Abstract
Herewith waste collagen was processed using a deep eutectic solvent (DES) obtained by the complexation between choline chloride (ChCl) and ethylene glycol 1:2 (ChCl:2EG) to form a material with properties similar to leather. The material generated using the waste tanned collagen processed with ChCl:2EG followed by mechanical extrusion was found to show characteristics similar to the one obtained from the material using animal hide powder. However, the tensile strength of extruded collagen was substantially higher (7.56 MPa vs. 0.23 MPa). The improved strength and structure may be due in part to traces of iron imparted into the material during extrusion of the acidic collagen sample. Nevertheless, the material thus generated was found to have a lower degree of crystallinity compared to commercial leather samples. Overall, the process demonstrates a facile method preparing a useful material out of waste collagen by the intervention of DESs.
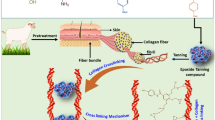
Graphical Abstract

Article Highlights
-
A simple method for preparing a useful biomaterial out of waste collagen via the inclusion of deep eutectic solvents.
-
Extrusion of the collagen resulted in substantially improved strength and structure.
-
The composite generated can be prepared from waste collagenic matter from the tanning industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Collagen is an abundant protein which can be obtained from a wide variety of sources. By far the largest collagenic material used is leather due to its availability and incomparable strength and flexibility. Leather is one of the largest global industries with a turnover of around $40 billion a year with an estimated 17 billion square meters of leather produced annually [1]. Unique properties of leather such as their robustness, durability, flexibility, ease of surface coating to impart new functionalities etc., make it suitable for various applications conventionally to make shoes, bags, seat covers in automobiles etc. [2]. Since leathers are produced from animal hides, its processing involves a number of steps such as removal of keratin and lipids (aka beamhouse processing), tanning and post-tanning; each step produces an aqueous waste stream—commonly known as tannery waste – that can be significantly polluted. Approximately 3 × 108 m3 of waste liquor is disposed of daily from a medium sized tannery [3]. Considering the volume of the tannery aqueous waste streams and the quantity of hides and skins that do not reach the leather value chain (approximately 16% of US production), it is important to find alternative tanning techniques and applications for waste collagen [4, 5].
It has been shown that deep eutectic solvents (DESs) can be used for number of applications such as metal processing, metal electroplating, metal polishing, purification and production of bio-diesel, [6] bio-polymer dissolution and functionalization etc. [7,8,9]. The ionic properties of DESs combined with their tunability and ease of handling make them interesting fluids for numerous applications. Type III eutectic mixtures can encompass a variety of amides and polyols such as urea, glycerol, ethylene glycol, fructose, and erythritol, which have low inherent toxicity where formulations can be produced using food grade ingredients [6]. So far, DESs have been used in leather processing to liquefy tanning agents to decrease in the use of toxic chemicals [10]. Furthermore, dyes soluble in the DESs showed very good penetration ability on the leather surface upon surface application [10]. DESs are viscous and can penetrate into leather so they have been used for fat liquoring as a replacement for the oils used during leather processing [10]. Although various ILs are used for the dissolution and processing of various proteins, the application of DESs in processing protein is limited except a report on the evaluation of partitioning efficiency of DESs for bovine serum albumin (BSA)[11] and one on the modification of gelatin.[12].
The advantages of using DESs in leather processing is the high solubility of tanning agents and low volume of liquid required to tan the hide resulting in fast tanning in a liquid that can be reused. The aim of this study is to show the same tanning approach can be used with waste collagen to produce a material with moderate strength and good flexibility, which is hypothesised to be used to produce fibrous materials for product, food, and biomedical applications. The objectives of this work are to: demonstrate effective production of a novel collagen biomaterial, and characterise its physiochemical properties in relation to other collagenic materials. However, more investigation into the full biocompatibility of the DESs investigated before they can truly be claimed as biocompatible. Section 1 of this article has introduced the field of waste collagen material and the potential role DESs can play in modifying sources of waste collagen in this regard. In Sect. 2 the production approach for the collagen extrudates and the testing regime are explained. In Sect. 3 the findings of this work are reported which are broken down into two areas of discussion: the production of sheet material, and the physio-mechanical properties. In Sect. 4 the conclusions and potential applications of the study are listed.
2 Experimental
2.1 Materials
Collagen gel was received from DEVRO, Glasgow, UK. Ethylene glycol and choline chloride (both Aldrich < 99%) Chestnut vegetable tanning agent (Castagno N, Silvateam, Italy) were used as received. ChCl:2EG was prepared following the method described by Abbott et al., (2004) [13], briefly choline chloride and ethylene glycol was heated in 1:2 molar ratios at 50 °C until a clear transparent homogeneous solution was obtained. The chemical components chosen are generally regarded as safe (GRAS) and considered to be biodegradable and biocompatible.
2.2 Sample preparation
The below conditions have been previously reported to significantly impact on the crystallinity and mechanical properties of the carbohydrate biomaterials and it had been hypothesised these conditions were also applicable to the generation of collagenic biomaterials [14, 15]. The collagen gel was made by dissolving collagen from bovine hide in 1 M HCl to form a thick slurry/gel. The collagen was then precipitated using ChCl:2EG containing 10 wt% chestnut tannin. The resulting mixture was stirred by hand until precipitation was observed. The solid was then extruded in using a Prism TSE-24 TC co-rotating twin screw (20 L/D) extruder with a Prism volumetric feeder and an air swept facecut pelletising system. The screw profile was as follows: 4.5 L/D conveying screw, 0.75 L/D 30° kneading, 0.75 L/D 60° kneading, 1.25 L/D 90° kneading, 7 L/D conveying screw, 0.5 L/D 60o kneading, 0.75 L/D 90° kneading, 3 L/D conveying screw, 1.5 L/D single lead discharge screws. All sections of the extruder were heated to 40 °C to allow the material to flow easily (higher temperatures were not used as this would denature the collagen). The speed was set to approximately 100 rpm, at which the die pressure was ca. 20 bar. The air swept face-cut pelletising system's blades were set at 95 rpm with only one blade set-up to cut.
The extruded pellets were placed between two copper plates lined with non-stick silicone sheets with a 1 mm copper separator (9 cm square aperture). The sandwich was then placed in a hydraulic press (Fontune Grotnes Laboratory Press TH400) and a force of 110 kN was placed on the sample for 10 min at the specified temperature. The sample was then cooled back to room temperature (water circulation) in 5–10 min in the press with the force still applied.. A schematic highlighting the sample preparation process can be found below in Fig. 1
2.3 Characterisation
A mechanical ‘dog bone’ press (Ceast Hollow Die Punch, Type 6051) to cut test shapes (test area size: L = 30 mm, W = 4 mm, D = as original material thickness). The tensile testing of dog bone shaped samples was carried out using an Instron 3343 tensile apparatus (Instron Ltd, Assembled, USA) with a load cell of 500 N. The material strain and stress were controlled by Instron Bluehill 2 software and average values were taken from 5 samples. In every case the thickness of each sample was measured using a micrometre and subjected to a strain rate between 2 and 10 mm min−1. Tensile strength and elongation at break were determined using an Instron tensiometer according to BS EN ISO 3376:2011.
Differential scanning calorimetric (DSC) studies were carried out on a Mettler Toledo DSC 1 STARe system using 5–10 mg sample in a sealed high pressure gold pan using temperature programme 25 °C (hold for 5 min) to − 120 °C at the rate of 10 °C/min (hold for 10 min) followed by raising the temperature to 150 °C at the rate of 10 °C/min (hold for 10 min). Inert nitrogen gas atmosphere was maintained for both TGA and DSC measurements. Dynamic mechanical analyses (DMA) were done on Mettler Toledo DMA 1 STARe Systems using dual-cantilever-bending mode by heating -100 °C to 100 °C at the rate of 10 °C/min. All thermal analyses were conducted in triplicate. Three-dimensional (3D) microscope (Zeta-200 Optical Profiler) was used to record the images of the cross-section of the samples at 50 × magnification. The images were taken in one location that seemed representative of the whole cross section.
3 Results and discussion
3.1 Production of high tensile strength collagen sheets
Collagen is often obtained from the split part of animal hides that occurs during processing and the most common method to extract the protein is by digesting using the enzyme pepsin. The collagen extracted is useful in various medical and food applications. In this study, the collagen was tanned (a quasi-cross-linking process) using a vegetable tanning agent in a mixture of choline chloride and ethylene glycol in a 1:2 molar ratio (ChCl:2EG). Figure 2A shows the collagen after it was precipitated with 10 wt% chestnut tannin dissolved in ChCl:2EG within 10 min of the two solutions being contacted with each other.
Figure 2B shows the appearance of the digested collagen (DC) gel after extrusion and it is interesting to observe that, upon extrusion the colour of the tanned DC gel became darker in appearance. Figures 2C shows the sample after pressing and drying. The intense colour could be due to accelerated tanning due to the extrusion, however analysis of the extruder screw post-extrusion showed some signs of mild corrosion from the acidic tanned collagen gel which could have incorporated traces of iron into the sample. Digestion of a sample of collagen followed by analysis using atomic absorption spectrometer (AAS) showed the presence of a small amount of iron which may also contribute to the colour. Figure S2 Shows that reaction of FeCl3 and Chestnut tannin does indeed lead to a deeper colour due to the complexation of the iron.
Further, the most obvious form of iron present on a metal surface is rust (Fe2O3·nH2O) and it has been observed during preparation of DNA based materials with Fe3O4 in ChCl:2EG that the Fe interact with the base pair of DNA [9]. In a similar mechanism it is possible that since the collagen backbone is made up of amino acids, the Fe might have interacted with this backbone as well as the documented electrostatic interaction with the amino acid side chains. The consequences of the presence of Fe in the biomaterial are at this stage unknown and would be the subject of future toxicity studies. The topic of tanning collagenic functional groups with plant tannin extracts has been extensively studied and related mechanisms can be found in the primary literature [3].
In order to evaluate the strength of collagen sheets obtained by extrusion and pressing, tensile strength measurements were carried out. It was observed that animal hide powder after dissolution and tanning followed by extrusion and pressing gave sheets having tensile strength of 0.27 MPa (Fig. 3). On the other hand, the tensile strength properties of DC gel upon tanning was 0.30 MPa before extrusion similar to one obtained with tanned hide powder gel (Fig. 3A). After extrusion and pressing the tensile strength values increased to 7.16 MPa (Fig. 3B). The increase in tensile strength was perhaps due to the formation of more compact material with ChCl:2EG and tannin in comparison to animal hide powder. It should be stressed, that while a relatively strong material was produced, the strength was significantly weaker than conventional leather tanned using the same tanning agent in the same liquid (43.2 MPa) [10]. This shows that the application is not for producing synthetic competitor to leather but more for utilising the hides and skins that do not reach the leather supply chain, and making materials with biomedical applications. Interestingly the tanned collagenic materials had properties similar to those recently reported for gelatin modified with DESs (UTS 14–22 MPa) [12].
Further, the shape of both curves conforms to the typical stress strain curve for single collagen fibres reported in the literature and does not exhibit the cree** behaviour that is seen in the leather samples [16]. It suggests that in the non-extruded sample there is a higher degree of cree** where the collagen strands are being pulled apart slowly during the stretching, whereas the extruded sample is much stronger but also more brittle and tends to break suddenly and not stretchable to the same extent. The 3D images of the cross section of the samples before and after extrusion showed presence of a fracture line on each sample (Fig. 4), the non-extruded sample showed presence of a much more fibrous cross-section which supports the theory that cree** of the fibres occurred (Fig. 4A). While the cross-sectional 3D image of the extruded sample showed presence of a much cleaner break with no fibrous structure present supporting the observation of the lower maximum extension (Fig. 4B) observed during tensile strength measurements.
The lack of fibrous structure in the extruded sample could be evidence that the more aggressive mechanical action of the extrusion process has disassembled the fibres into fibrils or microfibrils. It is also possible, that the high salt content of the DES combined with the presence of a hydrogen bond donor has disrupted the internal collagenic hydrogen bond and partially gelatinised the sample. This is contra to findings published using similar systems with whole skin samples but, in this case, maybe symptomatic of a more broken-down, quasi- dissolved, starting material [10]. The extruded sample cannot be fully gelatinised as it still exhibits an endothermic transition attributed to denaturation in the DSC.
Since thermal properties of leather or processed collagen is very important in deciding its suitable application areas, the measurement of thermal properties of the DC gel processed with ChCl:2EG before and after extrusion was carried out. The differential scanning calorimetric measurements (DSC) shown in Supporting Figure S3 exhibits a similar profile to the one obtained for the hide powder sample (Supporting Figure S4). In this case the thermogram has showed endothermic peaks at around 80 °C (C) indicating presence of an interaction between the collagen and the tanning agent in the sample. The other endothermic peak appeared at around 150 °C (D), which was not present in the case of the sample obtained from animal hide powder (Supporting Figure S4). This is perhaps due to the extra interaction in the structure of the former initiated by Fe. Furthermore, it is quite unlikely that the endotherm at 150 °C is due to traces of water present on the sealed DSC pan and it must be the inherent property of the material. The degradation temperature for Type 1 untanned collagen is estimated by Samouillan et al., to be around 69.8 °C, [17] the substantial increase in the shrinkage temperature after tanning indicate pronounced influence of the tanning agents on the collagen structure.
Cucos et al., have investigated the degradation temperature of parchment and vegetable tanned leather after it had undergone accelerated ageing by exposing the materials to high temperatures to generate a material that had similar properties to a material that was produced years ago [18]. In the study it was noted that prior to the accelerated ageing the degradation temperature for both materials was higher and the typical shrinkage temperature for vegetable tanned leather before any ageing was found to be 81–84 °C. Similar shrinkage temperature was observed for the extruded DC gel prepared in the presence of ChCl:2EG (cf. DSC & DMA measurements, Fig S3–S6). Further, Cucos et al., has observed decrement in degradation temperature by 4 °C for the one-day aged samples [18]. However, in the present research the DC gel samples were tested less than a week after the materials were made and substantial decrease in the shrinkage temperature was observed. Therefore, it could be an interesting avenue of research to determine whether the shrinkage temperatures of these materials will decrease as they age and are exposed to environmental factors.
Powder X-Ray diffraction measurements were carried out on the DC gel extruded material prepared in presence of ChCl:2EG along with a piece of genuine leather in order to see the similarities or differences in their crystallinity. It can be seen from the XRD plot that the leather and the extruded sample (Fig. 5) follow a similar pattern with appearance of crystalline peaks (2θ = 20°) in the same positions, however the intensity of the crystalline peak of the extruded DC gel sample was lower in comparison to that with leather probably due to the presence of moisture affecting the crystallinity. Alternatively, it may be possible the DES has compromised the crystallinity as observed with thermoplastic starch materials processed in a similar manner [13b]. The peak at 2θ = 8° is found in tanned leather and is associated with crystallinity resulting from quasi-cross-linking mechanism involved in tanning [19].
Most chemical cross linkers used with collagenic materials are used in biomimetic systems such as implants and bio-scaffolds. For this reason, the cross linkers used tend to be based on synthetic mimics of the compounds which cross-link collagen in nature such as lysine. Compounds such as glutaraldehyde, a common leather syn-tan or mixtures of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) are most commonly used. [20] UV irradiation and dehydration through thermal treatment are also common mechanisms. All these techniques tend to give more hydrophobic structures, whereas the aim of this work was to use a mechano-chemical approach more suited to large volume processing and potentially producing larger volumes of material which may find use in areas such as synthetic leather.
4 Conclusion
The waste collagen gel received from an industry was processed in the presence of a deep eutectic solvent, ChCl:2EG. The precipitated, extruded and pressed gel sample was found to have similar characteristics in comparison to the gel obtained directly from animal hide powder in the DES. The gel material was found to have comparable shrinkage temperature as well as crystallinity behaviour in comparison to commercially available leather samples. This is the first attempt to process waste collagenic material using a DES and while the materials are not as strong as leather, fibrous materials were produced which could have direct use in biomedical applications, such as a skin substitute in wound care. Given the tunability of DESs and their ability to solubilise a wide range of solutes there should be significant scope to create a range of materials from biocompatible sources. The full biocompatibility of the reported extrudate materials needs to be confirmed, as do improvements to the tensile and flexural properties. It is believed this can be achieved through fine tuning of the choice of DES components to provide physical and toxicity properties required of biomedical applications. Other potential applications including food packaging and collagen products, such as leather substitutes, are of interest and would be considered as part of future work.
Data availability
All data underpinning this publication are openly available from the University of Northampton Research Explorer at at 10.24339/4620715c-398f-4454-b6d2-f9da10afa12a.
References
Global Leather Goods Industry 2013–2018: Trend, Profit and Forecast Analysis, Lucintel, https://www.lucintel.com/leather-goods-market-2018.aspx (Accessed January 17th 2023)
Covington AD (1997) Modern tanning chemistry. Chem Soc Rev 26:111–126. https://doi.org/10.1039/CS9972600111
Covington AD, Wise WR (2019) Tanning chemistry: the science of leather, 2nd edn. The Royal Society of Chemistry, Cambridge
The Leather and Hide Council of America, Real Leather is the Sustainable Choice, https://www.usleather.org/press/Real-Leather-is-the-Sustainable-Choice (Accessed August 2023)
Nothing to Hide, Essay one: Hide and skin production around the world, http://nothing-to-hide.org/LeatherFacts (Accessed January 17th 2023)
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Mondal D, Sharma M, Mukesh C, Gupta V, Prasad K (2013) Improved olubility of DNA in recyclable and reusable bio-based deep eutectic solvents with long-term structural and chemical stability. Chem Commun 49:9606–9608. https://doi.org/10.1039/C3CC45849K
Bhatt J, Mondal D, Bhojani G, Prasad K (2015) Preparation of bio-deep eutectic solvent triggered cephalopod shaped silver chloride-DNA hybrid material having antibacterial and bactericidal activity. Mat Sci Eng C 56:125–131. https://doi.org/10.1016/j.msec.2015.06.007
Mondal D, Bhatt J, Sharma M, Chatterjee S, Prasad K (2014) A facile approach to prepare a dual functionalized DNA based material in a bio-deep eutectic solvent. Chem Commun 30:3989–3992. https://doi.org/10.1039/C4CC00145A
Abbott AP, Alaysuy O, Antunes APM, Douglas AC, Guthrie-Strachan J, Wise WR (2015) Processing of leather using deep eutectic solvents. ACS Sus Chem Eng 3:1241–1247. https://doi.org/10.1021/acssuschemeng.5b00226
Zeng Q, Wang Y, Huang Y, Ding X, Chen J, Xu K (2014) Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 139:2565–2573. https://doi.org/10.1039/C3AN02235H
Qu W, Häkkinen R, Allen J, D’Agostino C, Abbott AP (2019) Globular and fibrous proteins modified with deep eutectic solvents: materials for drug delivery. Molecules 24:3583. https://doi.org/10.3390/molecules24193583
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147. https://doi.org/10.1021/ja048266j
Abbott AP, Conde JP, Davis SJ, Wise WR (2012) Starch as a replacement for urea-formaldehyde in medium density fibreboard. Green Chem 14:3067–3070. https://doi.org/10.1039/C2GC36194A
Abbott AP, Abolibda TZ, Davis SJ, Emmerling F, Lourdin D, Leroy E, Wise WR (2014) Glycol based plasticisers for salt modified starch. RSC Adv 4:40421–40427. https://doi.org/10.1039/C4RA06220E
Gentleman E, Lay A, Dickinson D, Nowman E, Livesay G, Dee K (2003) Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 24:3805–3813. https://doi.org/10.1016/s0142-9612(03)00206-0
Samouillan V, Delaunay F, Dandurand J, Merbani N, Gardou J, Yousfi M, Gandaglia A, Spina M, Lacabanne C (2011) The use of thermal techniques for the characterization and selection of natural biomaterials. J Funct Biomater 2:230–248. https://doi.org/10.3390/jfb2030230
Cucos A, Budrugeac P, Miu L (2014) DMA and DSC studies of accelerated aged parchment and vegetable-tanned leather samples. Thermochim Acta 583:86–93. https://doi.org/10.1016/j.tca.2014.03.022
Zhang J, Chen W (2020) A faster and more effective chrome tanning process assisted by microwave. RSC Adv 10(39):23503–23509. https://doi.org/10.1039/D0RA04189K
Nair M, Best SM, Cameron RE (2020) Crosslinking collagen constructs: achieving cellular selectivity through modifications of physical and chemical properties. Appl Sci 10(19):6911. https://doi.org/10.3390/app10196911
Acknowledgements
K.P. acknowledges the Council of Scientific and Industrial Research, New Delhi for funding a Raman Research Fellowship. F.B would like to thank the University of Leicester for funding. The authors would like to thank DEVRO PLC for the contribution of raw materials and expertise.
Author information
Authors and Affiliations
Contributions
FB: Formal Analysis, Data Curation, Methodology; WRW: Validation, Conceptualisation; SJD: Writing—original draft, Writing—review & editing; KP: Visualisation, Investigation, Resources; APA: Funding Acquisition, Supervision, Project Administration, Resources.
Corresponding author
Ethics declarations
Conflicts of interest
The authors confirm they have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wise, W.R., Bevan, F., Davis, S.J. et al. Formation of a modified collagenic biomaterial using deep eutectic solvents. SN Appl. Sci. 5, 245 (2023). https://doi.org/10.1007/s42452-023-05460-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05460-2