the increased free amino acid and protein content in response to exogenous phenolic components. Turmeric curcumin is an effective antioxidant, due to its polyphenolic composition, and shows scavenging of free radicals through the phenolic group's donation of an H-atom, additionally, it increases the amounts of antioxidants including flavonoids and polyphenols [26, 33]. Increased nitrogen, phosphorus, and potassium levels and uptakes were seen in plants exposed to phenolic components. The sodium contents and uptakes showed a contrary trend. In this case, phenolic components, which have great selective permeability for plant cell membranes and positive charges, hence improving the reactivity of the plant system and maybe a decrease in the absorption of sodium and a rise in the uptake of potassium, phosphorus and nitrogen [10]. Research from the past demonstrated that plants treated with phenolic components such as turmeric curcumin produced more yield than untreated control plants; this could have been caused by a balance in the plant’s water content, as indicated by stomata changes, which may have accelerated photosynthesis and growth [24]. Exogenous turmeric curcumin enhanced growth and stress tolerance of plants [34]; the treatment of plants with phenols has been found to have a positive effect on the growth of lignin, hydroxyl proline and resistance enzymes, as well as increasing plant's resilience to stress [35]. Plant water relationships are improved by phenolic chemicals and their derivatives, increase the production of antioxidant enzymes, strengthen a variety of crops against biotic and abiotic threats including high temperatures, disease invasion, drought, heavy metals, salt, and change the expression of certain genes that are resilient to stress and efficiently reactive oxygen species (ROS) [36, 37]. Exogenous phenolic substances increased plant biomass, chlorophyll content, potassium retention, and the activities of antioxidant enzymes, all of which showed that plants became more resistant to stress [38]. In times of stress, phenolic chemicals are essential for the metabolism of aromatic plants as a result of the fact that it improves photosynthesis and the synthesis of carbohydrates, which constitute the terpenes units of the compounds that make up essential oils [39]. As evidenced by the increase in blooming, the utilization of essential oils and the components of those oils, the phenolic molecule's antioxidant qualities aid it in protecting plants from damaging circumstances [40]. Plants exposed to various biotic and abiotic stressors benefit from the antioxidant capabilities of phenolic compounds as a result of their effects on the plasma membrane and the physiological processes involved therein, such as reducing oxidative stress, drastically reduce the production of ROS, decreasing the disturbance of the calcium homeostasis, increasing net nitrate absorption, and promoting the survival of the H+ATPase in the plasma membrane [41, 42]. Both mint species (spearmint and horsemint) differed in the values of growth characters, as well as their contents of chemical components, also in their response to turmeric curcumin applications. This might be as a result of the two species' various genetics [43]. Some earlier investigations have supported these findings; they demonstrated that similar plant species had various morphological characters and chemical compositions depending on the environment [44]. Growth, synthesis and accumulation of secondary metabolisms in medicinal and aromatic plants were impacted by genetic variables from distinct species [45]. Each plant species has its own ideal light conditions (in terms of both quality and quantity), which are able to produce the greatest amount of secondary metabolism [45]. Different basil species differ in terms of morphological traits, mass production, and chemical components [43]. Different chamomile species vary from one another in terms of growth traits, essential oil, and chemical components [46]. According to the various species, the quantity and weight of tea flowers as well as their chemical components varied substantially [47]. It was discovered that different safflower species responded to agricultural treatments differently based on differences in their physical and chemical characteristics [48]. The major component revealed polymorphism in the essential oils of Cymbidium sinense flowers, which could be attributed to genetic factors [49]. On the other hand, under turmeric curcumin, mint species, and their interactions, additional factors like fertilizers treatment, irrigation, location, stages, and growing season may have an impact on mint productivity [50,51,52,53,54,55,56,57]. It is recommended to use turmeric curcumin as a viable method to lessen the detrimental effects of abiotic stress on the production of spearmint and horsemint. To decrease the detrimental effects of stressful situations and increase the possibility of exporting mint to other nations, many farmers would profit from this research as it will enable them to cultivate spearmint and horsemint in Egypt’s new areas. This study clearly shows that adding turmeric curcumin improved the yield of spearmint and horsemint herbs, which, as the output rose, are significant from both an economic and medicinal standpoint. Given its high production and medicinal value, the study advises expanding and promoting the growth of spearmint and horsemint in arid regions utilizing turmeric curcumin. Consider conducting more research, focusing on dosage and administration methods, to optimize the use of turmeric curcumin to boost the growth of spearmint and horsemint in dry areas. Providing farmers with information on growing spearmint and horsemint in dry areas through the holding of advisory seminars by agricultural authorities and research institutions and how turmeric curcumin helps plants withstand the damaging effects of stress, in addition to the benefits of spearmint and horsemint in food, cosmetics, drugs, and colognes, to reduce the expenses of production requirements would motivate farmers to increase the area under cultivation of spearmint and horsemint in the new sites. It is essential to consider the potential enduring impacts and sustainability of using turmeric curcumin in agricultural practices, as well as any risks associated with employing them in certain situations. Additionally, it has been stated that in order to produce mint and essential oil, different species or turmeric curcumin treatments are required; as a result, its synthesis undergoes a number of alterations, which amplify its biological domain as a source of essential oils naturally, which increase its biological domain and act as a natural source of essential oils.
5 Conclusion
This study demonstrated how the productivity of mint is affected by species, turmeric curcumin, and their interactions. It has been established that the horsemint and spearmint species constitute a new source of natural essential oils. This study suggests using modern breeding techniques to create new species in order to enhance the sources of natural essential oils that may be utilised for a range of biological purposes. Turmeric curcumin treatment also increases essential oil output and modifies their chemical, which modifies their biological action. This study showed that producing mint species under turmeric curcumin requires sandy soil. In order to achieve the maximum rate of herb production, essential oil output in both species, the predicted application concentration for turmeric curcumin in spearmint production was 90 mg L−1, but it was 60 mg L−1 in horsemint production. This study will help farmers, the ministry of agriculture, and pharmaceutical companies in the future in boosting the production of mint and essential oil as a source naturally occurring for the pharmaceutical industries. Conversely, based on the relevant components, this study serves as a reference for selecting mint species for use in essential oil-related industries.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on responsible request.
References
Ghorbanpour A, Varma A. Medicinal plants and environmental challenges. Berlin: Springer Nature; 2018.
Fatih B, Madani K, Chibane M, Due P (2017) Chemical composition and biological activities of Mentha Species. Aromatic and Medicinal Plants - Back to Nature. InTech doi: https://doi.org/10.5772/67291.
Pathirana R, Carimi F. Management and utilization of plant genetic resources for a sustainable agriculture. Plants. 2022;11(15):2038.
Stefanaki A, Cook CM, Lanaras T, Kokkini S. The oregano plants of ChiosIsland(Greece): essential oils of Origanum onites L. growing wild in different habitats. IndCrops Prod. 2016;82:107–13.
Aboukhalid K, Alfaiz C, Douaik A, Bakha M, Kursa K, Mołdoch AM, Machon N, Tomi F, Lamiri A. Influence of environmental factors on essential oil variability in O. compactum BENTH growing wild in Morocco. Chem Biodiver. 2017;14(9):e1700158.
Khalid AK, Darwesh OM, Ahmed AMA. Peel essential oils of Citrus types and their antimicrobial activities in response to various growth locations in Egypt. J Ess Oil Bear Plants. 2021;24(3):480–99.
Khalid AK, El-Gohary AE, Ahmed AMA. Effect of the interaction between salicylic acid and geographical locations on grapefruit essential oil. J Essent Oil-Bear Plants. 2018;21:1594–603.
Mayer MJA, Narbad AJ, Parr ML, Parker NJ, Walton F, Mellon A, Michael AJ. Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl-CoA hydratase/lyase enzyme function. Plant Cell. 1969;13:1669–82.
Payton F, Sandusky P, Alworth WL. NMR study of the solution structure of curcumin. J Nat Prod. 2007;70:143–6.
Zaki FS, Khater MA, El-Awadi ME, Dawood MG, Elsayed AE. Curcumin polyvinyl alcohol nano-composite enhances tolerance of Helianthus annuus L. against salinity stress. Beni-Suef Univ J Basic Appl Sci. 2023;12:60.
AOAC, Official Methods of Analysis of the Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. 21st ed. Washington DC: AOAC; 2019.
Clevenger JF. Apparatus for determination of essential oil. J Amer Pharma Assoc. 1928;17:346–9.
Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL: Allured Publ Corp; 1995.
Dubois M, Gilles KA, Hamilton JK, Roberts PA, Smith F. Phenol sulphuric acid method for carbohydrate determination. Ann Chem. 1956;28:350–9.
Moore S, Stein WH. Photometris ninhydrin method for use in the chromatography of amino acids. J Biol Chem. 1948;176:367–88.
Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–78.
Pourmorad F, Hosseinimehr S, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotech. 2006;5:1142–5.
Middha A, Purohit S. Determination of free radical scavenging activity in herbal supplement: Chyawanprash. Int J Drug Dev Res. 2011;3:328–33.
Chase P, Singh OP. Ethno medicinal plants used by Angami Tribe of Nagaland, India. Ind J Trop Biodiv. 2013;21:29–42.
Snell R, Snell G. Colorimetric method of analysis. New York: Van Nostrand Company; 1954.
Chapman HD, Pratt PF. Methods of analysis for soils, Plants and Water. Davis: Division of Agriculture Sciences University of California; 1978.
Snedecor GW, Cochran WG. Statistical methods. 11th ed. Ames, IA: Iowa State University Press; 1990.
Foucart T (1982) Analyse Factorielle, Programmatiol Sur Micro- ordinateur. Masson ITCF Paris. ISBN-13: 978-2225764509.
Barkosky RR, Einhellig FA, Butler J. Caffeic acid induced changes in plant water relationships and photosynthesis in leafy spurge Euphorbia esula. J Chem Ecol. 2000;26:2095–109.
Tanase C, Bara CI, Popa IV. Cytogenetical effect of some polyphenols compounds separated from industrial by-products on maize (Zea mays L.) plant. Cellul Chem Technol. 2015;49(9–10):799–805.
Einhellig FA, Rasmussen JA. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J Chem Ecol. 1979;5:815–24.
Burbott A, Loomis J. Evidence for metabolic turnover monoterpene in peppermint. Plant Physiol. 1969;44:173–9.
Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–7.
Cheng GW, Malencik DA, Breen PJ. UDP-glucose: flavonoid O-glucosyltransferase from strawberry fruit. Phytochem. 1994;35:1435–9.
Rowshan V, Bahmanzadegan A. Effects of salicylic acid on essential oil components in Yarrow (Achillea millefolium Boiss). Int J Sci Bas Appl Res. 2013;2:453–7.
Ahmed AMA, El-Kady FA, Khalid AK. Morphological and chemical characters of Petroselinum crispum (Mill) subjected to some biostimulants. Asian J Plant Sci. 2018;17(2):96–106.
Mansour SA, El Deeb FAA, Abd El-Rahman FA, Khalil MT. Impact of phenolic compounds on free amino acid contents and electrophoretic patterns of the Nile Tilapia, Oreochromis niloticus. Egypt J Aquat Biol Fish. 2021;25(4):301–12.
Barclay LRC, Vinqvist MR, Mukai K, Goto H, Hashimoto Y, Tokuanga A, Uno H. The antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org Lett. 2000;2:2841–3.
Zafar-ul-Hye M, Nawaz MS, Asghar H, Waqas M, Mahmood F. Caffeic acid helps to mitigate adverse effects of soil salinity and other abiotic stresses in legumes. Genet Genom. 2020;4:1–6.
Li S, Pi J, Zhu H, Yang L, Zhang X, Ding W. Caffeic acid in tobacco root exudates Defends tobacco plants From Infection by Ralstonia solanacearum. Front Plant Sci. 2021;12:1–14.
Wan YY, Zhang Y, Zhang L, Zhou ZQ, Li X, Shi Q. Caffeicacid protects cucumber against chilling stress byregulating antioxida nt enzyme activityand proline and soluble sugar contents. Acta Physiol Plant. 2015;37:1706.
Srinivasulu C, Ramgopa M, Ramanjaneyulu G, Anuradha CM, Kumar CS. Syringic acid (SA) a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother. 2018;108:547–57.
Mehmood H, Abbasi GH, Jamil M, Malik Z, Ali M, Iqbal R. Assessing the potential of exogenous caffeic acid application in boosting wheat (Triticum aestivum L.) crop productivity under salt stress. PLoS ONE. 2021;2:1–16.
Letchamo W, Xu HL, Gosselin A. Variations in photosynthesis and essential Oil in thyme. J Plant Physiol. 1995;147:29–37.
Talaat IM, Khattab HI, Ahmed AM. Changes in growth, hormones levels and essential oil content of Ammi visnaga L. plants treated with some bioregulators. Saudi J Biol Sci. 2014;21:355–65.
Yu JQ, Sun Y, Zhang Y, Ding J, **a X. Selective trans-cinnamic acid uptake impairs [Ca2+]cyt homeostasis and growth in Cucumis sativus L. J Chem Eco. 2009;35:1471–7.
Abenavoli M, Lupini A, Oliva S, Sorgonà A. Allelochemical effects on net nitrate uptake and plasma membrane H+ATPase activity in maize seedlings. Biol Plant. 2010;54:149–53.
Serafini LA, Pauletti GF, Rota LD, Santos ACA, Agostini F, Zattera F, Moyna P. Evaluation of the essential oils from nine basil (Ocimumbasilicum L.) cultivars planted in Southern Brazil. J Essent Oil-Bear Plants. 2009;12:471–5.
Koutsoukis C, Roukos C, Demertzis PG, Kandrelis S, Akrida-Demertzi K. The variationof the chemical composition of the main plant species in asubalpine grassland in northwestern Greece. Legume Sci. 2019;1: e23.
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80–9.
Shalaby AS, Hendawy SF, Khalil MY. Evaluation of some chamomile cultivars introduced and adapted in Egypt. J Essent Oil-Bear Plants. 2010;13:655–69.
Wang H, Cui X, Zhao X, Gao S, Zhao J, Yuan Z. Differences of biochemical constituents and contents of eight cultivars flowers of Camelliasinensis. J Essent Oil-Bear Plants. 2015;18:320–8.
Zari H, Babak P, Asad R. The effect of priming with nano-sliver on agronomic traits of safflower cultivars. J Essent Oil-Bear Plants. 2015;18:1148–56.
Li J, Zhu G, Wang Z. Chemical variation in essential oil of Cymbidiumsinense flowers from six cultivars. J Essent Oil-Bear Plants. 2017;20:385–94.
Khalid KA. Effect of macro and micro nutrients on essential oil of coriander fruits. J Mater Environ Sci. 2015;6:2060–5.
Yassen AA, Khalid KA. Influence of organic fertilizers on the yield, essential oil and mineral content of onion. Int Agrophys. 2009;23:183–8.
Behtari B, Gholami F, Khalid AK, Tilaki GD, Bahari R. Effect of growth stages and altitude on Artemisia herba-alba Asso essential oil growing in Iran. J Essent Oil-Bear Plants. 2012;15:307–13.
Khalid KA, Hussien MS. Effect of cattle and liquid manures on essential oil and antioxidant activities of celery (Apium graveolens L.) fruits. J Essent Oil-Bear Plants. 2012;15:97–107.
Ahmed AMA, Talaat IM, Khalid AK. Soil Moisture and glutamic acid affect yield, volatile oil and proline contents of oregano herb (Origanum vulgare L.). Int J Bot. 2017;13:43–51.
Said-Al Ahl AH, Khalid AK. Response of Coriandrum sativum L. essential oil to organic fertilizers. J Essent Oil Bear Plant. 2010;13:31–7.
Khalid AK, El-Gohary AE, Ahmed AMA. Effect of growing seasons on the leaf essential oil composition of Citrus species that are cultivated in Egypt. J Essent Oil Res. 2020;32:296–307.
Ibrahim ME, Mohamed MA, Khalid KA. Effect of growing locations on the essential oil content and compositions of lemon verbena shrubs under the conditions of Egypt. J Essent Oil Bear Plant. 2014;17:288–94.
Acknowledgements
The authors are grateful to the National Research Centre (NRC) for its cooperation with this scientific endeavor.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This essay’s research was made possible by a grant with Project No. 11080301, from the National Research Centre, which also gave us permission to experiments with horsemint and spearmint.
Author information
Authors and Affiliations
Contributions
FSAZ and AMAA: Participated in the design, carried out the experiments in the green house, chemical studies and performed the statistical analysis. KAK: Contribute to writing, drafted the manuscript and participated in the sequence alignment. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Both species of mint {horsemint (Mentha longifolia), and spearmint (Mentha spicata L)} were grown, and their samples were taken in accordance with all relevant institutional, governmental, and global regulations. Mint plants were exposed to turmeric curcumin which is a natural product and safe for human health. All specimens of treated plants used for analytical studies. The procedures employed to obtain the various samples (plant aerial parts: leaves, stems and flowers) of horsemint and spearmint were compliant with all applicable institutional, national, and international rules and law. There are no existing materials in institutional or museum collections.; not more than the very minimal number of specimens required to complete the research; when the research objectives allow it, used non-lethal sampling techniques instead of lethal collecting, and used preferential collection of post-reproductive individuals (or the life stage with the least reproductive value) when lethal collection is necessary to improve the chances of the species' survival; stored all gathered samples in facilities where they could be kept permanently and made available to other scientists, hence reducing the requirement for additional collections; timely provided permit-issuing organizations with copies of research-based studies and publications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaki, F.S.A., Khalid, K.A. & Ahmed, A.M.A. Mint species (Mentha longifolia and Mentha spicata L) growth, essential oil generation and chemical components are impacted by turmeric curcumin applications. Discov Appl Sci 6, 160 (2024). https://doi.org/10.1007/s42452-024-05810-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05810-8