Abstract
This work presents the manufacture of CKD-blended cement from raw materials such as clinker, gypsum, marble and cement kiln dust (CKD) obtained from CIMENCAM Figuil, North Region of Cameroon. The raw materials were first characterized through XRF chemical analyses. Chemical tests (proportion of free lime, loss on ignition, sulfate, and insoluble residues), physical tests (fineness, consistency, setting time, and expansion), and compressive strength tests were carried out on manufactured CKD-blended cements and mortars. Clinker is composed of CaO (65.30%) and SiO2 (21.13%), with significant MgO (2.71%), Na2O + K2O (1.29%), SO3 (0.81%) and lime saturation factor of (97.9); which make this clinker good for cement production. Gypsum is composed of SO3 (36.32%) and CaO (33.92%) but contains very low proportion of impurities; it is therefore classified as low-impurities gypsum suitable for cement productions. The marble is dominantly composed of CaO (37.09%) with significant SiO2 (7.26%), SO3(2.40%), (2.36%), Fe2O3 (1.82%) and MgO (0.18%); it is classified as low-CaO calcitic-aluminosilicate and ferrosilicate bearing marble. The CKD is dominantly made up of CaO (46.0%) and SiO2 (11.62%) with significantly low Na2O + K2O (1.02%), and SO3(2.16%); this classifies it as low alkalies-sulfate cement kiln dust. The proportion of free lime, SO3, loss on ignition, and insoluble residues in the manufactured CKD-blended cements increase with an increase in the proportion of CKD. The blaine specific surface area (BSSA) (4797–6346 cm2/g) and the sieved residues (26.14–30.36%) show an increase with the proportion of used CKD. The compressive strength tests carried out on the prepared brick-shape hardened CKD-cement mortars (at 2, 7, and 28 days) show that, the resistance depends on the proportion of the used CKD (0% control, 5%, 10%, 15%, 20%… up to 50%). Pressures of 17.5 to 11.6 MPa was obtained in 2 days, 28.9 to 20.0 MPa in 7 days, and 35.5–23.3 MPa in 28 days.
Article Highlights
-
1.
Raw materials (clinker gypsum, marble, and cement kiln dust) are characterized and used to process CKD-blended cements.
-
2.
Clinker is sintered and has a good resitance.
-
3.
Gypsum is a low-impurities gypsum.
-
4.
Marble is a low-CaO calcitic-aluminosilicate-ferrosilicate bearing marble.
-
5.
CKD is a low alkalies-sulfate ciment kiln dust.
-
6.
The chemical and physical tests carrried out on the cements mainly show an increase with the increase in the proportion of CKD.
-
7.
The comprehensive strenght tests carried out on brick-shape hardened CKD-blended cement mortars shows that
The resistance depends on the proportion of the used CKD
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cement industries in the world, are now focused on how to be highly performant by producing high quality products while reducing environmental and health pollution, and minimize cost account during different steps of cement production [1,2,3,4,5]. The perfection in cement production can be done in five ways [1,2,3, 5,6,7,8,9]: (1) use modern and sophisticated clinker production equipments; (2) a constant follow-up and improving the quality of produced clinker; (3) reduction of energy consumption during clinkerization; (4) improve the composition of the used cement raw materials; and (5) use of additives (both natural and industrial) to reduce the quantity of clinker. Additives such as rice husk ash, volcanic ashes, blast fumace slag, silica fume, coal fly ash, siliceous fly ash, calcareous fly ash, limestone fines, shale and metakaolin are used to produce blended cements [4, 9,10,11,12,13]. These blended cements are considered as environmentally friendly cements with reduced proportion of CO2 emission and energy saving during processing [14].
Cement production is the cause of many environmental problems such as land degradation, toxic metal pollution (e.g., Hg, Cd, Tl, As, Sb, Pb, Cr, Co, Cu, Mn, Ni, and V), and global warming effets, due to the release of CO2 to the atmosphere [4, 9, 14, 15]. Other emitted polluting substances during cement production include: dust, carbon monoxide, nitrogen oxides, sulphur oxides, polychlorinated dibenzo-p-dioxins, dibenzofurans, total organic carbon, metals, hydrogen chloride, and hydrogen fluoride [1, 16,17,18]. Air pollution by dust, is mainly recorded during milling of cement raw material and clinkerization [9, 19]. CO2 emissions are from two sources: when raw materials are heated with combustible (e.g., natural gas, coal, petroleum coke, fuel oil, and tires) and during clinkerization [14]. Waste from milling of cement raw materials are used for landfilling which also creates environmental problems [1, 8, 20]. Air pollution by dust can be minimized by capturing dust and their transportation to collectors; and later used as clinker raw material if their composition is suitable [1, 6, 8, 9].
In CIMENCAM Figuil (North Region of Cameroon), 10,000 tons of cement production generate 1400 tons of cement kiln dust in 2 months [21]. The waste causes environmental and health problems related to respiratory issues and it also incure a loss of milled and mixed raw materials estimated at 26 Euro/ton [21]. High alkaline dust are considered not suitable to be fed in a clinkerization process; except they are pretreated [9]. To avoid or reduce environmental pollutions and health problems caused by the produced CKD in Figuil, and minimize cost account during different steps of cement production, the CKD can be sampled and used to manufacture CKD-blended cements, as in recent studies (e.g., [1, 3, 6]). The current work aims to manufacture blended cement with cement kiln dust produced in CIMENCAM Figuil. It is organized into four steps including: determination of the chemical features of raw materials (clinker, gypsum, marble, and ciment kiln dust) used to manufacture the CKD-blended cements; processing of CKD-blended cements; determine the chemical features and physical properties of the manufactured CKD-blended cements and verify if these cements samples meet the required standard; and produce CKD-blended cement- sand hardened mortars and determine its mechanical properties.
The current manuscript is structured as followed: after the introduction, the manuscript includes the overview on cement kiln dust, cement raw materials used in CIMENCAM Figuil and type of dust produced in this company. It also includes materials and methods used to characterize the used cement raw materials, to manufacture the CKD-blended cements, and those used to carry out chemical, physical and mechanical tests on the manufactured cements and processed CKD-blended cement mortars. At its last stage, the manuscript presents and discusses results characterizing the raw materials used to manufacture the CKD-blended cements, and those for quality (chemical, physical, and mechanical) tests carried out on the manufactured CKD-cements and cement mortars.
2 Overview on cement kiln dust, cement raw materials used in CIMENCAM Figuil and type of dust produced
2.1 Characteristics of cement kiln dust
Cement is a hydraulic binder obtained by firing of mainly limestone and clay at approximately 1400 °C [9], 1450 °C [22], and 1450–1500 °C [14], with addition of gypsum, and in some cases, of additives [4, 13, 23]. Additives are natural occurring substances (minerals or rocks) and industrials products with properties suitable to partially replace clinker during the last stage cement manufacturing [4, 12, 14, 23]. Examples of used additives include volcanic scoria, blast fumace slag, silica fume, siliceous fly ash, calcareous fly ash, limestone fines, shale, metakaolin and cement kiln dust [4, 10, 11, 13, 14, 17]. The cement kiln dust is a highly alkaline material and a mineral by-product collected via the electrostatic filters during clinkerization [3, 17, 24]. It is also defined as a fine powdery material (mainly passing a 90 μm sieve) that is generated during the cement manufacturing process, then carried off in the flue gases, and subsequently collected in baghouses or electrostatic precipitators [25]. This dust is mainly composed of the free lime dust, volatile compounds, enriched salts of alkaline sulfate, and halide [3, 17, 24]. The chemical and physical properties of a cement kiln dust depend on many factors such as type of raw material, type of operation, type of dust collection facilities, and type of used fuel [3, 24]. Cement kiln dust is chemically the fingerprinting of the cement kiln raw feed which primarily consists of calcium carbonate and silicon dioxide with considerable amount of alkalies, chloride and sulfate in CKD [3]. The mineralogical composition of CKD presented in Bochenczyk [19] showed the presence of four main components from different sources: those from raw mill (e.g., quartz, calcite, aragonite, and/or dolomite); alkali condensation product (sylvine); clinker phases (alite and belite); and hydrates formed as a result of air exposure or Portland clinker phase hydrates (portlandite).
2.2 Type of cement raw materials and dust in CIMENCAM Figuil
CIMENCAM Figuil used the following raw materials [21]: main raw materials (marble 80 and clay 20%) and adjustment raw materials (iron ore, bauxite, and sand). Marble is mined at the Bidzar quarry found at about 27 km north to the cement industry [21]. In this quarry, marble deposit is overlain from down to the top by calcareous schist, chlorite schist, mica schist and quartzite [26,27,28]. Clay is mined in Figuil. Sand is mined from sand deposits found in and around Mayo-Louti River (flowing in the south and East of the town of Figuil). The other adjustment raw materials (iron ore and bauxite) are not found localy. They are probably imported [21].
Five main types of dusts are produced from exploitation of raw materials to transportation of the manufactured cement in CIMENCAM Figuil [21].These include: dust produced during exploitation of marble, clay and sand; dust emissions during transportation and storage of the mined raw materials; dust produced during crushing and milling of the raw materials; dust-gas generated from the oven during clinkerization (CKD); and dust produced during packaging and transportion of the manufactured cement. As presented in Khouan and Lebdiri [29] for study carried out in other cement industry, this dust-gas association is very fine-grained and rich in alkaline elements.
3 Materials and methods
The materials and methods for this study include those used: for chemical analysis of cement raw materials, to formulate CKD-blended cement, and to carry out chemical, physical and mechanical tests on the manufactured cements, cement pastes, or hardened sand-cement mortars.
3.1 Chemical analysis of raw materials used to manufacture CKD-blended cement
The following raw materials (marble, clinker, gypsum and CKD) used to manufacture CKD-blended cements in this study, were analyzed to determine their major element composition in CIMENCAN Figuil, North Cameroon. This chemical analysis was carried out with the aid of an XRF (German XRF9900 thermo spectrometer). It was done in two phases: sample preparation and sample analysis. The sample preparation began with a separate crushing (with STE French and PB Vibro Rocklabs crusher) of marble, clinker, and gypsum to a grain size of 1 cm. Crushed samples were milled and powdered to less than 1 µm grain size. The sample analysis began with the mixture of 1.0 g of each powdered sample (marble, clinker, gypsum, or CKD) with 8 g of lithium tetraborate. Each mixture was put in a platinium-gold alloy (called crucible) and homogenized. After this step, 1 ml of lithium bromide was added to the homogenized mixture with the aid of a micropipette. The obtained substance was immediately put and heated in an electric furnace at 1150 °C, for 20 min. The heated solution was removed, pour into a crucible to obtain a molten pearl, and allowed to cool in few minute in the ambient air. The analysis was launched following the instructions of the used X-ray florescence spectrometer and after having entered the value of the loss on ignition, followed by those of other quantified chemical elements in the analysis program. The results appear 5 min afterwards on the connected computer monitor.
3.2 Formulation of CKD-blended cements
The experimental processing of CKD-blended cement samples was carried in CIMENCAM Figuil. The type of chosen cement is Multix (CEM IV 32.5R). The proportions (%) of raw materials (clinker, gypsum, marble, and CKD) were obtained after a series of calculations based on Portland cement proportions (96% of clinker and 4% of gypsum) and that of multix produced in CIMENCAM (clinker: 62.5%, gypsum: 3.9%, and marble additive: 33.6%). Eleven cement samples (CEM1 to CEM11) were processed by replacing marble additive with CKD, following the proportion in % (Table 1) and in grams (Table 2).
After formulation, each produced cement was crushed and milled (with STE French and PB Vibro Rocklabs crusher) following this procedure (Fig. 1); 5 kg of each produced cement was weighed in a bucket, put in crushing-milling machine, and closed. Each sample was crushed and milled at a 45 min standardized time. The crushed-milled sample was collected and sieved with a 200 µm sieve to separate milled fractions from those which were not milled. After crushing and milling of each sample, the maching was cleaned, and emptied. A new sample was weighed and put in the cleaned machine for crushing and milling.
3.3 Chemical and physical tests on produced CKD-blended cements
Chemical and physical tests were carried out on each manufactured cement sample in order to know if they meet the cement’s quality control standard. The chemical tests carried out on the produced cements include: the determination of the proportion of free lime, loss on ignition (LOI), sulfate (SO3), and insoluble residues (IR). Physical tests include: the determination of cement fineness of cement powders and normal consistency, setting time, and expansion carried out on cement pastes.
3.3.1 Chemical tests
3.3.1.1 Determination of free lime
The free lime is the lime which never reacted and combined during clinkerization. The procedure of it’s determination by EN 196–2 [29] method was as follows: 1 g of milled cement was weighed and put in a 50 ml beaker. 50 ml of ethylene glycol was put in the cement-bearing beaker. The obtained solution was heated at 80 °C on a hot plate, and mixed with a magnetic stirrer for 30 s. After these steps, the obtained mixture was filtered with a filter paper (41) placed in an erlenmeyer flask. The filtered product was titrated with HCl at 0.1 N until a yellow color appeared after adding 2 to 3 drops of bromophenol blue. The value of the free lime was obtained from this formula:
%FCaO: percentage of free lime and V: volume of used HCl acid (ml).
3.3.1.2 Determination of loss on ignition
The determination of the loss on ignition (LIO) by NA 235 [31] method, was as follows: 1 ± 0.05 g (m0) cement was weighed and put in an ignited crucible (a total weight: m1). The cement-bearing crucible was placed in a controlled electric oven at 1150 ± 25 °C for 20 min. The heated-dried cement-bearing crucible was removed and allowed to cool in a desiccator (at a room temperature), and its constant weight (m2) was determined. The value of m1 and m2 were used to calculate that of loss on ignition from this equation:
With, m1: the weight before calcination; m2: the weight after calcination; and m0: the weight of sampled cement.
3.3.1.3 The determination of the proportion of sulfate (SO3)
The sulfate content was determined by the EN 196–2 [30] method. This method consists in determining the proportion of SO3 from a precipitated barium sulfate complex. Sulfate ions formed during the decomposition of cement by hydrochloric acid, are precipitated at a pH ranging from 1.0 to 1.5 produced by a barium chloride solution. Barium sulfate precipitation is done at the boiling point. The determination is completed gravimetrically, and the sulfate expressed as SO3. The procedure to determine the SO3 content in the produced cement samples was as follows: 1 ± 0.05 g (m1) of cement was weighed and put in a beaker containing 90 ml of cold distilled water. The mixture was stirred while adding 10 ml of diluted concentrated hydrochloric acid. The obtained solution was heated at a temperature below boiling point for 15 min. After the heating phase, the heated solution was filtered with a filter paper into a 400 ml beaker. The retained residues were washed with distilled water to a filtrate volume of approximately 250 ml. 10 ml of barium chloride solution (BaCl2) was added on filtrate when heating to a boiling temperature for 15 min. Barium sulphate (BaSO4) was formed during heating. The formed barium sulfate was filtered with a No. 41 filter paper and washed with hot distilled water. The barium sulfate-bearing filter paper was calcined for 15 min, and allowed to cool at a room temperature in the desiccator. The weight (m2) of BaSO4 was measured and used to calculate the SO3 content from this formula:
With, m1: the weight of the calcinated sample, m2: the weight of the empty crucible, and m: the weight of the sample before calcination.
3.3.1.4 Determination of the insolubles residues (IR)
The insoluble residues (by the EN 196-2 [30] method) was determined by treating each cement sample with dilute hydrochloric acid in order to minimize the precipitation of soluble silica. The obtained residues were treated again with a solution of sodium carbonate to eliminate trace of formed silica. The determination of the insoluble residues was as follows: 90 ml of cold distilled water was put on 1 g manufactured cement, mixed, and 10 ml of concentrated HCl acid was rapidly added. The obtained solution was heated on a hot plate, and crushed with the flatter end of a stirring glass rod until a complet decomposition. The heated solution was allowed to digest for 15 min below the boiling temperature, before being filtered through a N°41 filter paper into a 400 ml breaker. The filter paper and host residues were washed with a hot water and put in a beaker. After filtering, 100 ml of sodium carbonate was added in the beaker, and the solution stirred with the aid of a glass rod. The stirred solution was heated until it boils. The boiled mixture was filtered, and the residues were rinsed with hot water. The filter paper and its rinsed residues were calcined for 15 min in a crucible. The crucible was allowed to cool before measuring its weight. The proportion of insoluble residues was calculated from this equation:
With, m1: the weight of the calcinated sample, m2: the weight of the empty crucible, and m: the weight of the sample before calcination (m = 1 g of produced cement).
3.3.2 Physical tests
Physical tests carried out of the processed cement samples include the determination of cement fineness (blaine specific surface area and cement sieving) carried out on cement powders, and other properties (normal consistency, setting time, and expansion) carried out on cement pastes.
3.3.2.1 Determination of the blaine specific surface area
The blaine fineness or blaine specific surface area (BSSA) of a cement powder is a single parameter that is meant to characterize the specific surface area; and therefore, the fineness of a cement, and is assumed to be linked to its physical and mechanical properties, such as rheology, setting time, and strength of the fluid and hardened cement paste [32]. The determination of air permeability (by blaine EN 196-6 [33] method) or cement fineness is measured in specific surface area by observing the time required for a fixed amount of air to flow through a bed of compacted cement of specified dimensions and porosity. The procedure to determine the BSSA was as follows: The blaine measuring instrument (Blainemeter AGL6697) was placed at the temperature controlled room (20 ± 2 °C), while verifying if the cement temperature corresponds to that of the room. Each cement sample was homogenized to a uniform distribution in a cell, and a required proportion for the test was weighed. A maximum precaution was taken to avoid any loss of sample after weighing. One filter paper was placed at the top and a the other one at the bottom of the prepared cement bed. After entering the plunger, the cement sample was compressed slowly and carefully while being sure that the plunger collar was in contact with the top of the cell. The plunger was slowly pull out at the start to prevent the washer from being sucked in by creating a vacuum; while being sure that the liquid level at rest in tube U was in position M. Vaseline was induced in the knotted part of the cell. This cell was placed on the tube and the valve open. The air was sucked from the tube through the pear until the liquid reaches the upper mark M1. The tap was closed and the manometric fluid descended. The time (t) between level crossing marks M2 and M3 was measured with a stopwatch. The relationships between K and the blaine specific surface area calculations (BSSA) was determined from the following equations:
With, K: a calculated constant; Its value varies from one product to another. K = 2.6007. t = time (s), e = porosity of the layer (e = 0.500), Ρ = density of ciment ( g/cm3), n = air viscosity at laboratory temperature, and BSSA (cm2/g).
3.3.2.2 Determination of cement fineness by sieving method
The determination of the proportion of residues in a cement sample (with the aid of a 63 µm EN 196-1 [34, 35] Alpine sieve: Cap 200LS-N) was done by sieving each cement sample with standard sieves and weighing of the retained fractions. Approximately 10 ± 0.01 g (M1) of cement was weighed and carefully put in a clean-dried sieve. The sieve was shaken with swirling, planetary, and linear motions until no finer material passes through it. The residues in the sieve were sampled and weighed. The obtained weight (M2) was recorded and expressed in percentage.
3.3.2.3 Determination of normal consistency
The normal consistency was determined on 500 g ± 1 g of a pure cement paste (with a W/C ratio of 0.26) and mixed mechanically with the aid of a mixer. The procedure started with introducing water and cement in a bowl (done in 10 s maximum time). A mixer (L0032/A2) introduced in the bowl was used to mix cement and water to form a paste. The mixer started with a low speed immediately it was introduced in the bowl. The starting time (zero) was recorded. The mixing stopped after 90 s and wait for 30 s (a period which a plastic squeegee was used to remove all the cement paste adhering to the sides and bottom of the bowl and placed in the middle of the bowl). The mixing process restarted at low speed for 90 s, after each sample was processed. The total mixing time was 3 min.
3.3.2.4 Setting time
The setting time to be determined includes the initial setting and the finial setting time. The determination of the initial setting time was as follows: The filled mold and the base plate were placed in a container. Water was added into this container up to the level that the surface of the dough was covered with at least 5 mm of water. This container was stored in the temperature-controlled room at 20.0 ± 1.0 °C. After an appropriate time, the container with mold and base plate were placed under the needle of the Vicat apparatus (Xs 300G). This needle was lowered gently until it touches the paste (mold); and 1 to 2 s pause time was observed in this position to avoid giving an initial speed or a forced acceleration to the moving parts. The needle was allowed to penetrate vertically into the dough (Fig. 2a); and the scale was read at the end of penetration or 30 s after needle was released. The time elapsed from time zero until the distance between the needle and the base plate was 6 ± 1 mm. This time was considered to be the time at which the cement begins to set.
The determination of the final setting time was as follows. The filled mold was inverted on its base plate so as to perform the end-of-set test on the side of the specimen which was originally in contact with the base plate. The mold and base plate were immersed in a container and stored in the temperature-controlled room at 20.0 ± 1.0 °C. After an appropriate time, the mold, base plate and container were placed under the needle of the Vicat device. The needle was lowered gently until it touches the paste. 1 to 2 s pause time was observed in this position to avoid giving an initial speed or a forced acceleration to the moving parts; and the needle was allowed to penetrate vertically into the dough. The scale was red at the end of penetration or 30 s after needle was released. The penetration operation was repeated on the same specimen at regularly spaced points, located at least 8 mm from the edge of the mold or 5 mm from each other, and at least 10 mm from the last point of penetration, and at intervals suitable times, for example, 30 min. Between penetration operations, the test specimen was kept in the container in the temperature-controlled enclosure. The Vicat needle was cleaned immediately after each penetration. The time measured from time zero after which the needle penetrates, for the first time, only 4.5 mm into the test tube was recorded. This time corresponds to the moment when the annular accessory ceases to leave a trace on the specimen and it can be established with precision by reducing the time interval between penetrations as the final setting time approaches. The final setting time was confirmed by repeating the test at two other points. The nearest 15 min, the time measured from instant zero, at the end of which the needle only penetrates 4 ± 0.5 mm into the sample for the first time, was reported.
3.3.2.5 Expansion
According to the European Standard, the device used to measure expansion is the Chatelier mold (Fig. 2b). This test consists in making a paste of standard consistency which will be introduced into an expansion mold. After filling, the mold was kept for 24 h in the humid room or in a storage tank. After this time, the spacing between the tips of the needles was measured. The mold was immersed in water which is gradually brought to a boil. After boiling for 3 h, the final needle point separation was measured again without waiting for the samples to cool. Expansion was characterized by increasing the spacing of the ends of the two needles.
3.4 Mechanical tests on the CKD-blended cement mortars
The mechanical cement quality control test carried out in this study, is the compressive strength. It was done base on the EN-196-1 standard [35]. This test was carried out on thirty three (33) brick-shape hardened sand-cement mortars prepared with the mixture of CEN standardized sand, water and eleven (11) manufactured CKD-blended cement samples.
3.4.1 Sample preparation
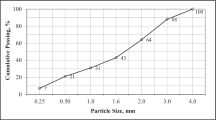
Sand used in preparing mortars is a CEN standardized sand (ISO [36] standardized sand), natural, clean, siliceous, fine-grained, and generally isometric and rounded in shape. This sand was dried, screened and 1350 ± 5 g was packaged in a polyethylene bag. Sieve grain size distribution analysis was performed on each sample with a total weight at least equal to 1350 g. Sieving continued until the amount of sand passing through each sieve was less than 0.5 g/min. The used CKD-blended cement was exposed to the ambient air for the shortest time and homogenized mechanically as described in NA 442 [37] before a fraction for the test was collected. Distilled water prepared in the Figuil cement plant laboratory was used for the validation tests, whereas, potable water was used for other tests, as a function of the water/cement ratio. Each manufactured CKD cement sample was used to prepare three brick-shape hardened mortars (length: 16 cm, width: 4 cm, and tickness: 4 cm). A total of 33 brick-shape hardened mortars were processed with eleven cement samples.
The brick-shape hardened mortars preparation proportionally by weight of used materials was done as follows. One part (CKD-blended cement), three parts (standardized sand), and one-half part (water: water/cement ratio = 0.50) were used. A mix for three test specimens consist of 450 ± 2 g of cement, 1350 ± 5 g of sand, and 225 ± 1 ml of water was required. After the steps presented above, the mixing procedure was as follows: Water and cement were introduced into a bowl (taking care to avoid any loss of water or cement). A mixer was put in the bowl as soon as the water and the cement come into contact. The mixing started at low speed with the timing of the mixing steps. The start time was recorded as ‘time zero’. After 30 s of mixing, all the sand was regularly introduced. The mixer was put on high speed and continued mixing for an additional 30 s. The mixer was stopped after 90 s. During the first 30 s, a plastic squeegee was used to remove all the mortar adhering to the sides and bottom of the bowl and placed in the middle of the bowl. The mixing process restarted at high speed for 60 s.
After mixing of the materials (sand, cement, and water), mortars specimens were molded (Fig. 3) as follows. The specimens were molded immediately after the preparation of the mortar. The first of the two layers of mortar (each of approximately 300 g) were introduced with a spoon into each of the mold compartments. The introduced coat was spread evenly with the aid of a large spatula, held almost vertically. The first layer of the spread mortar was tightening with 60 impacts of the impact device. The second layer of mortar was introduced with a small spatula and tightened again with 60 impacts, before being covered. Each covered mold was rapidly placed on a horizontal support in the humid room. Precautions were taken to be sure that the humid air reaches all sides of the mold; and that the molds are not tacked on top of each other, and make sure that each mold is removed from the storage medium at the scheduled time for its release. After the molding phase, each specimen was removed from its mold with care to avoid any damaging (demolding). This demolding was done with the aid of a plastic hammer. It was done not more than 20 min for a 24 h test, and, 8 and 24 h after molding for tests over 24 h. The demolded specimens were immersed immediately in water at 20.0 ± 1.0 °C. They were later stored horizontally when the molding faces were vertical. The stored specimens were kept in order to carry out the resistance tests at the different ages indicated, with the following tolerances: 24 h ± 15 min, 48 h ± 30 min, 7 days ± 2 h, and 28 days ± 8 h.
3.4.2 Compressive strength test
The compressive strength tests carried out on the molded specimens (brick-shape, length: 16 cm, width: 4 cm, and thickness: 4 cm, and 3 bricks for each CKD cement sample: 1 brick for 2 days test, 1 brick for 7 days test, and 1 brick for 28 days test) includes, the resistance to bending and resistance to compression (Fig. 4). The resistance to bending test was carried out as follows (Fig. 4a). Each brick was installed in the device (German 230 V/Hz/60/60 and controlab POO50.33) by placing a side face on the support rollers and its longitudinal axis perpendicular to the supports. The loading roller was used to apply the load vertically to the opposite side face of the brick, while increasing the load steadily at a rate of 50 ± 10 N/s, until failure.
The resistance to compression was determined as follows (Fig. 4b). The test was performed on both halves of the broken brick. The test on each half-brick was carried out by applying the load to the side faces. The half-brick was centered laterally in relation to the plates of the machine (at ± 0.5 mm), and longitudinally so that the end of the brick protrudes by approximately 10 mm, in relation to the plates or the auxiliary plates. The load was increased steadily at the rate of 2400 ± 200 N/s throughout the duration of the load application, until failure.
4 Results and discussion
This part presents and discusses chemical characteristics of raw materials used to manufacture the CKD-blended cement samples, chemical characteristics of the manufactured cements, physical properties of the manufactured cement powders and prepared pastes, and mechanical properties of the prepared brick-shape hardened CKD-blended cement mortars.
4.1 Chemical characteristics of raw materials used to manufacture CKD-blended cements
The chemical characteristics of raw materials used to manufacture cement kiln blended cement include that of clinker, gypsum, marble, and that of cement klin dust. The determined chemical features are mainly the major element composition, although the proportion of free lime and the calculated mineralogical features for used clinker are presented and also discussed.
4.1.1 Clinker
The determined features for the studied clinker include: the proportion of free lime, the major element composition, and the calculated mineralogy which were compared with those of other cement plants (Tables 3 and 4).
The proportion of free lime in the two analyzed clinker samples is 0.6 and 0.9%, respectively. The calculated average value is 0.75%. One of this values is within the range 0.8 to 2.5 of well cooked and resitant clinker (suitable to be used to process blended cement).
The major element composition in oxide for analyzed clinker (see Table 3) shows relatively high CaO (65.3%) and SiO2 (21.13%) contents. The content of Al2O3 (4.88%), Fe2O3 (2.87%) and MgO (2.71%) although relatively low, are significant. The K2O (1.06%), Na2O (0.23%), SO3 (0.81%), and P2O5 (0.13%) contents are the lowest. The lime saturation factor (LSF) is 97.9. The relatively high CaO and SiO2 contents in the clinker shows that its was processed from a combination of marble, clay and sand. Silica is originated from both clay and sand; whereas, CaO is from marble. The values of those two oxides are close to those of Azerbaijan and Amran-Yemen clinker (see Table 3). These high concentrations are registered during decarbonatation of the raw materials and clinkerization. This is confirmed by the LSF obtained for the analyzed clinker and calculated by this equation:
The required range limit values for LSF defined by Mekti et al. [40] are 90 ≤ LSF ≤ 100. If this value is too high, the raw materials would not be well cooked; and this would therefore increase the proportion of free lime. The value 97.9 for the analyzed clinker is within the range limit defined by Mekti et al. [40] which makes this clinker good for cement production. The relatively low alkaline (Na2O: 0.23%, K2O: 1.06%, and Na2O + K2O: 1.29%) and metallic oxide (TiO2: 0.23% and MgO: 2.71%) contents are due to their volatile nature and the low proportion of clays (the main source of alkalies) used during clinkerization (at 950–1400 °C) and decarbonatation of used carbonate rocks. The low SO3 content 0.81% can be due to the nature and composition of the used combustible (biomass). The low LOI (0.4% the required value in CIMENCAM Figuil) can be due to loss of water during cooking of the raw materials. With low LOI and decarbonatation, lime is combined with other mentioned compounds to form C3S, C2S, C3A and C4AF during pyro-processing. The calculated values with Bogue [41] equation, show some differences when compare with those of clinker presented in Dewanga and Gupta [39] (see Table 4). The C3S value is greater than 60; and place the studied clinker within the range limit of high resistant clinker. For Dewanga and Gupta [39], Portland cement with higher percentage of C3S will exhibit higher earlier strength, C2S hydrates and hardens slowly and is largely responsible for strength increases beyond 1 week, C3A hydrates and hardens the quickest, and liberates a large amount of heat almost immediately and contributes somewhat to early strength, and C4AF hydrates rapidly but contributes very little to strength, and its presence allows lower kiln temperatures.
4.1.2 Gypsum
The composition of selected major element in oxide (SiO2, Al2O3, Fe2O3, CaO, and MgO), SO3 and loss on ignition for gypsum (Table 5), shows that the highest quantified oxides are SO3 (36.32%) and CaO (33.92%). These values are followed by that of loss on ignition (13.83%). The SiO2 (1.28%), Al2O3 (0.42%), Fe2O3 (0.2%), and MgO (0.29%) contents are relatively low. The CaO and SO3 contents which are important characterizing features for gypsum (e.g., [42]), show some difference compare with those in Morocan, Azerbaijan, and Amran-Yemen gypsum (see Table 5). The CaO content in the studied gypsum, is higher than that of Morocan gypsum; but, is within the range in Azerbaijan and Amran-Yemen gypsums. The SO3 content is less than that of Morocan, Azerbaijan, and Amran-Yemen gypsums. This difference is also visible for other quantified oxides, which in general, are less than those in Morocan, Azerbaijan, and Amran-Yemen gypsums. For Bouzit et al. [42] element oxides other than CaO and SO3 in gypsum are impurities. The low concentration of oxides (impurities) in the studied gypsum places it within the range of low-impurities gypsum or pure gypsum, if base on Bouzit et al. [42] classification of Morocan gypsum. It is therefore mainly made up of calcium sulphate hydrate which does not require any purification.
4.1.3 Marble
The chemical composition of the used marble (Table 6) shows that CaO (37.09%) is relatively dominant; followed by silica (7.26%), SO3(2.40%), Al2O3 (2.36%), Fe2O3 (1.82%) respectively. The other quantified oxides Na2O (0.2%), MgO (0.18%), and K2O (0.12%) are relatively low; whereas, TiO2 (0.05%) is the lowest. The CaO content 37.09% precisely, is less than the CaO (55.3%) in marble powder presented in Belouadah et al. [43], CaO (55.42%) in marble powder presented in El-sayed et al. [44], CaO (40–56.12%) in typical calcitic marble and CaO (45.9–50.14%) in Itobe marble found in Nigeria (Onimisi et al. [45]) (see Table 6). It is more than the CaO (28.94–31.82%) in typical dolimitic marble presented in Onimisi et al. [45]. The difference is also visible for other quantifed oxides; notably, the SiO2, SO3, Al2O3, Fe2O3 Na2O, MgO, and K2O contents (which are higher than those of marble powder presented in Belouadah et al. [43], El-sayed et al. [44], and Onimisi et al. [45]). The MgO content which has been used to discriminate marble type [45, 46], is also different. In the calcitic type marble, the MgO content is less than 15%, and the CaO content ranges from 50 to 54% [46]. The CaO and MgO contents range from 30 to 31% and 15 to 21%, respectively in the dolomitic type marble [46]. The CaO and MgO contents in the Figuil marble are 37.09 and 0.18%, respecyively. These values are not within Goldschmidth et al. [46] marble type’s classification limit. This marble can therefore be a specific type. It can be classified within the typical calcitic type marble, precisely low-CaO calcitic marble, as the MgO content is lower than that of magnesian type marbles. The relatively high SiO2 content (7.26%) in the studied marble (almost ten times that of marble presented in El-sayed et al. [44], and almost five times that of marble powder presented in Belouadah et al. [43]), might classifies this marble as high-SiO2 type. The SiO2 content is within the range in typical calcitic marble (Table 6); which support the low-CaO calcitic marble classification. The relative high SiO2 content in the studied marble might be due to the presence of silicate minerals. The presence of silicate minerals is supported by the relatively high Al2O3 (2.36%) and Fe2O3 (1.82%) contents (more than values in all marbles presented in Table 6). These minerals could be aluminisilicates and ferrosilicates. The studied marble can be classified as low-CaO calcitic-aluminosilicate-ferrosilicate bearing marble. This marble was probably formed from metamorphism of a silica-bearing carbonate protolith or registered silica fluid contamination during metamorphism. The total alkaline (Na2O + K2O) content which is indicative of salinity level during the deposition and lithification of the protolith of a marble rock [45, 47], in the studied marble, is less 1%. This low total alkaline content might indicates that the depositional environment of the original carbonate materials source of the marble in Figuil was shallow depth with high salinity, if based on Clarke [47] and Onimisi et al. [45] studies (proposed a decrease in alkaline content in marble with the increase in salinity in shallow depth environment of the marble’s source rock).
4.1.4 Cement kiln dust
The quantified oxides in the studied cement kiln dust are dominantly CaO (46.0%) and SiO2 (11.62%) (Table 7). Other quantified oxides proportionally include Al2O3 (3.46%), MgO (2.20%), SO3(2.16%), Fe2O3 (1.19%), K2O (0.80%), Na2O (0.22%), TiO2 (0.17%), and the calculated Na2O + K2O (1.02%). These oxide’s compositions show some differences when compare with those of the analyzed clinker (this study: Table 3), and with those of typical CKD [48], Amran-Yemen CKD [17], CKD in Beltagui et al. [49], and those in Todres et al. [50] (Long-wet kiln, Long-dry kiln, Alkali by-pass from preheater/precalciner, and Typical Type I portland cement) (Table 7). The CaO and SiO2 contents in the study CKD are less than those of the analyzed clinker. This might be due to their reduction in the CKD during clinkerization. The CaO and SiO2 contents in the study CKD are within the ranges in CKD used to produced other CKD-blended cements presented in Table 7. In Adaska and Taubert [3] the CKD is primarily consisted of CaO and SiO2 which is similar to cement kiln raw feed, but the amount of alkalies, chloride, and sulfate is usually considerably higher in the dust. The SO3 content in the study CKD is higher than in the analyzed clinker (Table 3). Interpretation to this relatively high value is difficult as some analyses are still needed. It can be proposed that the relatively high SO3 content in the CKD (more than that of used clinker) is partly originated from used combustible (biomass). The total alkaline content (Na2O + K2O: 1.02%) in the study CKD is close that of the analyzed clinker (Na2O + K2O: 1.29%); but, dominantly lower than the values (Na2O + K2O: > 2 to 17.82%) for most of the CKD presented in Table 7. The low alkaline in the CKD and clinker can be due to the volatile nature of these elements, and the low proportion of used clay in CIMENCAM clinker manufacturing process. With its low alkalies and sulfate contents, the study CKD can be classified as low alkalies-sulfate cement kiln dust; not necessary to be pretreated. It is therefore suitable to be used as an additive for blended manufactring. For Dunuweera and Rajapakse [9], low alkaline CKDs are suitable for CKD-blended cement manufacturing as they would not be pretreated.
4.2 Chemical characteristics of the manufactured CKD-blended cements
Chemical features characterizing the processed CKD-blended cements (proportion of free lime, sulfate content, the loss on ignition, and the proportion of insoluble residues) are presented separately. The presented results are discussed and compared with some published works. This was aimed to test the quality of the manufactured cements; as the quality of a manufactured cement depends on its chemical features.
4.2.1 Free lime
The free lime result presented in Fig. 5, shows that the proportion of free lime varies with the addition of CKD in the formulated cement samples. The obtained values are within the range limit 0.8–2.5 standard presented in Table 8. This shows that the used CKD does not influence on the free lime contents; whose increase above the standard (0.8–2.5), could have had a negative impact on the quality of the manufactured cement, resulting in strength drop** and expansion increasing. It is possible that most limes in the CKD were combined with oxides during clinkerization process.
4.2.2 Sulfate (SO3)
The sulfate (SO3) content in the eleven manufactured cements is variable, and increases with addition of CKD (see Fig. 6). The increase in SO3 contents could be due to the fact that the quantified sulfate is originated from three different used raw materials gypsum, CKD, and limestome. Gypsum is probably the main source of SO3 in the manufactured cements, as its proportion is above 5%. The relative increase in SO3 can has a negative impact on the manufactured cement, as it could reduce the percentage of C3A and C3S and consequently the decrease in cement strength.
4.2.3 Loss on ignition
The loss on ignition (LOI) (Fig. 7) was obtained in an oxidizing atmosphere (air) by calcination in the oven at (1150 °C) in order to give an indication of the presence of water and organic matter in the particular mixture in the manufactured CKD-blended cement samples; based on similar studies presented in Trezzaa and Scianb [50]. The loss on ignition increases with the addition of CKD. This increase is due to two main parameters: (1) the fluidity and the alkalies contents in the used CKD; and (2) the humidity and proportion of organic matter in the used raw materials. The obtained values for LOI in all the manufactured cements are within the standard limit in CIMENCAM Figuil plant (Table 8). Coupled to the other observations presented above, it is clear that the addition of CKD does not act significantly on the content loss on ignition during the manufacturing of the CKD-blended cement samples.
4.2.4 Insoluble residues
The percentage insoluble residues (6.71–9.26%) varies from one manufactured cement sample to another (Table 9). Three groups can be distinguished: (1) those with the value of IR ranges from 6.71 to 7.9%; (2) those with the value of IR ranges from 8.16 to 8.96%; and (3) the one with the value of IR (9.26%). To control the non-cementing material in Portland cement, ASTM standard proposes that the value of IR should not exceeds 0.75%; whereas in British standard, the value of IR should be 1.5% [51]. The obtained values for the manufactured CKD-blended cement samples are greater than that of ASTM and Bristish standards. The impact on the compressive strenght would be examined in the paragraphs presenting and discussing the compressive strenght results.
4.3 Physical properties of the manufactured CKD-blended cements
The determined physical properties include: cement fineness (blaine specific surface area and sieving) carried out on cement powders, the normal consistency, the setting time and the expansion (carried out on cement pastes).
4.3.1 Cement fineness
Cement fineness is an important property that must be determined to verify the reaction of the manufactured CKD-blended cements in the concrete in the fresh and hardened state during mixing [32, 52]. It is determined either by sieving or by the blaine’s specific surface method [32]. The obtained data for the manufactured CKD-blended cement fineness (Table 10) include that of the blaine specific surface area (BSSA) (Fig. 8a), and that of cement sieving (Fig. 8b). The obtained values for blaine specific surface area for the eleven manufactured CKD-blended cement samples range from 4794 to 6343 cm2/g (Table 10) with most values being less than 6000 cm2/g. The plotted BSSA values in Fig. 8a shows an increase with the increase of CKD proportion. When the cement’s surface area is large, there is more contact with water, as well as a rapid and complete hydration of the mixture [52]. For Ferraris and Garboczi [32], the smaller the size of the particles, the larger is the specific surface area. The obtained BSSA for the manufactured CKD-blended cement is larger which reflect a smaller particle size. Figure 8b is a plot diagram of the sieved-weight data of the manufactured CKD-blended cement. The cements were sieved with an air stream (Alpine) 63 µm sieve. The proportion retained sieved fraction of the manufactured CKD-blended cements increases with the increase in quantity of the used CKD additive (Fig. 8b). This increase is mainly due to the fineness of the used CKD additive (feature obtained during crushing and milling).
4.3.2 Normal consistency
Increasing the ratio of waste materials in the blended cement paste significantly increased the amount of water required to produce a neat blend of normal consistency [1, 53]. The standardized consistency tests carried out on the manufactured cements to determine the optimum quantity of mixing water and to obtain a good mortar provided the results presented in Table 11 and Fig. 9. The mixing water varies from 26.14 to 30.36% (Table 11). Plotted data in Fig. 9 shows a step-like feature with just partially direct increase in proportion of mixing water with the increase of the proportion of used CKD. The direct increase in the proportion of mixing water with that of the used CKD is observed when the proportion of mixing water is above 27.62%. This therefore shows that the manufactured CKD-blended cements need more water compared to that of the control cement; and increases significantly when the CKD proportion increases. This increase might be due to the lime content (CaO) in the CKD, the alkali content, and its high fineness if based on Daous [1], Sharif [53] and Samir [54] studies.
4.3.3 Setting time
The setting times for the manufactured cements, are more than that of the normal cements (≥ 1h30 min). The setting time of the paste prepared with the different CKD-blended cement samples increases with the addition of CKD (Fig. 10). This increase in the setting time is generally due to the chemical and mineralogical compositions of the used CKD, and the high fineness of the CKD-cement [54, 55]. Indeed, the sulfur content in the form of SO3 and the magnesia contained in the CKD slow down the hydration of the cement and consequently the hardening of the paste, hence the increase in setting time, making the manipulation of the latter during the works.
4.3.4 Expansion
Expansion test is used to make evidence of the presence of expansive materials in the manufactured CKD-blended cements (precisely the magnesia and the free lime contents in the binder). These undesirable elements can cause serious threats to the durability of constructions. It is measured on the needles using a ruler and must not exceed the limit set by standard EN 196-3 [56]. The obtained result shows no increase in expansion with the addition of CKD. This can be due to the low free lime contents in the manufactured cements, low MgO (2.40%) in the used limestones and low MgO (2.20%) in the used CKD. It is therefore certain that manufactured cements are stable with zero expansion, have no significant effect on the stability and remain below the maximum limit imposed by standard EN 196-3 [56] which is 10 mm. This stability can be explained by the minimum rate of MgO and SO3 in the manufactured cements that does not exceed 5%.
4.4 Mechanical properties of prepared CKD-blended cement hardened sand mortars
The determined mechanical properties for the prepared brick-shape CKD-blended cement hardened mortars are the compressive strength. The result of compressive strength tests (at different periods 2, 7, and 28 days) carried out on 33 brick-shape hardened CKD-blended cement mortar specimens are presented in Table 12 and Fig. 11. This result shows that, the resistance depends on the proportion of the used CKD (0% control, 5%, 10%, 15%, 20%… up to 50%). With regard to compressive strength, a pressure of 17.5 MPa was obtained in two (02) days for the control sample. This strength is greater than the minimum limit value (15 MPa) set out in the Lafarge/Holcim standard. However, by injecting 10% of CKD, an increase in strength at 18.1 MPa is registered; and decreases each time the percentage of CKD in the cement increases. Resistances range from 18.1 MPa at 10% of CKD, to 11.6 MPa at 50% of CKD. For 28 days the obtained values are higher than the minimum value (32.5 MPa) (in the Lafarge/Holcim standard) for tests carried out on manufactured cements containing 5 to 10% CKD. The values drop when the proportion of CKD in the cement is above 10%. This drop in resistance is due to the impact of several chemical parameters, insoluble residues (6.71–9.26%) which are very high in the manufactured CKD-blended cement, to the chemical and mineralogical composition, as well as to the crystalline form of the alkalies present in the addition of CKD. Indeed, the insoluble residues high beyond the target influence the resistance of the cement just like SO3 and MgO. They slow down the hydration of the main minerals (C3S and C2S) which in cements, are responsible for the evolution of the resistance in a short and medium term. This consequently reduces the manufactured cement’s strength. Other negative and not negligeable factors are the proportion of organic matter from used additives and the alkalies percentage in the dust which form the periclase during the hydration of the cement (causing swelling and cracks, which reduce the strength of a processed cement).
5 Conclusion
-
1.
The raw materials (clinker, gypsum, marble, and CKD) used to manufacture the CKD-blended cements have interesting chemical features which make them suitable for cement production. The used gypsum is classified as low-impurities gypsum, whereas marble is low-CaO calcitic-aluminosilicate and ferrosilicate-bearing marble, and cement kiln dust (CKD) classified, as low alkalies-sulfate cement kiln dust.
-
2.
Chemical features of the formulated CKD-blended cements includes the free lime, SO3, loss on ignition (LOI), and insoluble residues generally increase with the increase of CKD in the formulated cement. The blaine specific surface area (BSSA) and refuses proportion, increase with the proportion of used CKD; which places the manufactured CKD-blended cements within high fineness cements. Normal consistency tests, setting time and expansion carried out on the prepared cement pastes all show an increase with the increase in proportion of used CKD; which can be due to the chemical and mineralogical composition of the manufactured CKD-blended cements.
-
3.
The compressive strength tests on hardened mortars shows that, the resistance depends on the proportion of the used CKD. The registered pressures decrease each time the percentage of CKD in the cement increases, which is probably due to the chemical and mineralogical composition of used CKD-blended cements and their high proportion of IR.
-
4.
This work lack mineralogical data for each of the used raw materials and that of the manufactured cements. The number of samples (33) is limited but acceptable as mentioned by one of the reviewers who proposed that a total of 99 samples could be sufficent. Future studies would include; XRD mineralogical characterization of the manufactured cements, physical and mechanical tests on a large quantity of manufactured CKD-blended cements. CIMENCAM and other cement manufacturing companies should recycle CKD in cement production in order to reduce the environmental and health problems, and waste in raw material.
Data availability
The datasets generated during the current study are available from the corresponding author (Dr KANOUO Sylvestre Nguo, E-mail; sylvestrekanouo@yahoo.fr) on reasonable request.
References
Daous MA (2004) Utilization of cement kiln dust and fly ash in cement blends in Saudi Arabia. JKAU Eng Sci 15:33–45
Holcim H (2006) Rapport développement durable. Holcim Liban, Dbayeh, p 36
Adaska SW, Taubet HD (2008) Beneficial uses of cement kiln dust. Presented at 2008 IEEE/PCA 50th Cement Industry Technical Conf., Miami, FL, May 19–22. https://doi:https://doi.org/10.1109/CITCON.2008.24
Chuan FL (2015) Innovative cement additives quality improvers in sustainable cement and concrete. Sains Malay 44:1599–1607
Duszak B, Adamski G, Foszcz T, Laska-Józefczak P (2015) Tests for contents of hard and harmful compounds in in the process of cement production in dust from rotary furnaces. Sci Works Inst Ceram Build Mater 22:9–22
Maslehuddin M, Al-Amoudi BSO, Shameem M, Rehman KM, Ibrahim M (2008) Usage of cement kiln dust in cement products-research review and preliminary investigations. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2007.09.005
Larbi CF (2012) Caractérisation des propriétés thermiques des liants hydrauliques au jeune âge: ciment et pouzzolane. Université Abdelhamid IBN Badis Mostaganem, Mémoire de Master II, pp 10–38
Abdulabbas HZ (2013) Utilization of cement kiln dust in concrete manufacturing. Jordan J Civil Eng 7:111–125
Dunuweera PS, Rajapakse GMR (2018) Cement types, composition, uses and advantages of nanocement, environmental impact on cement production, and possible solution. Adv Mater Sci Eng 4158682:1–11. https://doi.org/10.1155/2018/4158682
Elimbi A, Tchakoute H, Njopwouo D (2011) Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr Build Mater 6:2805–2812. https://doi.org/10.1016/J.CONBUILDMAT.2010.12.055
Mbessa M, Ndongo EC, Nga HN, Tatietse TT (2012) Influence of the powder of pozzolana on some properties of the concrete: case of the pozzolana of djoungolo (Cameroon). Int J Modem Eng Res 2:4162–4165
Al-Chaar KG, Alkadi M, Yaksic AD, Kallemeyn AL (2011) The use of natural pozzolan in concrete as an additive or substitute for cement. ERDC/CERL TR-11-46. https://apps.dtic.mil/sti/pdfs/ADA558534.pdf
Mambou NLL, Njimboum AS, Tchakouteu MT, Mache JR (2019) Valorization of pozzolans as partial additive of portland cement: a case of pozzolans from the localities of foumbot, penja and tombel (Cameroon). J Miner Mater Character Eng 7:18–33
Abdul-Wahab AS, Hassan ME, Al-Jabri SK, Kaan Yetilmezsoy K (2019) Application of zeolite/kaolin combination for replacement of partial cement clinker to manufacture environmentally sustainable cement in Oman. Environ Eng Res 2:246–253. https://doi.org/10.4491/eer.2018.047
Chen C, Habert G, Bouzidi Y, Jullien A (2010) Environmental impact of cement production: detail of the different processes and cement plant variability evaluation. J Clean Prod 5:478–485. https://doi.org/10.1016/j.jclepro.2009.12.014
Schuhmacher M, Domingo LJ, Garreta J (2004) Pollutants emitted by a cement plant: health risks for the population living in the neighborhood. Environ Res 2:198–206. https://doi.org/10.1016/j.envres.2003.08.011
Hussein MK, Ahmed E (2016) Formulation of the cement kiln dust (CKD) in concrete: studies of the physical-chemical and mechanical properties. Int J ChemTech Res 12:695–704
Elbaz AA, Aboulfotoh MA, Dohdoh MA, Wahba MA (2019) Review of beneficial uses of cement kiln dust (CKD), fly ash (FA) and their mixture. J Mater Environ Sci 11:1062–1073
Bochenczyk UA (2019) Chemical characteristics of dust from cement kilns. Gospodarka Surowcami Mineralnymi-Miner Resour Manag 2:87–102
Farmer MA (1993) The effects of dust on vegetation-a review. Environ Poll 1:63–75. https://doi.org/10.1016/0269-7491(93)90179-r
Hamadou T (2022) Valorisation des poussières de fitre four dans le ciment. Université de Maroua, Mémoire de Master en Ingénièrie, p 86
Labidi I, Megriche A (2022) Natural resources exploitation in sulfate-resisting Portland cement manufacturing: towards quality improvement and reduction of environmental impact. Front Chem 10:806433. https://doi.org/10.3389/fchem.2022.806433
Figmig R, Estokova A, Luptak M (2021) Concept of evaluation of mineral additives’ effect on cement pastes durability and environmental suitability. Materials 14:1448. https://doi.org/10.3390/ma14061448
Vignesh VA, Haresh B, Gowrishankar M (2019) Utilization of cement kiln dust and wood ash as a partial replacement of cement in concrete. Addendum 10:38–46
Khanna SO (2009) Characterization and utilization of cement kiln dusts (CKDs) as partial replacements of Portland cement. PhD Thesis, University of Toronto, p. 344
Schwoerer P (1962) Carte géologique du Cameroun au 1/500000, coupure de Garoua Est et notice d’explication. Direction des Mines et de la Géologie.
Basua AAE (2021) Geochronology and petrogenese of A-type Panafricain Granitoids of Babouri-Figuil Comple, NW Domaine of Cameroon: Implication of tectonic evolution of Central Africa. PhD Thesis, China University of Geosciences (Wuhan), p. 260
Kanouo SN, Nzeukou NA, Kouské PA, Bahané TC, Kringle R, Basua AAE, Fagel N (2021) Trace and REEs geochemistry, a tool to study mudrocks and quaternary deposits found in babouri-figuil intracontinental basin (North Cameroon): provenance, depositional conditions, and paleoclimate. SSRG Int J Geo-inf Geol Scie 2:67–88. https://doi.org/10.14445/23939206/IJGGS-V8I2P107
Khouan F, Lebdiri Z (2016) Caractérisation et utilisation de poussières de four de ciment CKD dans la Stabilisation/Solidification des déchets de l’unité B.C.R Bordj Ménaiel Boumerdès, Master de Master II en Mines et géologie, Université Abderrahmane Mira de Bejaia, Mines et géologie
EN 196-2 (2005) Methods of testing cement-Part 2: chemical analysis of cement, British Standards Institution
Norme Algérienne (NA 235) La présente norme spécifie pour les ciments, la méthode de détermination de la perte au feu
Ferraris C, Garboczi, E (2012) Identifying improved standardized tests for measuring cement particle size and surface area. TRB 2013 Annual Meeting, 1–14. http://onlinepubs.trb.org/onlinepubs/nchrp/nchrp_rrd_382.pdf
EN 196-6 (2010) Methods of testing cement-part 6: determination of FIneness
EN 196-1 (1995) Méthodes d’essai des ciments, Partie I: Détermination des résistances mécaniques. Comité Européen de Normalisation, 15-101-1
EN196-1 (2005) Methods of testing cement-Part 1: determination of compressive strength. British Standards Institution.
ISO 13322-1 (2010) Particle size analysis-image analysis methods-part 1: static image analyses methods
Norme Algérienne (NA 442) Composition, spécifications et critères de conformité des ciments courants
Onat OL, Valiyev RK, Agapov VR, Kangarli ML (2016) Analysis of anhydrite gypsum effects on quality of cement. Int Res J Eng Tech 8:824–829
Dewangan A, Gupta PD (2018) Significance of marble and Portland cement. Int J Eng Tech Manag Res 5:255–265. https://doi.org/10.29121/ijetmr.v5.i2.2018.170
Mekti Z, Bounouala M, Chettibi M, Boutemedjet A (2018) Industrial application of bast furnace slag as substitute for sand at the cement plant of Hadjar-Soud (Algeria). Sci Bull Natl Mining Univ 1:43–50. https://doi.org/10.29202/nyngu/2018-1/22
Bogue RH (1955) The chemistry of Portland cement. Soil Sci 79(4):322
Bouzit S, Laasri S, Taha M, Laghzizil A, Hajjaji A, Merli F, Buratti C (2019) Characterization of natural gypsum materials and their composites for building applications. Appl Sci 9:2443. https://doi.org/10.3390/app9122443
Belouadah M, Rahmouni AEZ, Tebbal A (2019) Influence of the addition of glass powder and marble powder on the physical and mechanical behavior of composite cement. Procedia Comp Sci 158:366–375. https://doi.org/10.1016/j.procs.2019.09.064
El-Sayed AH, Farag BA, Kandeel MA, Younes AA, Yousef MM (2016) Characteristics of the marble processing powder waste at Shap El-Thoaban industrial area, Egypt, and its suitability for cement manufacture. HRBC J. https://doi.org/10.1016/j.hbrcj.2016.06.002
Onimisi M, Obaje NG, Daniel A (2013) Geochemical and petrogenetic characteristics of the marble deposit in Itobe area, Kogi state, Central Nigeria. Adv Appl Sci Res 4(5):44–57
Goldschmidth JR, Graff L, Joensu OI (1955) The occurrence of magnesium calcite in nature. Geochim Cosmochim Acta 1:212–230. https://doi.org/10.1016/0016-7037(55)90033-8
Clarke FW (1972) The data of geochemistry, 2nd edn. Washington Government Printing Office, Seattle, p 782
Haynes BW, Kramer GW (1982) Characterization of U.S. cement kiln dust, Bureau of Mines, United States Department of Interior, Washington, DC, USA, Information Circular. https://digital.library.unt.edu/ark:/67531/metadc17086
Beltagui H, Sonebi M, Maguire K, Taylor S (2017) Utilisation of cement kiln dust the activation of fly ash low strength applications. AJCE 2:549–553
Todres HA, Mishulovich A, Ahmed J (1992) Cement kiln dust management: permeability, RD103, Portland Cement Association, Skokie, Illinois, USA. https://www.semanticscholar.org/paper/CEMENT-KILN-DUST-MANAGEMENT
Kaittikomol K, Jaturapitakkul C, Tangpagasitn J (2000) Effect of insoluble residue on properties of Portland cement. Cem Concr Res 8:1209–1214. https://doi.org/10.1016/S0008-8846(00)00315-X
Hlobil M, Kumpová I (2022) Surface area and size distribution of cement particles in hydrating paste as indicators for the conceptualization of a cement paste representative volume element. Cem Concr Comp 134:104798. https://doi.org/10.1016/j.cemconcomp.2022.104798
Sharif AFS (2011) Influence of cement kiln dust as partial replacement on some properties of ordinary and Portland cement. Tikrit J Eng Sci 3:23–32
Samir B (2016) Recherche bibliographique sur la valorisation de métakaolin dans les matériaux de construction. Université Mohamed Boudiaf, Mémoire de Master en Ingénièrie, p 105
Kiptum KC (2018) Analysis of Some physical and chemical properties of Bad cement delivered to a construction site in Eldoret, Kenya. Int Adv Res J Sci Eng Tech 4:107–109. https://doi.org/10.17148/IARJSET.2018.5419
EN196-3 (2017) Methods of testing cement-Part 3: determination of setting time and soundness, British Standards Institution.
Acknowledgements
The authors extend their gratitude to CIMENCAM Figuil (Cameroon) for providing raw materials, laboratory and industrial facilities. Many thanks to the anonymous reviewers, whose useful comments help to deeply rework and improve the manuscript.
Funding
This work was partly funded by CIMENCAM Figuil Cameroun by providing the laboratory facilities. The other part was funded by the authors.
Author information
Authors and Affiliations
Contributions
TH carried out the experimental and graph work and reviewed, NSK supervised the experimental work, wrote, reviewed and revised the article, JD reviewed and revised the article, ABT reviewed the article, and LLD reviewed the article.
Corresponding author
Ethics declarations
Competing interests
There is no financial or other relationship with people or organizations that may inappropriately influence this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamadou, T., Kanouo, N.S., Dikwa, J. et al. Cement kiln dust in CIMENCAM Figuil (North Cameroon): characteristics and recycling as additives for blended cement production. SN Appl. Sci. 5, 181 (2023). https://doi.org/10.1007/s42452-023-05401-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05401-z