Abstract
This study presents a theoretical model to mimic heat transfer and nanoparticle transport through the tumour interstitium surrounding an inclined cylindrical blood vessel exposed to an alternating magnetic field. Using similarity transformations, we convert the governing equations (partial differential equations) into a system of ordinary differential equations, which we solve numerically with a MATLAB built-in solver (bvp4c). The converence of the numerical solution is proved using the mesh convergence test. All parameters and their effects on fluid flow, heat, and mass transfer in the interstitium are studied and investigated. For instance, the nanoparticle penetration into the deep tissue can be enhanced by exposing the tumour to a magnetic field, increasing the tumour temperature and the nanoparticle Brownian motion, which is a consequence of increasing the tumour temperature. Moreover, we consider the case of non-Newtonian interstitial fluid in the tumour to mimic the nonlinearity of the fluid flow in the tumour tissue. The findings of this manuscript may optimise tumour ablation using hyperthermia by optimising nanoparticle delivery to deep tumour tissue and tumour temperature.
Article Highlights
-
Heat transport via thermophoresis in the tissue increases the nanoparticle accumulation in the tissue.
-
Nanoparticle transport due to Brownian motion reduces the nanoparticle delivery to the deep tissue.
-
The tumour-specific absorption coefficient plays a significant role in optimising the efficiency of thermal therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanofluids have received much attention recently because of their potential use as physical heat transfer in fluids [1] and their importance in medical applications such as drug delivery [2, 3], cancer treatment [4], neurological diseases [5] and breaking down blood clots formed in blood vessels [6]. The main advantage of using nanofluids in biomedical applications is their enhanced heat transfer coefficient compared to other liquids [5]. The heat transfer efficiency in nanofluids depends on the fluid (blood or interstitial fluid) thermal conductivity, the fluid dynamic viscosity, and the nanoparticle (NP) shape thermal properties [6]. The enhancement of the thermal properties of the interstitial fluid or the blood due to the introduction of nanoparticles can be evaluated using mathematical or empirical correlations deduced from various experimental studies in the literature [7, 8].
The idea of enhancing the thermal conductivity of fluids by introducing nanoparticles (NPs) was proposed at the end of the twentieth century. Choi and Eastman [9] introduced the definition of nanofluids and proposed new classes of innovative fluids to improve heat transfer using suspending metallic NPs with conventional fluids. A comprehensive analysis of the factors that enhance the nanofluid thermal properties was presented and analysed by Buongiorno [10].
Introducing NPs into non-Newtonian fluids requires the employment of constitutive models distinct from those employed for Newtonian fluids [11]. For example, the tangent hyperbolic model, first introduced by Pop and Ingham [12], is a non-Newtonian fluid model used to understand and predict various flow properties of some industrial fluids such as paints, nail polish, ketchup, whipped cream, and other fluids [13]. Williamson model is also a non-Newtonian model that describes the flow of pseudoplastic fluids which have various applications in engineering and industry [14]. Second-grade viscoelastic model is another non-Newtonian fluid model used in the literature to study heat and mass transfer in non-Newtonian nanofluids [15]. Furthermore, the Casson model is a non-Newtonian model with less shear stress than the yield stress, but it starts to deform when shear stress becomes more significant than the yield stress. Rafique et al. [16] used the Buongiorno model to investigate Casson nanofluid flow over a nonlinear slanted stretching sheet with chemical reaction and heat generation/absorption. They found that the Casson factor reduced the fluid velocity, while the sheet inclination factor increased heat and mass transfer.
Studying fluid flow in biological tissues can be modelled as flow in a porous medium. Izadi and Sheremet [17] presented the phenomenon of thermal radiation transmission of nanofluid and thermal gravitational through a permeable material exposed to a magnetic field. They noticed a decrease in the Nusselt number value at large values of the Hartmann number. Aboud et al. [18] investigated the nanofluid flow through a hollow cylinder in a magnetic field. Their study showed that the fluid flow increased with the increase in the energy law indicators. In addition, they found a strong influence of the magnetic field on the fluid flow, which improved the heat transfer process by forced convection. Hatami et al. [19] simulated the heat and mass transfer in blood, a non-Newtonian fluid. They incorporated the effect of Brownian motion and thermal separation coefficient into their model. The results revealed that the increase in the thermal conductivity coefficient led to an increase in the temperature of the fluid and an increase in the concentration of the NPs.
On the other hand, they found a noticeable decrease in velocity due to the influence of the magnetic field. Islam et al. [20] analysed the flow of nanofluids in a permeable material surrounded by a cylinder exposed to a magnetic field. They found that the temperature of the nanofluids increased due to an increase in thermal electrophoresis, Brownian motion of the NPs, and the Eckert number, whereas the temperature decreased due to the increase in the Prandtl number. Mirza et al. [21] presented a mathematical model for blood transfusion in hydrodynamic and electromagnetic phases. They studied the influence of magnetic and electrical parameters on the heat transfer in blood and particles and the blood flow. They concluded that the electrical parameters significantly affected blood velocity and NP transport. Azhar et al. [22] studied mathematically the influence of a magnetic field on a Prandtl fluid flow and heat transfer. They found that increasing the Prandtl number improved the heat transfer in the fluid. In addition, they observed that the skin friction increased linearly with the Grashof number.
Mitragotri [23] presented various examples illustrating the importance of physical properties in biology, including size, shape, mechanical properties, and biological applications of biological materials. He concluded that the drug should penetrate the tissue well to obtain effective results for cancer treatment. According to the physiological aspects of cancer, several studies and strategies have been proposed to improve drug delivery efficiency through tumours [24]. For example, the structure of blood vasculature around the cancer is irregular, with a relatively small number of vessels. So, several therapeutical strategies incorporate antibodies or ligands to be compatible with tumour cell receptors to improve drug delivery [25]. Hence, introducing NPs to clinical techniques showed promising results over conventional clinical and preclinical techniques [26].
This paper presents a mathematical model that investigates the NP extravasation from an inclined blood vessel surrounded by tissue and subjected to a magnetic field. Additionally, we mimic the interaction between the magnetic field and the NPs, which generates heat in the tissue. This study also examines the tissue temperature that induces cell death in tumours. We found that the tumour-specific absorption coefficient plays a significant role in NP delivery to the tumour; and the efficacy of the tumour treatment using thermal therapy. The manuscript is structured as follows: Sect. 1 (the introduction) provides information on magnetohydrodynamic nanofluids, intrinsic thermal properties of nanofluids, and applications of nanofluids in biomedicine. The description of the physical model and the corresponding mathematical model are presented in Sect. 2 presents a description of the physical model and its associated mathematical model. Following is an explanation of the numerical solution approach in Sect. 3. Section 4 contains the outcomes of the mathematical model and the discussion, while Sect. 5 provides the conclusion of the paper.
2 Description of the physical model
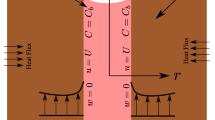
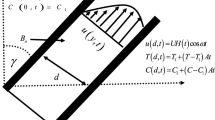
We consider the problem of an extended, circular cylinder inclined at an angle \(\alpha\), placed inside an incompressible viscous liquid and exposed to a magnetic field \({B}_{0}\), as shown in Fig. 1. We assume that both the concentration of NPs (\({C}_{w}\)) in the blood vessel and the blood temperature (\({T}_{f}\)) are constants. Moreover, we simulate the NP transport from the blood vessel to the adjacent tissue exposed to heat flux at the external tissue edge, the problem boundary. The heat source in this model is assumed to be an external heat source, such as an alternating magnetic field, that irradiates tissue at infinity, which is the heat flux source at the boundaries in this model.
We defined the incompressible Casson nanofluid by Casson [27] as;
where, \({\tau }_{ij}\) is the shear stress tensor (Casson yield stress tensor), \({\tau }_{0}\) is an infinite value of the Casson yield stress, \(\pi ={ e}_{ij}{ e}_{ij}\) is the product of the component of the deformation rate with itself, \({\pi }_{c}\) is the critical value of \(\pi\) based on the non-Newtonian model, \({e}_{ij}=\frac{1}{2} \left(\frac{\partial {u}_{i}}{\partial {x}_{j}}+\frac{\partial {u}_{j}}{\partial {x}_{i}}\right)\) is the strain tensor rate where \({u}_{i}\) denoting the components of fluid velocity, and \({\mu }_{{\varvec{B}}}\) is the plastic dynamic viscosity of the non-Newtonian fluid. The kinematic viscosity \(\upsilon\) of the Casson nanofluid is given by
where \(\rho\) is the density of fluid and \(\beta =\frac{{{\varvec{\mu}}}_{{\varvec{B}}\boldsymbol{ }\sqrt{2{\pi }_{c}}\boldsymbol{ }}}{{\tau }_{0}}\) is the Casson parameter, which represents the viscosity index of the non-Newtonian Casson nanofluid. Also, in the case \(\pi >{\pi }_{c}\) the shear stress is defined as [28, 29]
According to the above assumptions, we consider the system of governing equations as follows [30]:
where \((w,u\)) are the velocity components of the fluid in the radial direction and along with the vessel axial, respectively, and \(C\) is the concentration of NPs. Furthermore, \(g\) is the gravitational force,\({k}^{*}\) is the mean of the thermal parameter, \(\sigma\) is the electric conductivity, \({\sigma }^{*}\) is the Stefan–Boltzman constant, \({D}_{B}\) is the Brownian diffusion coefficient, \({D}_{T}\) is the thermophoretic diffusion coefficient, \(\alpha\) is the angle of inclination from horizontal, \({\beta }_{c}\) is the concentration expansion coefficient, \({\beta }_{T}\) is the thermal expansion, and \(SAR\) is the specific absorption rate (the rate of energy absorbed by the biological tissue). The heat capacities ratio of the NPs and the base fluid is denoted by \(\tau\), which is defined as \(\tau =\frac{{(\rho {c}_{p})}_{p}}{{(\rho {c}_{p})}_{f}}\), where \({c}_{p}\) denotes the heat capacity at constant pressure.
The boundary conditions corresponding to the model are given as follows:
where \(R\) is the radius of the cylinder.
We reduce the system of PDEs (4)–(9) into a system of ODEs using the subsequent similar transformations [30]:
The Stokes stream function \(\psi\) is introduced in the form
The Cauchy Riemann equation \(\psi\) is given by
Substituting Eq. (11) into Eq. (12), we get
Also, we define the dimensionless temperature and NP concentration in the form
Applying the transformations (10–14) to the momentum conservation equation (Eq. 5) we get
where \(K=\sqrt{\frac{\upsilon L}{{R}^{2} {U}_{0}}}\) is the curvature of the cylinder, \(H{a}^{2}=\frac{\sigma L {B}_{0}^{2}}{\rho {U}_{0}}\) is the magnetic field parameter, \({G}_{r}=\frac{g {\beta }_{T} \left({T}_{f}-{T}_{\infty }\right) {L}^{2}}{z {U}_{0}^{2}}\) is the thermal Grashof number and \({G}_{m}=\frac{g {\beta }_{c}\left({C}_{w}-{C}_{\infty } \right) {L}^{2}}{z {U}_{0}^{2}}\) is the mass Grashof number, \({K}^{*}=\frac{\upsilon L}{K {U}_{0}}\).
Similarly, applying the transformations (10–14) into the energy equation (Eq. (3)) we get
where \(Pe=Re Pr\) is the Peclet number, \(Pr=\frac{\upsilon }{{\alpha }_{f}}\) is the Prandtl number, \(Re=\frac{{U}_{w} z}{\upsilon }\) is the local Reynolds number, \({\alpha }_{f}=\frac{{k}_{f}}{{(\rho {c}_{p})}_{f}}\) is the nanofluid thermal diffusivity, \({R}_{d}=\frac{4 {\sigma }^{*}{\mathrm{T}}_{\infty }^{3}}{{K}_{f} {K}^{*}}\) is the thermal radiation parameter, \({N}_{b}=\frac{\tau {D}_{B}({C}_{w}-{C}_{\infty })}{\upsilon {\rho }_{p}}\) is the Brownian motion parameter, \(Nt=\frac{\tau {D}_{T }{(T}_{f}-{T}_{\infty })}{\upsilon { T}_{\infty }}\) is the thermophoresis parameter, \(Ec=\frac{{{U}^{2}}_{w}}{{({c}_{p})}_{f} {(T}_{f}-{T}_{\infty })}\) is the Eckert number, and finally, \(\lambda =\frac{{D}_{T} SAR {U}_{0}{L}^{2}}{{{k}_{f} D}_{B} {T}_{\infty } }\) is the specific absorption rate.
The dimensionless NP volume friction can be derived by applying the transformations (10–14) into the concentration equation (Eq. (7)) in the form
where \(Sc=\frac{\upsilon }{{D}_{B}}\) is the Schmidt number.
The boundary conditions (Eqs. (8–9)) can also be transformed using the dimensionless transformations (10–14) in the form:
We can explicitly estimate the dimensionless heat flux across the blood vessel wall (see Eq. 19) in the form
where\({A}_{1}=\frac{1}{Pr}\), \({A}_{2}=\frac{1}{Sc}\), \({k}^{*}=\frac{-k}{{U}_{0}\left({C}_{w}-{C}_{\infty }\right)}\), \({Q}_{H}^{*}=\frac{-{Q}_{H}}{{U}_{0}\left({T}_{f}-{T}_{\infty }\right)}\) \({\xi }_{1}=\frac{{T}_{\infty }}{{T}_{f}-{T}_{\infty }}\) and \({\xi }_{2}=\frac{{C}_{\infty }}{{C}_{w}-{C}_{\infty }}\).
The skin friction coefficient \({C}_{f}\) and the local Nusselt number \(Nu\), which are defined as:
where
\({\tau }_{w}\) is shear stress and \({q}_{w}\) is the heat flux at the surface of the stretching cylinder. Substituting Eq. (22) into Eq. (21) we get the skin friction and local Nuselt number in the form
3 Method of solution
We reduce the system of ordinary differential equations (ODEs) into a system of first-order ODEs using the following transformations [31].
So, the Eqs. (15–17) and (18–19) can be written in the form:
The boundary conditions (18, 19) can be written in the form
We impose the initial values of the model variables as: \({s}_{1}={s}_{2}={s}_{3}={s}_{4}={s}_{5}={s}_{6}={s}_{7}=0\). We solve the system of Eqs. (24 and 25) using bvp4c, a MATLAB function within a relative error \({10}^{-5}\) which is adequate to achieve convergence [32]. The convergence of the code results was proven using different spatial grids, using the parameter values listed in Table 1. We discuss the outcomes of the mathematical model in the section that follows.
4 Results and discussion
We have solved the governing Eqs. (15–17) according to the boundary conditions (18 and 19) using a 300-point spatial grid, which corresponds to a relative error of O(\({10}^{-4}\)). The numerical solution has been validated against two published papers in the literature as shown in Table 2. The findings of the model are depicted graphically in Figs. 2, 3, 4 and 5.
Figure 2 depicts the influence of the curvature of the cylinder coefficient (\(K\)) and the thermal radiation parameter \((Rd)\) on the axial velocity of the interstitial fluid (\(f{\prime}\)), the effect of the nanofluid temperature (\(\theta\)), and the NP concentration in the interstitial fluid (\(\phi\)). As expected, both \(\theta\) and \(\phi\) are maximal at the blood vessel wall but monotonically decrease across the tissue, see Fig. 2A, B and D, since the blood vessel is the source of the interstitial fluid and the NPs. However, as \(K\) increases, axial fluid flow in the tissue is resisted due to the increase in the electromagnetic force (Fig. 2C). As illustrated in Fig. 2B, with large values of \(K\), the interstitial fluid temperature elevates since more NPs accumulate in the tissue, increasing the thickness of the mass boundary layer. This spread of NPs across the tumour is due to the reduction in the viscous forces. Therefore, increasing \(K\) assists in delivering more NPs into the tumour, see Fig. 2A.
Interestingly, increasing the radiation parameter \((Rd)\) we observed that \(Rd\) has a negligible effect on the interstitial fluid velocity but a considerable impact on the temperature, as shown in Fig. 2D. Therefore, thermal radiation enhances the nanofluid temperature in the tissue (Fig. 2D).
Figure 3 shows the influence of the velocity of the interstitial fluid at the blood vessel wall (\(\gamma\)) and the influence of the thermophoresis parameter \((Nt)\) on the nanofluid temperature (\(\theta\)) and the NP concentration (\(\phi\)) in the interstitial fluid. The model indicates that the increase in the value of \(\gamma\) causes a considerable decrease in the concentration of NPs in the deep tumour cells (Fig. 3A) and also decreases the nanofluid temperature (Fig. 3B). Therefore, large pores at the blood vessel wall reduces the NP concentration in the tumour, consequently reducing the effectiveness of thermal therapy as a cancer treatment. In Figs. 3C and D the model demonstrates that the increase in the thermophoresis parameter (\(Nt\)) increases the temperature of the nanofluid (Fig. 3D), and the concentration of NPs within the tumour (Fig. 3C), which supports the stability of the NPs within the tumour tissue as a result of an increase in the thickness of the mass boundary layer.
Figure 4 visualise the effect of the of thermophoresis parameter \((Nt)\) on the axial velocity of the interstitial fluid (\({f}{\prime}\)) and the influence of the Brownian motion parameter \((Nb)\) on the nanofluid temperature and the NP concentration in the interstitial fluid. The model results demonstrate that the increase in the thermophoresis parameter raises the axial velocity of the interstitial fluid (Fig. 4A). Similarly, as the Brownian motion of the NPs increases, so does the temperature of the interstitial fluid (Fig. 4C), which raises the tumour temperature, so enhancing the thermal therapy efficacy. However, it significantly reduces the thickness of the mass boundary layer that resists the NP transport in the tissue, as shown in Fig. 4B.
The influence of the specific absorption rate coefficient parameter (\(\lambda\)) on nanofluid temperature, NP concentration and the velocity of interstitial fluid is illustrated in Figs. 4D and 5A and B. The results indicate that increasing \(\lambda\) decreases the NP concentration (Fig. 4D), reducing the thickness of the mass boundary layer. In contrast, increasing \(\lambda\) in the tumour increases both the fluid temperature and the interstitial fluid velocity, as shown in Fig. 5A and B, which improves the NP accumulation in the tumour. Finally, the effect of the Nusselt number (\(Nu\)) and heat flux (\({Q}_{H}^{*}\)) across the vessel wall on the different value of \(Rd\) is depicted in Fig. 5C and D. The results reveal that the increase of parameter \(Rd\) raises the Nusselt number, representing the heat transfer across the vessel wall (Fig. 5C). Similarly, Fig. 5D shows the heat flux at wall of the vessel against the radiation parameter \(Rd\) which confirms the enhancement of heat transfer across the vessel boundaries due to the increase in the radiation parameter.
Finally, in Table 3 we illustrate the impact of the radiation, the radial velocity of the interstitial fluid, and the thermophoresis on the heat transfer (\(Nu\)) and the skin friction at the wall of the vessel. The radiation parameter and the radial velocity of the interstitial fluid at the vessel wall increase the heat transfer from the blood vessel to the surrounding tissue, while the thermophoresis parameter reduces it. On the other hand, the skin friction is increased by the radiation coefficient and the thermophoresis parameter but decreased by the interstitial fluid extravasation velocity.
5 Conclusions
In this work, we studied the numerical analysis of a Casson nanofluid over an inclined cylindrical vessel surrounded by a hot tumour tissue. We looked into the effects of nanofluid velocity, tissue-specific heat absorption, thermal radiation and thermophoresis on NP transport and heat transfer in the tumour tissue. We developed a system of partial differential equations (PDEs) to simulate the fluid flow, NP transport, and heat transfer in the interstitial space. We closed the mathematical model with appropriate boundary conditions to determine the heat and mass flux across the tissue boundaries. Then, using similarity transformations, we converted the system of PDEs to a system of ODEs which were solved numerically by MATLAB.
We observed that NPs could easily permeate tissue at large curvature values of the cylinder, which increases NP concentration in the neighborhood of the vessel. In addition, increasing the velocity of the interstitial fluid reduces the thickness of the mass boundary layer, which resists the mobility of NPs within the tissue. Heat transport by thermophoresis in the tissue, on the other hand, increases the concentration of NPs inside the tissue, stabilising the NPs within the tumour tissue due to the increase in the thickness of the mass boundary layer. In addition, the Brownian motion of NPs raises the temperature in the tumour but reduces the NP concentration in the tissue. Furthermore, enhancing the tumour specific absorption coefficient optimises the efficacy of heat therapy by increasing the interstitial fluid velocity and enhancing the tumour tissue temperature.
However, this study assumed that the fluid flow, NP transport, and heat transfer are homogeneous over the circumference direction. Furthermore, we also assumed a steady state with a continuous supply of NPs from the vessel, which should be generalised in future work to imitate NP elimination by the immune system.
Data availability
Not applicable.
Abbreviations
- C:
-
Nanoparticle concentration
- \({C}_{w}\) :
-
Nanoparticle concentration at the upper wall
- \(C_{p}\) :
-
Specific heat at constant pressure
- \({C}_{\infty }\) :
-
Concentration in ambient flow
- \({T}_{\infty }\) :
-
Ambient fluid temperature
- DT :
-
Thermophoresis coefficient
- DB :
-
Brownian motion coefficient
- Gr :
-
Grashof number
- Gr1,..,7 :
-
Dimensionless constants
- g :
-
Acceleration due to gravity
- Ha :
-
Hartmann number
- \(Rd\) :
-
Radiation parameter
- \({Re}_{z}\) :
-
Local Reynolds number
- \(k\) :
-
Thermal conductivity
- \(k^{*}\) :
-
Coefficient of the mean absorption
- \(K\) :
-
Curvature of cylinder
- \(Nb\) :
-
Brownian motion parameter
- \(Nt\) :
-
Thermophoresis parameter
- \(p\) :
-
Fluid pressure
- \(P\) :
-
Dimensionless pressure
- \(\Pr\) :
-
Prandtl number
- \({Nu}_{z}\) :
-
Nusselt number
- Re:
-
Reynolds number
- \({B}_{0}\) :
-
Magnetic field strength
- \({q}_{w}\) :
-
Surface heat flux
- T:
-
Temperature
- \(Q\) :
-
Heat generation/absorption parameter
- u, v :
-
Velocity components in x, y directions
- \(U,V\) :
-
Dimensionless velocity components
- \(x,y\) :
-
Cartesian coordinates, m
- \({e}_{ij}\) :
-
Deformation rate
- \(\alpha\) :
-
Thermal diffusivity
- \(\beta\) :
-
Thermal expansion coefficient
- \(\phi\) :
-
Dimensionless nanoparticle concentration
- \(\sigma\) :
-
Effective electrical conductivity
- \(\theta\) :
-
Dimensionless temperature
- \(\mu\) :
-
Dynamic viscosity
- \(\nu\) :
-
Kinematic viscosity
- \(\rho\) :
-
Density
- \(\lambda\) :
-
Dimensionless constant velocity
- \(\gamma\) :
-
Dimensionless constant velocity
- \(R\) :
-
Radius of cylinder
- \(0\) :
-
Reference
- \(f\) :
-
Pure fluid
- w:
-
Wall
References
Lane LA, Qian X, Smith AM, Nie S (2015) Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Ann Rev Phys Chem 66:521–547. https://doi.org/10.1146/annurev-physchem-040513-103718
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171. https://doi.org/10.1038/nrc1566
Kim B, Han G, Toley B, Kim C, Rotello V, Forbes N (2010) Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol 5:465–472. https://doi.org/10.1038/nnano.2010.58
Hirsch L, Stafford R, Bankson J, Sershen S, Rivera B, Price R, Hazle J, Halas N, West J (2003) Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci 100:13549–13554. https://doi.org/10.1073/pnas.2232479100
Huang L, Hu J, Huang S, Wang B, Siaw-Debrah F, Nyanzu M, Zhanga Y, Zhugeac Q (2017) Nanomaterial applications for neurological diseases and central nervous system injury. Prog Neurobiol 157:29–48. https://doi.org/10.1016/j.pneurobio.2017.07.003
Hu J, Huang W, Huang S, ZhuGe Q, ** K, Zhao Y (2016) Magnetically active Fe3O4 nanorods loaded with tissue plasminogen activator for enhanced thrombolysis. Nano Res 9:2652–2661. https://doi.org/10.1007/s12274-016-1152-4
Huang D, Wu K, Zhang Y, Ni Z, Zhu X, Zhu C, Yang J, ZhuGe Q, Hu J (2019) Recent Advances in Tissue plasminogen activator-based nanothrombolysis for ischemic stroke. Rev Adv Mater Sci 58:159–170. https://doi.org/10.1515/rams-2019-0024
Hu J, Huang S, Zhu L, Huang W, Zhao Y, ** K, ZhuGe Q (2018) Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl Mater Interfaces 10:32988–32997. https://doi.org/10.1021/acsami.8b09423
Choi U, Eastman J (1995) Enhancing thermal conductivity of fluids with nanoparticles, No. ANL/MSD/CP-84938; CONF-951135-29. Argonne National Lab.(ANL), Argonne, IL (United States). https://digital.library.unt.edu/ark:/67531/metadc671104
Buongiorno J (2006) Convective transport in nanofluids. ASME J Heat Transf 128:240–250. https://doi.org/10.1115/1.2150834
Brujan E (2011) Cavitation in non-Newtonian fluids: with biomedical and bioengineering applications. Springer-Verlag, Berlin Heidelberg. https://doi.org/10.1007/978-3-642-15343-3
Pop I, Ingham D (2001) Convective heat transfer: mathematical and computational modelling of viscous fluids and porous media. Elsevier, Amsterdam
Mernone A, Mazumdar J, Lucas S (2002) A mathematical study of peristaltic transport of a Casson fluid. Math Comput Model 35:895–912. https://doi.org/10.1016/S0895-7177(02)00058-4
Khuram R, Alotaibi H (2021) Numerical simulation of Williamson nanofluid flow over an inclined surface: Keller box analysis. Appl Sci 11:11523. https://doi.org/10.3390/app112311523
Abid K, Azhar E (2022) Numerical outlook of a viscoelastic nanofluid in an inclined channel via Keller box method. Int Commun Heat Mass Transf 137:106260. https://doi.org/10.1016/j.icheatmasstransfer.2022.106260
Rafique K, Imran M, Anwar M, Misiran M, Ahmadian A (2021) Energy and mass transport of Casson nanofluid flow over a slanted permeable inclined surface. J Therm Anal Calorim 144:2031–2042. https://doi.org/10.1007/s10973-020-10481-9
Izadi M, Sheremet M, Mehryan S, Pop I, Oztop H, Abu-Hamdeh N (2020) MHD thermogravitational convection and thermal radiation of a micropolar nanoliquid in a porous chamber. Int Commun Heat Mass Transf 110:104409. https://doi.org/10.1016/j.icheatmasstransfer.2019.104409
Aboud E, Rashid H, Jassim H, Ahmed S, Khafaji S, Hamzah H, Ali F (2003) MHD effect on mixed convection of annulus circular enclosure filled with non-Newtonian nanofluid. Heliyon 6:e03773. https://doi.org/10.1016/j.heliyon.2020.e03773
Hatami M, Hatami J, Ganji D (2014) Computer simulation of MHD blood conveying gold nanoparticles as a third grade non-Newtonian nanofluid in a hollow porous vessel. Comput Methods Programs Biomed 113:632–641. https://doi.org/10.1016/j.cmpb.2013.11.001
Islam S, Khan A, Kumam P, Alrabaiah H, Shah Z, Khan W, Zubair M, Jawad M (2020) Radiative mixed convection flow of maxwell nanofluid over a stretching cylinder with joule heating and heat source/sink effects. Sci Rep 10:1–18. https://doi.org/10.1038/S41598-020-74393-2
Mirza I, Abdulhameed M, Vieru D, Shafie S (2016) Transient electro-magneto-hydrodynamic two-phase blood flow and thermal transport through a capillary vessel. Comput Methods Programs Biomed 137:149–166. https://doi.org/10.1016/j.cmpb.2016.09.014
Azhar E, Kamran A, Atif S (2022) Exploring the dynamics of natural convective Prandtl fluid flow subjected to induced magnetic field. Waves Random Complex Media. https://doi.org/10.1080/17455030.2022.2089931
Mitragotri S, Lahann J (2009) Physical approaches to biomaterial design. Nat Mater 8:15–23. https://doi.org/10.1038/nmat2344
Minchinton A, Tannock I (2006) Drug penetration in solid tumours. Nat Rev Cancer 6:583–592. https://doi.org/10.1038/nrc1893
Allen T (2002) Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2:750–763. https://doi.org/10.1038/nrc903
Davis M, Chen Z, Shin D (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 8:771–782. https://doi.org/10.1038/nrd2614
Casson N (1952) A flow equation for pigment-oil suspensions of the printing ink type. Rheol Disperse Syst (1952) 84–104
Eldabe N, Salwa M (1995) Heat transfer of MHD non-Newtonian Casson fluid flow between two rotating cylinder. J Phys Soc Jpn 64:41–64
Mustafa M, Hayat T, Pop I, Hendi A (2012) Stagnation-point flow and heat transfer of a Casson fluid towards a stretching sheet. Z Naturforsch 67:70–76. https://doi.org/10.5560/ZNA.2011-0057
Tadesse W, Eshetu G, Tesfaye K, Assaye W (2020) Analytical study of heat and mass transfer in MHD flow of chemically reactive and thermally radiative Casson nanofluid over an inclined stretching cylinder. J Phys 4:125003. https://doi.org/10.1088/2399-6528/abcdba
Ismaeel A, Mansour M, Ibrahim F, Hady F (2022) Numerical simulation for nanofluid extravasation from a vertical segment of a cylindrical vessel into the surrounding tissue at the microscale. Appl Math Comput 417:126758. https://doi.org/10.1016/j.amc.2021.126758
Cai W, Su N, He X (2016) MHD convective heat transfer with temperature-dependent viscosity and thermal conductivity: a numerical investigation. J Appl Math Comput 52:305–321. https://doi.org/10.1007/s12190-015-0942-2
Murthy MK, Raju CSK, Nagendramma V, Shehzad SA, Chamkha AJ (2019) Magnetohydrodynamics boundary layer slip Casson fluid flow over a dissipated stretched cylinder Defect and Diffusion. Forum. https://doi.org/10.4028/www.scientific.net/DDF.393.73
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The Science, Technology & Innovation Funding Authority (STDF) provides open-access funding in cooperation with The Egyptian Knowledge Bank (EKB). However, during the preparation of this manuscript, no funds, grants, or other support was received by the authors.
Author information
Authors and Affiliations
Contributions
Writing, formal analysis, and computation of the numerical results were handled by RS; review and editing by AM; and final revision and supervision by FM and MR
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismaeel, A.M., Kamel, R.S., Hedar, M.R. et al. Numerical simulation for a Casson nanofluid over an inclined vessel surrounded by hot tissue at the microscale. SN Appl. Sci. 5, 223 (2023). https://doi.org/10.1007/s42452-023-05436-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05436-2