Abstract
This present study investigates the heat-assisted extraction (HAE) of bioactive antioxidants from Enantia chlorantha stem bark (ECSB). The effects of process parameters of operating temperature (OT: 35–55 °C), extraction time (ET: 100–200 min) and solid: liquid ratio (S/L: 1:20–1:50 g/mL) are determined on the total polyphenol content (TPC), antioxidant activity (AA) and extract yield (EY) using the D-Optimal design. Multi-criteria optimization is also conducted to determine the HAE optimum conditions. The integrated process for E. chlorantha stem bark extracts (ECBEs) production is thereafter designed with the optimum HAE conditions and techno-economically analysed. Three industrial ECBEs production setups (5, 100 and 1000 L extractor capacities) are analysed for probable scale-up and the risk & sensitivity analyses are conducted via Monte Carlo simulation. The HAE parameters affect the extraction process significantly. The OT, ET and S/L that maximize the TPC, AA and EY simultaneously are: OT = 54.10 °C, ET = 120.16 min and S/L = 1:50 g/mL. The 1000 L plant extractor setup is the most economical capacity having the least unit production cost (UPC) of 763.52 US$/kg ECBEs. The certainty of achieving 763.52 US$/kg ECBEs for the 1000 L capacity is 55.43% and the only variable with negative contribution (− 37.9%) on ECBEs UPC is the extract recovery variable.
Article Highlights
-
Heat-assisted extraction technology is capable of recovering polyphenols from E. chlorantha stem bark.
-
The extract is rich in chlorogenic acid, betulinic acid, ellagic acid. gallic acid and rutin.
-
The 1000 L extractor plant capacity is the most economical scale with the unit production cost of 763.52 US$/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The bioactive compounds of medicinal plant sources have received considerable attention in recent years for their remarkable functions in the prevention of chronic diseases and because of their actions in the promotion of good health. These bioactive compounds are chemicals with biological activities that are available in small quantity in plants and provide health benefits exceeding the basic nutrition [1]. These phytochemicals are distributed in several parts of plants such as in the leaves, roots, stem bark, flower and fruits [2]. Although the bioactive compounds encompasses classes of chemicals (such as the terpenoids, alkaloids, phenolics and tannins [2, 3], the phenolic class has well documented scientific proofs of medicinal and chemo preventive characteristics. Polyphenols are diverse group of plant secondary metabolites and have been shown to have numerous health benefits in human nutrition. There are investigations pointing to their anti-sickling, anti-malaria, anti-diabetic, anti-cancer, anti-inflammatory, antioxidant, anti-microbial and anti-hypertensive properties [1, 4, 5]. The bioactive antioxidants have wide applications in many commercial sectors such as in functional ingredients (for functional food) developments [6, 7], in the formulation of new therapeutic drugs [8], as nutraceuticals [9, 10], in cosmetics and food preservation [11, 12] and as flavorant, pesticides and colorants [2, 13, 14]. Recent investigations have therefore focused on the recovery and characterization of this class of bioactive compounds from potent and cheap medicinal sources because of their wide industrial applications. Among the established prominent methods of phenolic compounds recovery, quantification and characterization are solid–liquid extraction [15], the Folin-Ciocalteu technique [2] and high performance liquid chromatography (HPLC) [16] respectively.
Enantia chlorantha remains one of the potent, abundant and cheap sources of ethno-medicinal origin in Nigeria. E. chlorantha belong to the Annonaceae family, and commonly known as African Yellow Wood [17]. The plant is very important in the Nigerian traditional medicine and the traditional healers have used the leaves, stem back and roots of the plant to treat a number of diseases [18]. The extracts of E. chlorantha is potent and being used for the treatment of malaria [18], tuberculosis, anaemia, typhoid fever, yellow fever, jaundice, urinary infection, infective hepatitis and leprosy [17, 19]. The E. chlorantha stem back (ECSB) is nutritive and reported to contain approximately 72.25% crude fiber, 6.29% carbohydrate, 10.78% crude protein, 3.78% crude fat, 2.48% ash content and 3.85% moisture [20]. The ECSB also contains micronutrients such as manganese, iron, zinc and copper [20, 21]. There are scientific proofs that the aqueous extracts of ECSB possessed antibacterial [22], anti-plasmodial [23], gastro-protective [24], antipyretic [25], antiulcer [26], anticonvulsive [27], anti-inflammatory [28], analgesic [28], and antioxidant [29] properties. The extract also has testiculoprotective [30], hepatoprotective [29], antitumor [31] and anti-diabetic [17] activities and it is a potent male fertility enhancer [20]. Hence, with the numerous pharmacological activities displayed by the ECSB, there is a need for detailed protocol for the optimal recovery and characterization of these bioactive compounds that are responsible for these activities which are yet to be reported.
The recovery of these phytochemicals and their corresponding activities from plant matrices are influenced by the engaged extraction technology and solvent type. Basically the extraction technologies for recovering bioactive antioxidants in plant materials are divided into the conventional/traditional and emerging technologies [2]. Generally, the emerging extraction technologies have been ranked superior to their counterpart conventional extraction technologies. The emerging extraction technologies have documented proofs of high extract integrity and quantity [2]. They are also reported to be very economical in extraction time and solvents used. The prominent emerging extraction technologies that have been previously employed for the recovery of bioactive compounds include the microwave-assisted extraction, supercritical and subcritical fluid extractions, high-pressure processing, pulsed electric field processing, high-voltage electrical discharge and infrared- and ultrasound-assisted extractions [2]. However, these emerging technologies are less tested for industrial scale-up and have high initial implementation cost making them less favored in the industry. Conversely, the conventional extraction technique like the heat-assisted extraction (HAE) method is an established extraction technology and has been widely reported for bioactive compounds recovery from plant matrices. For instance, phenolic-rich extracts had been successfully recovered from Carica papaya leaves, Azadirachta indica leaves and Parkia speciosa pod [2, 32, 33] by adopting the HAE technology (HAE-T). Although prolonged heating or high temperatures generally can cause some degradation or loss of beneficial properties of phenolic compounds, some phenolic compounds that are relatively heat stable are gallic acid, caffeic acid, quercetin and rutin while others such as catechins, epicatechins, anthocyanins and resveratrol are heat-unstable in nature. There are also numerous advantages of HAE-T which made it very relevant till date at industrial scale such as ease of scalability, low maintenance and purchase cost, low instrumentation cost and relative ease of implementation at larger capacity [2].
Another yet very important aspect of bioactive recovery from plant matrices is solvent selection [34]. Bioactive compounds have been extracted by a range of organic and inorganic solvents like ethanol, methanol, ethyl acetate, acetone and water and solvent mixtures (such as ethanol and water and methanol and water) [35,36,37]. While some of these extracting chemicals (e.g. ethyl acetate, acetone and methanol) are regarded as toxic and therefore bioactive extracts obtained using these chemicals have limited acceptability especially in food applications because of the residual chemicals which might be present in the extracts even after purification. Some of these extracting organic chemicals are also very costly, non-renewable, non-sustainable, and relatively unavailable and may cause fouling in process equipment at industrial setup. Water on the other hand is safe and has been extensively explored for phytochemicals recovery [32, 38, 39]. Water has been explored as extracting solvent for the wide range of phytochemicals present in plant and has subsequently emerged a versatile solvent for organic compounds recovery in recent years. The ability of purified water has been documented in the extraction of phenolic acids, flavonoids, tannic acids [32, 40], alkaloids [41, 42] and saponins [43, 44]. Water is also a relatively cheap, environmental friendly, safe, renewable and sustainable extraction solvent.
The HAE of bioactive extracts is often affected by multi extraction factors such as the operating temperature, extraction time, solvent to plant sample ratio [32, 33] and particle size [45,46,47]. The small particle size of the plant samples has however been established to contribute greatly to solute (bioactive compounds) mass transfer yield, antioxidant activities and phytochemical composition as compared to bigger particle size [45, 46]. Therefore, since these extraction factors may affect both the integrity and quantity of bioactive compounds during recovery, a robust optimization protocol is a necessity [39]. Literature search revealed a number of documented robust optimization tools for bioactive compounds recovery from plant matrices such as the one-factor-at-a-time (OFAT) procedure, Taguchi method and response surface methodology approach [48], however, the response surface methodology (RSM) approach is widely used [33, 39, 49, 50] and has fascinating advantages. The RSM approach can be used to evaluate the influences and interactions of multiple process factors on the target response (s) with fewer numbers of experimental runs [2]. RSM also has capabilities of maximizing and/or minimizing simultaneously the multiple process factors using the desirability function algorithm to obtain a set of process conditions that optimizes the phytochemicals recovery from plant materials. The global optimum extraction data that are achieved therein can serve as a foundational basis for the preliminary integrated process design, scale-up investigations, technical process assessment and economic analysis for the commercialization feasibility assessment of the industrial bioactive extracts production [39].
Process integration plays a major role in the preliminary technical and economic feasibility assessments for the commercialization of a product [2, 38]. In this regards, all adjoining unit operations that are involved in bioactive extracts production are holistically considered to interact with one another like in a typical production plant. Therefore, detailed techno-economic analysis of an encompassing process that integrates an extraction technology (such as HAE-T) can provide a robust and more accurate data driven assessment of such production process for probable product commercialization evaluation. The assessment can also provide guidance for process optimization to obtain production process that is viable economically. Computer aided process simulation (CAPS) is a recently introduced tool for the design, up scaling and technical and economic analyses of manufacturing processes. CAPS has capabilities to achieve mass and energy balances of a production process, conduct sizing of process equipment, achieve cost assessment of a production process and assist in the optimization of an integrated process [2, 34, 50]. CAPS has been successfully used for the integrated process designs and assessment of the economics of various bioactive recovery technologies such as in the analysis of supercritical CO2 bioactive compound extraction from Eugenia uniflora L. leaves [51], techno-economic analysis of ultrasound- and agitated bed extraction technologies for the production of natural antioxidant from Euterpe edulis [52], production of phenolic-rich extracts from C. papaya leaves [2] and in the recovery of anthocyanins from Dioscorea alata [53]. Presently, there are available process simulation software for the design of integrated processes, investigations of process scale-ups and assessment of the economics of manufacturing processes. The Aspen plus [54], Aspen batch [50] and SuperPro Designer [55, 56] are among the most engaged process simulation software. In addition, during the deterministic integrated process design and techno-economic analysis, some technical as well as cost variables used for model building have tendencies of introducing uncertainties in the results and therefore the risk assessment and sensitivity analysis are normally performed for the reasons of process refining and optimization.
Although there are several published reports of bioactive compound recovery from diverse plant sources, there is however, a continuous need to understand and establish the material-processing condition relationship that is necessary to obtain bioactive compounds of appreciably high quantity and activities from each specific plant material. These pieces of information are needed in bioactive ingredient manufacturing industry for process and product design and optimization. This is as a result of diverse intrinsic properties and inherent chemical compositions of the medicinal plant materials. Thorough understanding of the recovery process (plant material-processing condition relationship) can further lead to improved process and product control. The water extracts of ECSB have been used extensively in traditional medicine to cure and manage broad scope of diseases and ailments, however, the extraction protocol which is a precursor to product standardization has not been established. The extraction conditions that optimize the recovery of bioactive antioxidants of high integrity and quantity from ECSB have also not been reported. In addition, the global bioactive ingredient market has been projected to reach 71.98 billion US$ by 2031 and grow by 6.6% annually over 2021–2031 [57] and therefore in order for a product of this kind to benefit from the increasingly growing market, detailed engineering works (via techno-economic profitability assessment) that evaluate the commercialization potentials and associated risks of the venture at industrial scale is a necessity, however, these are lacking at present. This present investigation is of high importance since commercialization of ECSB antioxidants in a country like Nigeria (where the ECSB is available abundantly at relatively low cost) can promote industrialization and boost the country’s foreign exchange.
Therefore, this present investigation focuses on establishing the ECBS bioactive antioxidants recovery protocol that enables in-depth understanding of the material -processing condition relationship by using a relatively easy-to-implement technology (HAE-T) in a develo** country like Nigeria and further evaluates the commercialization potential of the venture at various industrial setups. Also, since the HAE-T is electricity demanding (electricity being used for extraction, raw material transfer, fluid transfer, product concentration and product drying) and its cost is a key contributor in plant operating cost, fluctuation in the unit cost of electricity is evaluated among others in the stochastic profitability modeling and sensitivity analysis. The research objectives are therefore to (1) study the effects of HAE process factors on the bioactive antioxidants recovery from ECSB using the RSM D-Optimal design and conduct process optimization with the assistance of the desirability function algorithm (2) identify the phenolic compounds present in the optimum ECSB extracts with HPLC (3) design the integrated process and techno-economically analyzed three industrial setup capacities of ECSB bioactive extracts production with SuperPro Designer software (4) perform the uncertainty analysis and sensitivity study of the technical and cost variables on the selected most profitable industrial setup to determine associated risks and identify/quantify significant important variables with the assistance of Oracle Crystal ball software.
The remaining part of this paper is organized into three robust sections. The immediate section is dedicated to detailed description of the methodology employed in the course of bioactive recovery experiments, integrated process simulation, process risk analysis and analytical procedures for the bioactive antioxidant quantification and characterization. Section 3 presents the process optimization, techno-economic and risk assessment results with appropriate explanations and justification as the case may be. Lastly, Sect. 4 concludes the investigation based on the objectives of this present study.
2 Materials and methods
2.1 Plant material and chemical reagents
The stem bark of E. chlorantha plant (whose leaves have been prior identified at the botany Department of Michael Okpara University of Agriculture, Umudike (MOUAU)) was harvested from a farm in Ogbomosho, South Western Nigeria. The E. chlorantha stem bark (ECSB) was washed under running water and spread on a perforated tray to dry (for approximately six weeks at room temperature) in the Analysis Laboratory of Chemical Engineering Department (CEAL), MOUAU. The dried ECSB (which had earlier been ground and screened with a cascade laboratory sieve (Octave sieve: OCT-DIGITAL 4527-01, Endecotts Ltd, London, UK) to particle size of 0.1 mm) was kept in a polythene bag. The oven drying method [58] was used for the moisture content (or water content (WaC)) determination of the dried ECSB and was found to be 11%. All laboratory chemicals and HPLC standards were analytical grades and were purchased from Sigma Aldrich, Poole, England. These chemicals were sodium carbonate (Na2CO3), ascorbic acid, Folin Ciocateu phenol reagent, glacial acetic acid, 2, 4, 6-tripyridyl-s-triazine, sodium acetate trihydrate, HCl and FeCl3. 6H2O. The HPLC standards were betulinic acid, gallic acid, caffeic acid, ellagic acid, chlorogenic acid, ferulic acid, rutin and quercetin. The distilled water was obtained from the CEAL, MOUAU.
2.2 Extraction of bioactive compounds
The heat-assisted extraction (HAE) of bioactive compounds from ECSB was investigated similar to the method of Pinela et al. [59] using a water bath (Equitron, India). This was carried out by charging a known amount of ECSB and distilled water into a beaker and incubated at elevated temperature for a definite residence time under continuous stirring. The beaker was covered with foil to prevent solvent loss during experimentation. Each set of extraction experimental conditions scenario (experimental run) was as prescribed by the D-Optimal design according to Sect. 2.3. After the stipulated residence time, the ECSB fiber was removed from the aqueous extracts by centrifugation (at 25 °C, 500 rpm and for 10 min). The ECSB extracts (ECBEs) was then decanted and kept in plastic bottles.
2.3 D-Optimal experimental design and statistical analysis
The D-Optimal design (DOD) was adopted at three levels to investigate the roles of operating parameters on the extractability of bioactive antioxidants from ECSB. The key extraction parameters (operating temperature (OT), solid: liquid (S/L) and extraction time (ET)) and their respective ranges (which were prior determined through preliminary experiments and literature) are displayed in Table 1. The selected responses for the HAE of bioactive antioxidants from ECSB were bioactive extract yield (EY, %), total polyphenol content (TPC, mg GAE/g d.w) and antioxidant activity (AA, µM AAE/g d.w). All investigated response parameters were thereafter fitted to a second order model presented in Eq. (i) to develop the relationship between HAE process and response parameters. The variable Y in Eq. (1) represents investigated response parameter (TPC, EY or AA).
The constant term in Eq. (1) is \({b}_{o}\); the linear coefficient is \({b}_{i}\),; the quadratic coefficient is \({b}_{ii}\) and the interactive coefficients is \({b}_{ij}\). All the model coefficients were determined by Design-Expert software; \({X}_{i}\) and \({X}_{j}\) are the coded studied process parameters. The significance of all regressed model coefficients was statistically determined at confidence level of 95% using the analysis of variance (ANOVA) test [32].
The model adequacies were measured by the \({\mathrm{R}}^{2}\)(Eq. 2), \({\mathrm{R}}_{\mathrm{adj}}^{2}\) (Eq. 3) and Pred \({\mathrm{R}}^{2}\) (Eq. 4).
In the above equations, the \({SS}_{residual}\) is the residual sum of squares, \({SS}_{model}\) is model sum of squares, \({DF}_{residual}\) is residual degree of freedom, \({DF}_{model}\) is model degree of freedom, \(\mathrm{PRESS}\) is predicted residual sum of squares and \({SS}_{Total}\) is the total sum of squares.
2.4 Numerical process optimization and validation
The simultaneous optimization of the HAE process and response parameters was achieved with the assistance of the numerical optimization algorithm in Design-Expert software and was according to Oke et al. [50]. The criteria for ECBEs process optimization were manually selected in the software’s numerical optimizer. The optimization goal, weight and importance of all process variables and responses for the ECSB HAE are presented in Table 2.
All suggested optimization solutions were ranked according to their desirability values. In addition, a validation experiment was conducted to ascertain/verify the suggested optimal solution. The statistical parameter used for the comparison of the predicted and laboratory validated results was relative standard deviation (RSD). The RSD between the experimental and suggested optimal response variables greater than 10 was regarded as similar [39]. The RSD was computed using Eq. (5).
Here, \({SD}_{1-2}\) represents standard deviation and \({M}_{v}\) is the mean value of the model predicted and experimental laboratory values.
2.5 E. chlorantha stem bark extracts yield quantification
The EY was determined in accordance with the procedure of Alara et al. [15]. Briefly, the decanted aqueous extracts in Sect. 2.3 was concentrated in a rotary evaporator and dried until a constant weight was obtained in a convective oven (Stangas SG-90526, Stangas Italy). The EY of ECSB was calculated according to Eq. (6).
The EY, \({W}_{1}\) and \({W}_{2}\) in Eq. (6) are the ECBEs yield (%), ECBEs weight (g) and ECSB weight (g) used in the HAE process, respectively.
2.6 Total polyphenol content determination in aqueous E. chlorantha stem bark extracts
The TPC in the aqueous ECBEs was determined in accordance with Gan and Latiff [33]. The Folin-Ciocalteu reagent (approximately 1.8 mL) was mixed with 1 mL (initial extract was diluted in distilled water at 1:10) of ECBEs in a tube. The content of the tube stood for 5 min and about 1.2 mL of sodium carbonate (7.5% (w/v)) was afterwards introduced into the tube containing the mixture, stirred well and incubated for 1 h in a cupboard at temperature of 20 °C. The TPC in ECBEs was measured spectrophotometrically at 765 nm absorbance and interpreted using the pre-constructed gallic acid curve [39]. The Eq. (7) [15] was employed to quantify the amount of TPC (mg GAE/g) in each experimental run.
In the equation above, the C (mg/mL) is the calibration curve interpreted gallic acid concentration; V (mL) is the solvent volume and m (g) is the dried ECSB weight that was used during laboratory experimentation.
2.7 Antioxidant activity determination in aqueous E. chlorantha stem bark extracts
The AA was quantified in accordance with Uddin et al. [60]. 1.8 mL of ferric ion reducing antioxidant power (FRAP) reagent (consisting of 0.1 M acetate buffer, 0.02 M FeCl3 and 0.01 M TPTZ in the ratio of 10:1:1, respectively) was added to a test tube containing 50 µL (ECBEs pre-diluted with distilled water in 1:10) extracts. The content of the test tube was mixed well and stood for 30 min at 37 °C. The AA (expressed as µM AAE/g d.w) was determined spectrophotometrically at 593 nm and interpreted using the ascorbic acid standard constructed calibration curve [39].
2.8 Identification of phenolic compounds in E. chlorantha stem bark extracts with HPLC
The HPLC analysis was employed to determine the phenolic profiling of ECBEs obtained at optimum process conditions in accordance with the method of Krishna and Manohar [61]. Here, the 0.45 µm membrane filter was used for the filtration of ECBEs to achieve 100% removal of fine fibrous particles. About 10 µL of the particle free ECBEs was then introduced into the reverse VP-ODS column (with dimension 150 × 4.6 mm and 5 µm particle size) of a Shimadzu HPLC (Shimadzu Corporation, Kyoto Japan) at 40 °C. The 0.2% v/v formic acid and acetonitrile were the chromatographic mobile phase solvent A and B respectively. The conditions of operation for the HPLC machine were: isocratic elution mode (mobile phase solvents A: B = 80:20); constant flow rate = 0.6 mL/min; total run time = 15 min and wavelength of detection = 220 nm. The reference phenolic acid standards were also measured similarly with the aqueous ECBEs. The retention times (RT) of the extracts and phenolic acid standards were compared to establish the phenolic profiling of the ECBEs.
2.9 Integrated batch process computer-aided simulation and economics
2.9.1 E. chlorantha stem bark extracts production process description
The proof-of-concept of ECBEs laboratory production was simulated with the aid of SuperPro Designer software [62] by adopting the reported plant configuration of Adeyi et al. [2]. Here, the optimum process conditions obtained in the earlier section (Sect. 2.4) formed the basis of operating parameters for the heat-assisted extractor simulation. Integration of other unit operations/procedures was also made in accordance with those available in a typical bioactive ingredient production plant. Superpro Designer which contains various models of equipment and chemicals in its databases/libraries was employed for the material and energy balances calculation and sizing of all featured process equipment. Superpro Designer software was also used for the steam requirement estimation, heat transfer agents quantification, and determination and quantification of electricity demands, cooling agents and other utilities needed for the investigated ECBEs setup capacities. More so, mode of operation of 100% batch and 7920 h annual operating time (AOT) were assumed for this study [39, 50]. ECSB was modeled as user-defined and its laboratory determined density of 1050 g/mL was employed for this simulation. Featured process equipment included the ECSB grinder (GR-101), ECSB fiber-extract centrifuge (BC-101), heat-assisted ECBEs extractor (R-101), ECBEs-solvent pump (PM-101), solvent evaporator (TFE-101), ECBEs dryer (SD-101) and storage tanks (V-101, V-102, V-103 and V-104).
Figure 1 is the base case integrated process flowsheet developed for the production of ECBEs. The designed base case proposed that the ECBEs be recovered from already dried ECSB. The profile of ECSB was formed based on this present investigation. Briefly, the process commenced by grinding dried ECSB (modeled to contain 27% ECBEs, 62% fiber and 11% WaC) for 5 min with the grinder GR-101, to 0.1 mm ECSB size. The pulverized ECSB and extracting distilled water solvent (which was stored temporarily in V-101) were transferred into the extractor, R-101, at the ratio of 1:50 g/mL. In R-101, extraction of ECSB took place for 120.6 min at temperature of 54.1 °C. The extraction mixture (consisting ECBEs-water and fiber) was transferred into BC-101 for 100% fiber (stored in V-103) and 40% water (which accounted for solvent loss) removal [39, 63]. The ECBEs-water mixture (which was stored temporarily in V-102) was transferred by the pump, PM-101 (efficiency = 70%), into TFE-101 (transfer coefficient = 2104.913 W/(m2K; efficiency = 90%; operating temperature = 65 °C) for ECBEs concentration. The Peng-Robinson cubic Equation of State (EOS) was used for the simulation of TFE-101 and was according to Vieira et al. [52]. This was achieved by selecting the Peng-Robinson Equation of State from the VLE model option interface of the TFE-101. The operating pressure of TFE-101 was determined by the SuperPro Designer as 170.70 mm Hg by iteration method and the TFE-101 ECBEs concentration was 1 h to obtain a high solid concentration in solution. The concentrated ECBEs was thereafter transferred to SDR-101 (operating temperature = 60 °C; time = 60 min) to obtain dried ECBEs (less than 5% WaC) and was stored in V-104. Both the batch and annual production rates of ECBEs were calculated and recorded.
Flowsheet and mass balance for the base case production of ECBEs [2]
2.9.2 E. chlorantha stem bark extracts production process economics
The economics of ECBEs production was conducted by estimating the major production costs and process profitability index. The two production costs that were calculated for the ECBEs production process were the total capital investment cost (TCC) and total operating cost (TOPC). The unit production cost (UPC) of ECBEs in US$/kg was the only profitability index that was assessed. The method of Baral and Shah [64] was used to assess the economics of ECBEs production setups. The TCC, TOPC and UPC of ECBEs production process were estimated with the aid of SuperPro Designer software [62]. Also, SuperPro Designer software equipment cost library was used to determine the cost of the featured process equipment for the ECBEs production setup (Fig. 1). The present design made assumption that all the pieces of equipment featured in the production line were constructed with stainless steel to achieve high corrosion resistance and minimum product contamination. The SuperPro Designer software has constantly maintained robust data bases/libraries and therefore, highly reliable and has been widely used by several authors [52] in the course of economic analysis of integrated processes.
The total equipment purchase cost (EPC) which formed the basis for the computation of production costs was the addition of all featured and unlisted equipment costs.
The TCC was equivalent to the sum total of the direct fixed capital cost (DFC), the working capital (WC) and the start-up and validation cost. The DFC was established by summing up the total plant direct cost (TPDC), total plant indirect cost (TPIC) and miscellaneous cost (MC). The TPDC encompassed the cost of process pi** (35% of EPC), instrumentations cost (40% of EPC), insulation cost (3% of EPC), electrical cost (10% of EPC), buildings cost (45% of EPC), yard improvement cost (15% of EPC) and auxiliary facilities cost (40% of EPC). The TPIC was estimated as the addition of engineering cost (25% of TPDC) and construction cost (35% of TPDC) while the MC was the total of the contractor’s fees and contingency which were calculated as 5% (TPDC + TPIC) and 10% (TPDC + TPIC) respectively. The WC was estimated as the total cost that covered for material procurement of 30 days, waste disposal and labor. Also the start-up cost was computed as 5% of DFC.
The TOPC which represents the total annual operating cost included the addition of raw material cost (RMC), total labor cost (TLC), total utility cost (TUC), facility dependent cost (FDC) and the laboratory QA/QC cost (LQA/QC). Since the ECBEs production plant was proposed to be sited in Nigeria, the RMC, TLC and TUC were estimated in Nigerian context. Therefore, the cost of dried ECSB was obtained at 0.5 US$/kg (although the cost varies with season) at a medicinal plant market in Ogbomosho, Nigeria while the cost of the extracting solvent (water) was 0.1 US$/kg. The utilities required in the production of ECBEs were cooling water (0.05 US$/MT), chilling water (0.4 US$/MT), processing steam (12 US$/MT) and electricity (0.05 US$/kWh). The operators proposed for the ECBEs plant were three (3) at the rate of 1.2 US$/h [39, 50]. The 1.2 US$/h was made up of the basic rate, administrative, benefits and supervision costs. The guide to labor cost was the 2020 minimum wage of national salaries, income and wage commission, Nigeria. The cost of waste treatment was excluded from TOPC computation because of the organic nature of the waste generated which can serve as fertilizers on farms. Also, the ECBEs production plant was proposed to be sited close to the source of raw materials (probably to E. chlorantha plantation) and therefore, the transportation cost was assigned zero. However, because of the uncertainty attached to the cost of ECSB due to its seasonal variation in market price and erratic fluctuations in the cost of electricity in Nigeria, these cost variables were taken into consideration (in the uncertainty and sensitivity analyses). The FDC was the summation of the insurance cost (1% of DFC), maintenance and repair cost (6% of DFC), factory (5% of DFC) and local taxes (1% of DFC) expenses. The LQA/QC was assumed to be 15 percent of TLC. The UPC of ECBEs which was the computed profitability parameter was computed as the total of the fixed and variable costs divided by the annual amount of ECBEs manufactured [38]. In addition, other assumptions were: project lifetime = 15 year; construction period = 30 months; inflation rate = 4%, equity = 40%; income tax rate = 40% and depreciation method = straight line [34, 64].
2.9.3 Stochastic simulation and sensitivity & risk analyses of unit production cost of E. chlorantha stem bark extracts
The stochastic modeling and sensitivity & uncertainty analyses of the base case ECBEs UPC were performed with the assistance of Monte Carlo simulation in Oracle Crystal Ball (OCB) software and were according to Adeyi et al. [38]. The interest in the UPC of ECBEs was because of its importance as a yard stick for measuring the cost effectiveness during the industrial scale-up investigations towards achieving a profitable and economically viable ECBEs production process [34]. Therefore, process and cost variables used during the deterministic ECBEs integrated process simulation that have tendencies to vary widely because of uncertainties (experimental errors and constant price change in the market) were selected for this analysis. In brief, the technical selected variables were the ECSB/water (g/mL), ET (min), OT (°C) and extract recovery (%) while the cost variables were the major equipment costs (US$), ECSB purchase cost (US$/kg) and). The HAE-T of recovering bioactive compounds is electricity demanding and hence a surge (or decrease) in unit cost of electricity may have notable effects on the unit production cost. All electricity cost (US$/kw-h these selected variables have tendencies to influence the ECBEs UPC which could subsequently affect the profitability of ECBEs process. When carrying out the analysis in OCB, the uncertain technical and cost variables were made the assumptions of OCB while the profitability parameter (UPC of ECBEs) was the forecast variable. All uncertain variables were investigated between − 20 and + 20 of their initial deterministic values and triangular distribution was used for both uncertain and profitability variables. Also, about 30,000 stochastic trials were made to obtain low mean standard error in ECBEs UPC.
3 Results and discussion
3.1 Model fittings, statistical analysis and adequacies for total polyphenol content, extract yield and antioxidant activity
The DOD was used to investigate the effects of HAE variables (OT: 35–55 °C; S/L: 1:20–1:50 g/mL and ET: 100–200 min) on responses (TPC (mg GAE/g d.w), EY (%) and AA (µM AAE/g). The same DOD (which produced 18 experimental runs) was adopted for the construction of predictive models and optimization of ECBEs recovery process. Table 3 presents the experimental values for all the studied selected responses. The TPC, EY and AA ranged between 30.11 and 96.18 mg GAE/g dw, 10.87–21.74% and 1.69–5.68 µM AAE/g respectively. Experimental run 5 (OT = 35 °C, S/L = 1:50 g/mL, ET = 200 min), run 18 (OT = 35 °C, S/L = 1:20 g/mL, ET = 100 min) and run 2 (OT = 55 °C, S/L = 1:20 g/mL, ET = 200 min) possessed the least TPC, EY and AA respectively. Conversely, run 3 (OT = 55 °C, S/L = 1:50 g/mL, ET = 100 min), run 14 (OT = 45 °C, S/L = 1:50 g/mL, ET = 150 min) and run 15 (OT = 35 °C, S/L = 1/50 g/mL, ET = 100 min) achieved the highest TPC, EY and AA respectively. The obtained ranges of TPC, EY and AA were comparable to the heat-assisted extraction of phenolic bioactive compounds from A. indica leaves [32], C. papaya leaves [2] and Eleutherine bulbosa bulbs [65].
When compared with other conventional extraction techniques like the maceration and Soxhlet extraction techniques, satisfactory results were obtained for the performance of HAE in recovering bioactive compounds from plant matrices [66]. For example, the mean yield of TPC of garlic, oregano and parsley leaves were in the range of 0.094–0.245; 10.770–27.057 and 3.311–8.501 mg GAE/g dw respectively when extracted by the maceration method (OT: 30–50 °C; ET: 4—6 h; S/L = 1:30 g/mL; solvent = 95% ethanol) [66]. In another investigation by Tunchaiyaphum et al. [67] using the Soxhlet extraction technique (OT: 160–220 °C; ET: 0.5–4 h; S/L = 1:40 g/mL; solvent = 95% ethanol), the recovery of TPC (17.23–30.62 GAE/g dw) from mango peels were far below the range obtained in the present study. However, the crude bioactive extract yield range (11.65–20.43%) reported for the Soxhlet extraction (OT: 60–80 °C; ET: 6–8 h; S/L = 1:8 g/mL; solvent = hydro-ethanol) of Zarawand Mudaharaj root [68] was comparable with results obtained in this study. Although process factors such as OT, ET, S/L, the polarity of the employed solvent and nature of the plant (e.g. the inherent concentration of bioactive compound in the plant matrix) may play a great role in the bioactive recovery process.
The quadratic models that defined the dependence between the studied process parameters and responses in coded terms, obtained with the assistance of RSM, for TPC, EY and AA are as presented in Eqs. (8), (9) and (10) respectively.
The alphabet A, B and C represent OT, S/L and ET respectively in the quadratic models. The ANOVA results for the constructed models, the model coefficients and parameters for measuring model adequacies are as displayed in Table 4. All the developed response models possessed significant model F-values (TPC = 59.92, EY = 56.00, AA = 193.31) (p values < 0.0001) and non-significant lack of fit (p value > 0.05). The models also have high R2 (0.9854, 0.9844 and 0.9954), Adj R2 (0.9689, 0.9668, and 0.9903) and Pred R2 (0.9110, 0.8920 and 0.9766) values for the TPC, EY and AA respectively.
All the Adj R2 values obtained were in close agreements with the corresponding Pred R2 for all the developed models. This indicated that the developed quadratic models have the capabilities of predicting the observed experimental data within the investigated range [65]. All linear effects (OT, S/L and ET), quadratic effect of OT and all interactive effects (OT X S/L, OT X ET and S/L X ET) were all significant (p < 0.05) in the TPC model. Likewise, all linear effects (OT, S/L and ET), quadratic effects of temperature and time, and all interactive effects (OT X S/L, OT X ET and S/L X ET) were significant (p < 0.05) in the EY model.
However, only the linear effects of OT and ET, quadratic effects of OT, S/L and ET and interactive effect of OT X ET were the only significant (p < 0.05) models terms in AA response model. The positive and negative signs in Eqs. (1), (2) and (3) indicated an increase and a decrease in the response values respectively as the process parameters increased. Therefore, increased OT, S/L and AA resulted in increased TPC and AA while increased S/L only caused increased AA. However, an increase in OT and EY resulted in significant reduction in AA. The parity graphs of the predicted versus experimental data for TPC, EY and AA are depicted in Fig. 2(a), (b) and (c) respectively. It is clear from Fig. 2 that all the constructed quadratic models predicted the observed experimental data excellently. Both the predicted and observed data sets fell on the diagonal line which indicated perfect prediction [50].
3.2 Numerical optimization of process variables for E. chlorantha stem bark extracts recovery
Multi-objective process optimization was conducted according to Oke et al. [50] to investigate operating variables that simultaneously maximized the TPC, EY and AA of ECBEs. Figure 3 presents the ramp and desirability values results of the best solution (solution with the highest combined desirability) suggested by the Design-Expert software. The optimum TPC (87.46 mg GAE/g dw), EY (21.09%) and AA (5.68 µM AAE/g dw) of ECBEs was achieved at OT of 54.10 °C, ET of 120.16 min and S/L of 1:50 g/mL. The desirability bar graphs (Fig. 3b) indicated OT, S/L and ET possessed desirability values of 1 (one) each. However, the response variables of TPC, EY and AA possessed desirability value of 0.8767, 0.9403, and 1 respectively. The obtained combined desirability value was 0.9376. The validation experiment was carried out at the optimum predicted process parameters (OT = 54.10 °C, S/L = 1:50 g/mL and ET = 120.16 min) to ascertain the Design-Expert software prediction. The RSD between the validation results (TPC = 91.23 mg GAE/g dw; EY = 23.11% and AA = 6.21 µ M AAE/g dw) and predicted results (Fig. 2a) were estimated as 2.98%, 6.47% and 6.30% for the TPC, EY and AA respectively. More so, since the RSDs obtained were less than 10, these results can therefore be considered not different from the predicted values [69]. Therefore, the constructed models for the TPC, EY and AA were well fitted and robust for the HAE ECBEs recovery prediction. The disparities obtained in the TPC, EY and AA of the predicted and validation results may be as a result of uncontrolled environmental (e.g. changes in atmospheric temperature), instrument (e.g. limitation in spectrophotometer measurement) and human errors (e.g. mistakes made by misreading a measurement).
3.3 Elucidation of bioactive compounds in E. chlorantha stem bark extracts
The main reason for the HPLC characterization of ECBEs was to gain information regarding the phenolic acids present in the extracts. To this end, eight (8) phenolic acids with well documented bioactivities in the literature were selected as references. The retention times (RT) for the reference phenolic acids and ECBEs at optimum HAE conditions were established and compared to inform the phenolic profiling of the ECBEs. Figure 4 is the HPLC chromatogram for the ECBEs recovered at the optimum process conditions (OT = 54.10 °C, S/L = 1:50 g/mL and ET = 120.16 min) and detailed the phenolic acids in ECBEs when compared with the reference phenolic acids. It should be mentioned that the presented chromatogram showed various peaks and absorption areas at different RT. The phenolic acids present in ECBEs based on RT therefore, are the betulinic acid (RT = 2.490 min), gallic acid (RT = 2.859 min), chlorogenic acid (RT = 3.690 min), ellagic acid (RT = 5.803 min) and rutin (RT = 6.594 min).
The reason for conducting qualitative identification of the phenolic compounds in the extracts was to determine the extracts potential medicinal values and to establish the basis for producing the extracts on an industrial scale. All the phenolic acids present in the ECBEs have well documented therapeutic potentials. There were reported scientific evidences that betulinic acid possesses potent anticancer effects due to its ability to induce apoptosis (programmed cell death) in cancer cells [70], gallic acid has anti-inflammatory and antioxidant activities and may also have potential as a treatment for certain types of cancer [71] and chlorogenic acid is an antibacterial agent as well as an antioxidant and may have potential as a treatment for type 2 diabetes [72]. In the same vain, recently published works have reported that the ellagic acid is active against the colorectal cancer [73], possesses antioxidant and anti-inflammatory properties and can protect against oxidative stress [74]. More so, rutin is effective for the treatment of varicosities, cardiovascular diseases, haemorrhoids and internal haemorrhage and can prevent retinal haemorrhage and cerebral accidents in patients [75].
3.4 Technicalities and economics of E. chlorantha stem bark extracts production using the heat-assisted extraction technology
3.4.1 Techno-economics of E. chlorantha stem bark extracts base case production process
Economic feasibility studies are essential for practical industrial applications as they assist in determining the financial viability of the project, facilitate sound decision making, help identify potential risks and challenges, provide a framework for project planning and help secure funding for the project. Therefore, this analysis is intended to evaluate the industrial production of ECBEs from ECSB in order to assess the viability of commercialization of natural medicinal extracts of high economic value from an indigenous medicinal plant of low economic importance. The base case which represents proof-of-concept of the laboratory production of ECBEs was synthesized with the optimum process conditions for the recovery of ECBEs obtained in Sect. 3.2 in accordance with the works of Oke et al. [50] and Adeyi et al. [39]. The integration of the evaporator and spray dryer was necessary to achieve low water content (WaC) (< 5%) in the ECBEs [2, 32, 34, 39]. The low WaC in the ECBEs was anticipated to obtain long term activity of the bioactive compounds in ECBEs. The base case flowsheet of ECBEs was synthesized with the assumption that the large capacity of ECBEs production will have similar behavior with the laboratory capacity provided same operating conditions are used for the design [39, 50, 76]. Figure 5 is the operational Gantt chart for the dried ECBEs production using HAE-T. It is evident from Fig. 5 that 5.2 h was used to produce a dried batch of 0.22 g ECBEs in an extractor of 0.057 L capacity. The cycle time of the process was 3 h and 2636 batch /y of ECBEs was achieved when the AOT was based on 7920 h. Figure 1 (base case flowsheet) detailed the streams’ material balances in the ECBEs production using HAE-T. Therefore, a batch production of 0.22 g (which translates to 0.58 kg/y) ECBEs required 0.001 kg (3 kg/y) ECB, 0.050 kg (131 kg/y) water and 0.149 kg (394 kg/y) drying air. The 0.22 g/batch (0.58 kg/y) of produced dried ECBEs comprised of 0.2109 g (0.56 kg/y) bioactive extracts and 0.00897 g (0.024 kg/y) WaC and this agreed with the optimum ECBEs yield data obtained in Sect. 3.2. The low WaC of 4.08% was designed to achieve minimum microbial activities in the produced dried ECBEs.
On annual basis, the ECBEs base case of 0.58 kg/y required 452 kW-h/y electricity demands, 31 MT/y cooling demands, and 0.1668 MT/y steam demand. The laboratory proof-of-concept economics was however, not interesting due to the outrageously high UPC of 3,825,955.1727 US$/kg ECBEs as a result of a huge TOPC of 2,217,388 US$/y and TCC of 9,732,891 US$ needed to produce 0.22 g ECBEs/batch (0.58 kg ECBEs/y). This therefore indicates that a process up scaling is a necessity if the ECBEs production will be profitable.
3.4.2 Effects of plant extractor setup capacity of E. chlorantha stem bark extracts production on material and energy balances
Three other scales of production of ECBEs using the HAE-T were investigated to have the knowledge of the profitability of ECBEs production at larger scales. The investigated scales of ECBEs production as a function of extractor capacity was according to the works of Ochoa et al. [53] and Adeyi et al. [38]. Therefore ECBEs production plants of extractor setup capacities of 5, 100 and 1000 L which translated to 0.019 kg/batch (50.084 kg/y), 0.386 kg/batch (1,017.496 kg/y) and 3.857 kg/batch (10,167.052 kg/y) respectively were considered for the scale-up studies. Table 5 detailed the material and energy requirements at batch and annual references for the investigated production setup capacities of ECBEs.
As shown in Table 5, the three key raw materials needed for the production of ECBEs were the ECSB, water and drying air. The ECSB was the plant material from where ECBEs were recovered, water was the solvent for ECBEs recovery while air served as the ingredient for the drying of ECBEs in the spray dryer. The production plants with extractor setups of 5, 100 and 1000 L required 0.088 kg/batch (231 kg/y) ECSB, 4.363 kg/batch (11,500 kg/y) water, and 13.107 kg/batch (34,551 kg/y) drying air; 1.754 kg/batch (4,625 kg/y) ECSB, 87.255 kg/batch (230,004 kg/y) water, and 262.147 kg/batch (691,021 kg/y) drying air and 17.544 kg/batch (46,246 kg/y) ECSB, 872.548 kg/batch (2,300,036 kg/y) water and 2,621.474 kg/batch (6,910,206 kg/y) drying air respectively. It is obvious from Table 5 that raw material requirements increased with increased ECBEs production capacities. This observation is in accordance with the reports of Adeyi et al. [2, 34, 38].
The energy demands in the utility category are the electricity demand, heating demand, cooling demand and heat transfer agents. The ECBEs production plant with 5 L extractor setup required 1.2 kW-h/batch (3412 kW-h/y) electricity demand, 0.007 MT/batch (14 MT/y) heating demand, 0.1 MT/batch (250 MT/y) cooling demand and 100 kg/batch (263,600 kg/y) heat transfer agents. Likewise the 100 and 1000 L ECBEs production extractor setups required 9 kW-h/batch (23,898 kW-h/y) electricity demand, 0.11 MT/batch (289 MT/y) heating demand, 0.75 MT/batch (1956 MT/y) cooling demand and 851 kg/batch (2,243,236 kg/y) heat transfer agents; and 86 kW-h/batch (227,939 kW-h/y) electricity demand, 1 MT/batch (2887 MT/y) heating demand, 7 MT/batch (18,802 MT/y) cooling demand and 8228 kg/batch (21,689,008 kg/y) heat transfer agents respectively. Although not scaled proportionately with respect to the scaling factor, the electricity demand, heating demand, cooling demand and heat transfer agents increased with increased ECBEs production capacities. The 5 and 1000 L extractor setup plant capacities required the least and highest electricity demand, heating demand, cooling demand and heat transfer agents respectively.
3.4.3 Effects of plant extractor setup capacity on production costs and unit production cost of E. chlorantha stem bark extracts
The TOPC and TCC were the production costs determined in the course of ECBEs production at different plant extractor setups. Also, the UPC of ECBEs was the profitability parameter assessed for the ECBEs production using the HAE-T. Both the production costs (TOPC and TCC) and UPC were determined in accordance with the method of Baral and Shah [64]. Table 6 summarized the production costs (TOPC and TCC), and other cost components such as the TPIC, TPDC, DFC, CFC, WC and start-up cost. The EPC (which was the seed for the computation of production costs) and UPC of the investigated ECBEs production extractor setup capacities were also presented alongside. It is noteworthy to mention that the sizing of equipment required by each ECSBEs production setup had been done based on the material balances displayed in Table 5. Although all the investigated ECBEs setups required the same set of process equipment, their sizes and numbers however, differ.
The breakdown of the equipment type, sizes, numbers and costs of the respective process equipment requirements for all the setups are detailed in Table 7. As can be seen in Table 7, the equipment size is a function of ECBEs production setup capacity. Here, all the process equipment type sizes increased with increased ECBEs production setup capacity. This is expected and it is indicative of the relative scales of ECBEs production by each setup capacity. The 1000 L plant extractor setup for the production of ECBEs required set of equipment with the highest throughputs (in terms of BC-101 and GR-101), volumes (in terms of SDR-101, R-101, V-101, V-102, V-103 and V-104) and surface area (in terms of TFE) among the investigated ECBEs production setups. The BC-101 was also required in multiple (Nu = 8) for the 1000 L extractor ECBEs production setup. The purchased costs of major equipment were also affected by their respective sizes. For example, the cost of BC-101, SDR-101 and R-101 showed a clear increase with an increase in ECBEs production extractor setup capacity.
Therefore, the EPC which represents the total equipment purchased cost is expected to vary with ECBEs production setup capacity. The disparity in the EPC as function of ECBEs production setup capacity is presented in Table 6. The EPC for the ECBEs production plants with 5, 100 and 1000 L extractor capacities are US$ 1,545,000, US$ 1,755,000 and US$ 4,999,000 respectively. The EPC was found to increase with an increase in ECBEs production capacity.
The TCC and its components (DFC, WC and start-up & validation costs) were observed to increase with increased ECBEs production setup capacity as presented in Table 6. The plant with 5 L ECBEs production setup possessed the least TCC of US$ 9,733,033 while the plant with 1000 L extractor setup possessed the highest TCC of US$ 32,254,528. Although when ECBEs setup capacity was increased by 20 folds (5–100 L), and 10 folds (100–1000 L), the plant TCC increased 1.14 folds (US$ 9,733,033 to US$ 11,106,834) and 2.90 folds (US$ 11,106,834 to US$ 32,254,528). This showed a non-linear relationship between the plant TCC and ECBEs plant extractor setup capacity. Adeyi et al. [2] and Adeyi et al. [34] reported similar observations on the scale up studies of natural colorants recovery from calyxes of Hibiscus sabdariffa and bioactive extracts production from C. papaya leaves respectively. The non-linear relationship exhibited by the plant TCC (which also increased disproportionately and manifested as non-linear increase in the DFC with extractor setup capacity) as a function ECBEs plant extractor setup capacity could be as a result of the disproportionate increase in EPC. Also, the two key components (WC and start-up & validation cost) in the plant TCC, increased non-linearly with ECBEs production plant extractor setup capacity.
The information regarding the TOPC as a function of ECBEs production plant setup capacity can as well be found in Table 6. The TOPC of the 5, 100 and 1000 L ECBEs production plant extractor setups were obtained as US$ 2,218,951, US$ 2,553,087 and US$ 7,539,527 respectively. The production plant with ECBEs extractor setup capacity of 5 L possessed the least TOPC while the 1000 L plant capacity had the highest TOPC. It is evident from Table 6 that the TOPC, just like TCC, also increased but non-linearly with ECBEs production setup capacity. Analysis of TOPC data in the table showed that when the setup capacity was scaled up by 2000% (5–100 L) and 1000% (100–1000 L), the TOPC increased by 15% and 195% respectively. This non-linear relationship between the TOPC and ECBEs plant setup capacity indicated that one or more TOPC cost components (MC, TUC, TLC, LQA/QC and FDC) were also scaled up non-linearly. The observed non-linearity between the ECBEs setup capacity and TOPC may likely be introduced from the TUC and FDC. The reasons being that equal operators were allocated to ECBEs process plant regardless of capacity, the raw material requirements were linearly scaled up as observed in Table 5 and the LQA/QC was calculated as percentage of TLC. The non-linear dependency observed is obvious in the TUC since the energy requirements was not a linear function of ECBEs production plant setup capacity and FDC was estimated as a fraction of DFC which itself increased disproportionately with ECBEs production setup capacity. The stacked graph of the TCC and TOPC is presented in Fig. 6. Figure 6 showed that the TOPC was higher than the corresponding TCC of all the investigated ECBEs production capacity.
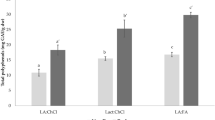
The TOPC/TCC for the 5, 100 and 1000 L ECBEs production setup capacity are 0.228, 0.229 and 0.234 respectively. The UPC of ECBEs, which is indicative of the total cost of production that was incurred in order to manufacture a kilogram of ECBEs, is detailed in Table 6. The UPC has been displayed as a function of plant extractor setup capacity in Table 6. The UPC was used in this study as an economic parameter to evaluate the cost effectiveness of each setup capacity of ECBEs production. The UPC is a widely used profitability parameter and has been reported for the assessment of many conceptual integrated processes in the literature [2, 56, 77]. The UPC of ECBEs production plant extractor setup capacity of 5, 100 and 1000 L are 43,646.6282, 2510.9536 and 741.5103 US$/kg ECBEs respectively. The plant setup capacity with the least ECBEs UPC is the 1000 L while the highest is the 5 L extractor capacity. Figure 7 presents the variation of the ECBEs extractor plant setup capacity with ECBEs UPC.
It is clear from the figure that UPC decreased with increased ECBEs production plant setup capacity. The power law relationship (R2 = 0.994) of -3.74 was established between the UPC of ECBEs and the investigated plant extractor setup capacity range. The observation made in Fig. 7 is an indication of economy of scale effect as the ECBEs production setup capacity increases. Similar observation was documented by Adeyi et al. [2, 34, 39]. However, since the 1000 L plant extractor setup capacity possessed the least ECBEs UPC of 714.5103 US$/kg, it was adjudged the most economical setup of ECBEs production. The UPC of 714.5103 US$/kg obtained for 1000 L (which is equivalent to 10,167.052 kg/y) extractor setup capacity of ECBEs production is comparable with US$ 756.36 reported for 6,384 kg/y of C. papaya leaves extracts (CPLEs) production obtained by HAE-T [2]. The disparity in the UPC of ECBEs and CPLEs are functions of different HAE conditions (HAE conditions for ECBEs and CPLEs are OT = 54.10 °C, S/L = 1:50 g/mL, ET = 120.16 min and OT = 35 °C, S/L = 1:40.25 g/mL, ET = 100 min, respectively) and scale of production (10,167.052 kg/y and 6,384 kg/y for ECBEs and CPLEs respectively). The UPC of US$ 714.5103 per kilogram of ECBEs obtained using HAE-T is however, higher than the cost of manufacturing of 475 US$/kg reported for the Eugenia uniflora leaves extracts using supercritical extraction (OT = 80 °C, P = 25 MPa, extractor capacity = 100 L) [51]. Although the UPC of ECBEs obtained is comparable with other reported works using similar HAE-T, the uncertainty and sensitivity analyses for an integrated process of this nature is a necessity in order to achieve further process engineering improvement. Hence, the 1000 L extractor ECBEs production setup, being the most economical (possessing the least UPC among the three ECBEs production setup capacities investigated) was therefore considered for the risk and sensitivity analyses in Sect. 3.5.
3.5 Stochastic modeling, profitability risk assessment and sensitivity analysis of variables on E. chlorantha stem bark extracts unit production cost
The 1000 L ECBEs production setup was considered for the profitability risk assessment and sensitivity studies being the most economically viable industrial capacity of producing ECBEs among the investigated capacities. One of the reasons for the risk assessment and sensitivity analysis of an integrated process of this nature is to pinpoint technical and cost variables of significant implications on the process techno-economics for further refinement and optimization. This is because the preliminary techno-economic study was deterministic in nature and therefore, some of the deterministic variables employed for the economic analysis can fluctuate and take different data value in reality. Hence, the technical and cost variables that have high probability to change greatly in nature were carefully selected and subjected to risk assessment and sensitivity analysis. The process variable of OT, ET, ECSB/water and ECBEs recovery were selected as technical variables and this was because all these factors are very liable to laboratory errors due to human, instrument or environment. In the same vain, the selected cost variables deemed to be prone to constant price change, were the major equipment purchase costs (the centrifuge, extractor, spray dryer and grinder costs), raw material (ECSB) purchase cost and electricity cost. The centrifuge, extractor, spray dryer and grinder purchase costs contributed 53.5%, 13.1%, 4.06% and 1.82% to EPC respectively and therefore justified their selection. It should be mentioned that these equipment costs varied widely in the market and formed the basis for the computation of TCC. The ECSB purchase price is season dependent while the electricity cost fluctuates here in Nigeria and as such were the reasons for their selection. Both the ECSB cost and electricity cost formed integral part of TOPC of ECBEs production process. All the selected variables (both technical and cost) do impact the profitability of the ECBEs production process tremendous. Figure 8 presents the stochastic distribution (30,000 ensembles) of ECBEs UPC. The distributions were the different ECBEs UPC values resulting from the Monte Carlo prediction using combinations of the selected technical and cost variables values (varied between − 20 and + 20% of their initial values). The ECBEs UPC probability, cumulative probability and reverse probability views are presented on the left hand side of Fig. 8 while the right hand side view presented the probability data statistics and UPC probability model fitting parameters. In order to have a robust understanding of the ECBEs UPC probability distribution, some statistical parameters such the UPC distribution mean, median, standard deviation and range were assessed. Figure 8 showed that the distribution is normal with data mean, median and standard deviation of US$ 763.52, US$ 757.88 and US$ 110.56 respectively. The kurtosis and skewness of the ECBEs UPC distribution are 2.88 and 0.277 respectively. The ECBEs UPC distribution also possessed a clearly defined range with minimum UPC of US$ 377.58 and maximum of US$ 1541.85.
The distribution was best described by beta model with Anderson Darling coefficient of 0.44789. The difference that existed between the mean values of the Monte Carlo predicted ECBEs UPC and the UPC obtained through techno-economic analysis was approximately 2.96%. The certainty of achieving the ECBEs UPC of 763.52 US$/kg was 55.43%. Also, the ranking of the relative importance of selected variables to the ECBEs UPC was achieved through sensitivity analysis. The dynamic sensitivity graph is presented in Fig. 9. Figure 9 showed that the investigated variables have different contributions to the perturbation in ECBEs UPC. The sensitivity bars to the right implied that a rise in the variable resulted into a corresponding rise in the ECBEs UPC and this indicated a positive contribution. In the same vain, a sensitivity bar to the left hand side implied that an increase in the variable decreased the ECBEs UPC and thus caused a negative contribution.
Analysis of Fig. 9 therefore indicated that ECSB/water, ET, centrifuge purchase cost, OT, extractor purchase cost, electricity cost and ECSB purchase cost contributed positively to the variance in ECBEs UPC. This invariably suggested that increase in these parameters resulted in increased ECBEs UPC since these costs will proportionately increase the production cost of ECBEs. For instance, an increase in ECSB/water is expected to cause an increase in material and equipment costs (since the ECBEs production plant will require higher equipment specification and capacities to achieve a batch size as ECSB/water increases) which subsequently impacted the UPC of ECBEs positively. Conversely, extract recovery negatively contributed to the perturbation in ECBEs UPC. This is logical and implied that an increase in the ECBEs yield will significantly reduce the ECBEs UPC. Also the dryer and grinder purchase costs did not contribute to the variance in ECBEs UPC. However, in numerical terms, the ECBEs/water, ET, centrifuge purchase cost, OT, extractor purchase cost, electricity cost, ECSB purchase cost, extract recovery, dryer purchase cost and grinder purchase cost contributed + 36.7%, + 13.6%, + 10.6%, + 0.55%, + 0.37%, + 0.2%, + 0.08%,—37.9%, 0% and 0% respectively to the perturbation in ECBEs UPC.
4 Conclusion
The optimization protocol for bioactive compounds recovery from E. chlorantha stem bark using HAE-T was investigated in the present study. The effects of important extraction factors (operating temperature, solid: water and extraction time) were examined on the heat-assisted extraction of bioactive extracts. The multi-criteria optimization was performed to establish the global optimum conditions for bioactive extract recovery from ECSB. In addition, the global optimum HAE data were employed for the design, scale-up and techno-economic analysis of an integrated process for the bioactive antioxidants production from ECSB. The risk assessment of the most economically viable production setup was established. All the investigated HAE parameters influenced the bioactive antioxidant recovery from ECSB. The phenolic profiling showed that ECBEs consisted of chlorogenic acid, betulinic acid, ellagic acid. gallic acid and rutin. The 1000 L industrial setup capacity possessed the least unit production cost (UPC) and therefore was selected as the most profitable ECBEs production capacity. The certainty of achieving the 1000 L base case ECBEs UPC of 763.52 US$/kg was 55.43%. The only variable that contributed negatively was extract recovery (− 37.9%) while other variables such as ECBEs/water (36.7%), ET (13.6%), centrifuge purchase cost (10.6%), OT (0.55%), extractor purchase cost (0.37%), electricity cost (0.2%) and ECSB purchase cost (0.08%) contributed positively to the variance in ECBEs UPC. Both dryer and grinder purchase costs did not perturb the ECBEs UPC.
Based on the present availability and need, the extracts had been characterized with HPLC, however, future work can elaborate on the elucidation of bioactive compounds present in the extracts with other state of the art analytical equipment such as the quadrupole time of flight mass spectrometry. Furthermore extracts purification method that is of interest from industrial point of view such as a solid-phase extraction (SLE) technique should be employed for the purification of the crude extracts. An encompassing integrated process incorporating SLE purification procedure can thereafter be designed, scaled-up and techno-economically analysed. The aforementioned will provide a more robust comprehensive data-driven assessment of an optimized profitable integrated process that is implementable industrially.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ECSB:
-
Enantia chlorantha Stem bark
- ECBEs:
-
Enantia chlorantha Stem bark extracts
- WaC:
-
Water content
- TPC:
-
Total phenolic content
- AA:
-
Antioxidant activity
- EY:
-
Extract yield
- GAE:
-
Gallic acid equivalence
- AAE:
-
Ascobic acid equivalence
- d.w:
-
Dry weight
- HAE:
-
Heat assisted extraction
- HAE-T:
-
Heat assisted extraction technology
- EPC:
-
Equipment purchase cost
- DFC:
-
Direct fixed capital
- TUC:
-
Total utility cost
- TCC:
-
Total capital cost
- TPIC:
-
Total plant indirect cost
- TPDC:
-
Total plant direct cost
- TOPC:
-
Total operating cost
- TLC:
-
Total labor cost
- FDC:
-
Facility dependent cost
- LQA/QC:
-
Laboratory QA/QC
- MC:
-
Miscellaneous Cost
- RMC:
-
Raw material cost
- CFC:
-
Contractor’s Fees and Contingencies cost
- WC:
-
Working capital
- UPC:
-
Unit production cost
References
Teodoro AJ (2019) Bioactive compounds of food: their role in the prevention and treatment of diseases. Oxid Med Cell Longev 2019:1–4. https://doi.org/10.1155/2019/3765986
Adeyi O, Oke EO, Okolo BI, Adeyi AJ, Otolorin JA, Nwosu-Obieogu K, Adeyanju JA, Dzarma GW, Okhale S, Ogu D, Onu PN (2022) Process optimization, scale-up studies, economic analysis and risk assessment of phenolic rich bioactive extracts production from Carica papaya L leaves via heat-assisted extraction technology. Heliyon 8(4):1–14. https://doi.org/10.1016/j.heliyon.2022.e09216
Loi M, Paciolla C, Logrieco AF, Mulè G (2020) Plant bioactive compounds in pre-and postharvest management for aflatoxins reduction. Front Microbiol 11:1–16. https://doi.org/10.3389/fmicb.2020.00243
Serrano A, Ros G, Nieto G (2018) Bioactive compounds and extracts from traditional herbs and their potential anti-inflammatory health effects. Medicines 5(3):1–9. https://doi.org/10.3390/medicines5030076
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, Li HB (2016) Natural polyphenols for prevention and treatment of cancer. Nutrients 8(8):1–35. https://doi.org/10.3390/nu8080515
Sun-Waterhouse D, Wadhwa SS (2013) Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: a review. Food Bioprocess Technol 6(3):607–627. https://doi.org/10.1007/s11947-012-0829-2
Castro-Alatorre NC, Gallardo-Velázquez T, Boyano-Orozco LC, Téllez-Medina DI, Meza-Márquez OG, Osorio-Revilla G (2021) Extraction and microencapsulation of bioactive compounds from muicle (Justicia spicigera) and their use in the formulation of functional foods. Foods 10(8):1–16. https://doi.org/10.3390/foods10081747
Ugbogu AE, Okezie E, Uche-Ikonne C, Duru M, Atasie OC (2016) Toxicity evaluation of the aqueous stem extracts of Senna alata in wistar rats. Am J Biomed Res 4(4):80–86. https://doi.org/10.12691/ajbr-4-4-1
Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf 12(1):5–23. https://doi.org/10.1111/1541-4337.12005
Babbar N, Oberoi HS, Sandhu SK (2015) Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit Rev Food Sci Nutr 55(3):319–337. https://doi.org/10.1080/10408398.2011.653734
Kumar S, Kalita S, Das A, Kumar P, Singh S, Katiyar V, Mukherjee A (2022) Aloe vera: a contemporary overview on scope and prospects in food preservation and packaging. Prog Org Coat 166:106799. https://doi.org/10.1016/j.porgcoat.2022.106799
Ko K, Dadmohammadi Y, Abbaspourrad A (2021) Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: a review. Foods 10(3):657. https://doi.org/10.3390/foods10030657
Kaur K, Sharma R, Singh S (2019) Bioactive composition and promising health benefits of natural food flavors and colorants: potential beyond their basic functions. Pigment Resin Technol. https://doi.org/10.1108/PRT-02-2019-0009
Beier RC (1990) Reviews of environmental contamination and toxicology. Springer, New York
Alara OR, Abdurahman NH, Ali HA, Zain NM (2021) Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: an optimization study and LC-QTOF-MS analysis. Future Foods 3:100035. https://doi.org/10.1016/j.fufo.2021.100035
Saeed Alkaltham M, Hayat K, Asif Ahmed M, Mohammad Salamatullah A, Sami R, Khojah E, Al-Mushhin AA (2021) Bioactive compounds, high performance liquid chromatography screening of phenolic compounds, and antioxidant potential activity of saffron (Crocus sativus L.). J Biobased Mater 15(5):700–704. https://doi.org/10.1166/jbmb.2021.2106
Davares AKL, Arsene MMJ, Viktorovna PI, Shommya D (2021) Enantia chlorantha and its multiple therapeutic virtues: a mini review. Pharm Res Int 33:254–259. https://doi.org/10.9734/JPRI/2021/v33i45A32741
Agbaje EO, Onabanjo AO (1991) The effects of extracts of Enantia chlorantha in malaria. Ann Trop Med Parasitol 85(6):585–590. https://doi.org/10.1080/00034983.1991.11812613
Etame RE, Mouokeu RS, Pouaha CLC, Kenfack IV, Tchientcheu R, Assam JPA, Poundeu FSM, Tiabou AT, Etoa FX, Kuiate JR, Ngane RAN (2018) Effect of fractioning on antibacterial activity of Enantia chlorantha Oliver (Annonaceae) methanol extract and mode of action. Evid Based Complement Alternat Med 2018:1–13. https://doi.org/10.1155/2018/4831593
Akinwale SG, Chukwu OE, Chioma OP, Chukudi AJ, Olubunmi AG (2022) Enantia chlorantha: a review. J Pharmacogn Phytochem. 11(3):34–38. https://doi.org/10.22271/phyto.2022.v11.i3a.14406
Adeyemi AI (2015) Isolation and screening of endophytic fungi from three plants used in traditional medicine in Nigeria for antimicrobial activity. Int J Green Pharm 9(1):58–62. https://doi.org/10.22377/ijgp.v9i1.439
Adesokan AA, Akanji MA, Yakubu MT (2007) Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr J Biotechnol 6(22):1684–5315. https://doi.org/10.5897/AJB2007.000-2397
Abubakar A, Ahmad NS, Akanya HO, Abdulkadir A, Abubakar AN (2020) Antiplasmodial activity of total alkaloids and flavonoids of stem bark extracts of Enantia chlorantha in mice. Comp Clin Pathol 29(4):873–881. https://doi.org/10.1007/s00580-020-03138-4
Siminialayi IM, Agbaje EO (2004) Gastroprotective effects of the ethanolic extract of Enantia chlorantha in rats. West Afr J Pharmacol Drug Res 20:35–38. https://doi.org/10.4314/wajpdr.v20i1.14743
Adesokan AA, Akanji MA (2010) Haematological changes following chronic administration of aqueous extract of Enantia chlorantha to albino rats. Trop J Health Sci 17(1):1117–4153. https://doi.org/10.4314/tjhc.v17i1.52791
Mesmine KTM, George EOE, Christophe M, Ernestine NZ, Paul TV, Barthelemy N (2015) Aqueous extract of Enantia chlorantha (Annonaceae) prevents the delay in chronic gastric ulcer healing caused by indomethacin in rats. Br J Pharm Res 8:1–13. https://doi.org/10.9734/BJPR/2015/19035
Agbaje EO, Tijani AY, Braimoh OO (2003) Effects of Enantia chlorantha extracts in laboratory-induced convulsion and inflammation. Orient J Med 15(1):68–71. https://doi.org/10.4314/ojm.v15i1.29050
Otimenyin SO, Uguru MO (2006) Acute toxicity studies, anti-inflammatory and analgesic activities of the methanolic extract of the stem bark of Enantia chlorantha and Nauclea latifolia. J Pharm Bioresour 3(2):111–115. https://doi.org/10.12816/0001627
Adebiyi OE, Abatan MO (2013) Protective effects of Enantia chlorantha stem bark extracts on acetaminophen induced liver damage in rats. Jordan J Biol Sci 147(916):1–7. https://doi.org/10.12816/0001627
Oyewopo AO, Saalu LC, Dare BJ, Oyewopo CI, Jimoh AA, Yama OE, Kadir RE, Olaniyan OT (2012) Testiculo-protective effect of stem bark extract of Enantia chlorantha on lead induced toxicity in adult Wistar rat (Rattus norvergicus). Reprod Syst Sexual Disord 1(2):1000107. https://doi.org/10.4172/2161-038X.1000107
Alford CM, Onyejiaka A, Myles EL (2016) Cytotoxic activity of Enantia chlorantha, Nauclea latifolia, and Citrus medica extracts on carcinoma cells. Cancer Res. https://doi.org/10.1158/1538-7445.AM2016-614
Oke EO, Adeyi O, Okolo BI, Adeyi JA, Ayanyemi J, Osoh KA, Adegoke TS (2020) Phenolic compound extraction from Nigerian Azadirachta indica leaves: response surface and neuro-fuzzy modelling performance evaluation with Cuckoo search multi-objective optimization. Res Eng. 8:100160. https://doi.org/10.1016/j.rineng.2020.100160
Gan CY, Latiff AA (2011) Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem 124(3):1277–1283. https://doi.org/10.1016/j.foodchem.2010.07.074
Adeyi O, Oke EO, Adeyi AJ, Okolo BI, Olalere AO, Otolorin JA, Aremu OS, Qwebani-Ogunleye T (2022) Microencapsulated anthocyanins powder production from Hibiscus sabdariffa L. calyx: process synthesis and economic analysis. Res Eng 13:100371. https://doi.org/10.1016/j.rineng.2022.100371
Laeliocattleya RA, Estiasih T, Griselda G, Muchlisyiyah J (2018) The bioactive compounds and antioxidant activity of ethanol and ethyl acetate extracts of Candi Banana (Musa paradisiaca). In: IOP conference series earth environ science 131(1): 012013. https://doi.org/10.1088/1755-1315/131/1/012013.
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Comp Altern Med. https://doi.org/10.4314/ajtcam.v8i1.60483
Lezoul NEH, Belkadi M, Habibi F, Guillén F (2020) Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules 25(20):4672. https://doi.org/10.3390/molecules25204672
Adeyi O, Adeyi AJ, Oke EO, Okolo BI, Olalere AO, Otolorin JA, Okhale S, Taiwo AE, Oladunni SO, Akatobi KN (2022) Process integration for food colorant production from Hibiscus sabdariffa calyx: a case of multi-gene genetic programming (MGGP) model and techno-economics. Alex Eng J 61(7):5235–5252. https://doi.org/10.1016/j.aej.2021.10.049
Adeyi O, Okolo BI, Oke EO, Adeyi AJ, Otolorin JA, Olalere OA, Taiwo AE, Okhale S, Gbadamosi B, Onu PN, Aremu OS, Qwebani-Ogunleye T (2022) Preliminary techno-economic assessment and uncertainty analysis of scaled-up integrated process for bioactive extracts production from Senna alata (L.) leaves. S Afr J Chem Eng 42:72–90. https://doi.org/10.1016/j.sajce.2022.07.007
Mohd Jusoh NH, Subki A, Yeap SK, Yap KC, Jaganath IB (2019) Pressurized hot water extraction of hydrosable tannins from Phyllanthus tenellus Roxb. BMC Chem 13(1):1–10. https://doi.org/10.1186/s13065-019-0653-0
Mokgadi J, Turner C, Torto N (2013) Pressurized hot water extraction of alkaloids in Goldenseal. Am J Anal Chem 4(8):398–403. https://doi.org/10.4236/ajac.2013.48050
Khunnawutmanotham N, Chimnoi N, Nangkoed P, Hasakunpaisarn A, Wiwattanapaisarn W, Techasakul S (2021) Facile extraction of three main indole alkaloids from Mitragyna speciosa by using hot water. ChemistrySelect 6(38):10221–10225. https://doi.org/10.1002/slct.202102590
Mohaddes-Kamranshahi M, Jafarizadeh-Malmiri H, Simjoo M, Jafarizad A (2019) Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods. Green Process Synth 8(1):62–67. https://doi.org/10.1515/gps-2017-0185
Güçlü-Üstündağ Ö, Balsevich J, Mazza G (2007) Pressurized low polarity water extraction of saponins from cow cockle seed. J Food Eng 80(2):619–630. https://doi.org/10.1016/j.jfoodeng.2006.06.024
Prasedya ES, Frediansyah A, Martyasari NWR, Ilhami BK, Abidin AS, Padmi H, Fahrurrozi AB, Juanssilfero S, Widyastuti AL, Sunarwidhi AL (2021) Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci Rep 11(1):1–9. https://doi.org/10.1038/s41598-021-95769-y
Gião MS, Pereira CI, Fonseca SC, Pintado ME, Malcata FX (2009) Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem 117(3):412–416. https://doi.org/10.1016/j.foodchem.2009.04.020
Rajha HN, El Darra N, Hobaika Z, Boussetta N, Vorobiev E, Maroun RG, Louka N (2014) Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Sci Nutr 5(4):397–409. https://doi.org/10.4236/fns.2014.54048
Tan YH, Abdullah MO, Nolasco-Hipolito C, Zauzi NSA (2017) Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew Energy 114:437–447. https://doi.org/10.1016/j.renene.2017.07.024
Ismandari T, Kumalaningsih S, Wijana S, Mustaniroh SAU (2020) Optimization of bioactive compound extraction from rose myrtle fruit (Rhodomyrtus tomentosa,(W. Ait), Myrtaceae) as the antioxidant source. Sci World J 2020:1–8. https://doi.org/10.1155/2020/9105847
Oke EO, Adeyi O, Okolo BI, Ude CJ, Adeyi JA, Salam KK, Nwokie U, Nzeribe I (2021) Heterogeneously catalyzed biodiesel production from Azadiricha Indica oil: predictive modelling with uncertainty quantification, experimental optimization and techno-economic analysis. Bioresour Technol 332:125141. https://doi.org/10.1016/j.biortech.2021.125141
Canabarro NI, Veggi PC, Vardanega R, Mazutti MA, do Carmo Ferreira M (2020) Techno-economic evaluation and mathematical modeling of supercritical CO2 extraction from Eugenia uniflora L. leaves. J Appl Res Med Aromat Plants 18:100261. https://doi.org/10.1016/j.jarmap.2020.100261
Vieira GS, Cavalcanti RN, Meireles MAA, Hubinger MD (2013) Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis). J Food Eng 119(2):196–204. https://doi.org/10.1016/j.jfoodeng.2013.05.030
Ochoa S, Durango-Zuleta MM, Osorio-Tobón JF (2020) Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod Process 122:111–123. https://doi.org/10.1016/j.fbp.2020.04.007
Adeniyi AG, Ighalo JO, Amosa MK (2021) Modelling and simulation of banana (Musa spp.) waste pyrolysis for bio-oil production. Biofuels 12(7):879–883. https://doi.org/10.1080/17597269.2018.1554949
Harun N, Othman NA, Zaki NA, Rasul NM, Samah RA, Hashim H (2019) Simulation of anaerobic digestion for biogas production from food waste using SuperPro designer. Mater Today Proc 19:1315–1320. https://doi.org/10.1016/j.matpr.2019.11.143
Gómez JA, Sánchez ÓJ, Correa LF (2020) Techno-economic and environmental evaluation of cheesemaking waste valorization through process simulation using SuperPro designer. Waste Biomass Valorization 11:6025–6045. https://doi.org/10.1007/s12649-019-00833-4
Insights on the Bioactive Ingredients Global Market to 2031 - Featuring Aijnomoto, BASF and Cargill Among Others (2022). https://www.globenewswire.com/en/news-release/2022/02/09/2381740/28124/en/Insights-on-the-Bioactive-Ingredients-Global-Market-to-2031-Featuring-Aijnomoto-BASF-and-Cargill-Among-Others.html. Accessed July 14 2022
Association of Official Analytical Chemists and Kenneth Helrich. 1990. Official Methods of Analysis of the Association of Official Analytical Chemists 15Th ed. 1990 ed. Arlington VA: Association
Pinela J, Prieto MA, Pereira E, Jabeur I, Barreiro MF, Barros L, Ferreira IC (2019) Optimization of heat-and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem 275:309–321. https://doi.org/10.1016/j.foodchem.2018.09.118
Uddin MN, Mitra K, Haque MZ (2016) Comparative bio-active compounds determination and in vitro antioxidant properties of newly developed soy mixed wheat flour and traditional wheat flour. Int J Food Prop 19(9):2113–2126. https://doi.org/10.1080/10942912.2015.1110163
Krishna Murthy TP, Manohar B (2014) Optimization of supercritical carbon dioxide extraction of phenolic compounds from mango ginger rhizome (Curcuma amada Roxb.) using response surface methodology. Biomed biotechnol 2(1):14–19. https://doi.org/10.12691/bb-2-1-3
Intelligen Inc., SuperPro Designer‐User's Guide, Scotch Plains, NJ, 2014.
Athimulam A, Kumaresan S, Foo DCY, Sarmidi MR, Aziz RA (2006) Modelling and optimization of Eurycoma longifolia water extract production. Food Bioprod Process 84(2):139–149. https://doi.org/10.1205/fbp.06004
Baral NR, Shah A (2016) Techno-economic analysis of cellulosic butanol production from corn stover through acetone–butanol–ethanol fermentation. Energy Fuels 30(7):5779–5790. https://doi.org/10.1021/acs.energyfuels.6b00819
Kamarudin AA, Esa NM, Saad N, Sayuti NH, Razak NAA (2020) Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind Crops Prod 144:112064. https://doi.org/10.1016/j.indcrop.2019.112064
Anne R, Nithyanandam R (2016) Optimization of extraction of bioactive compounds from medicinal herbs using response surface methodology. Int Proc Chem Biol Environ Eng 99:76–85. https://doi.org/10.7763/IPCBEE.2016.V99.9
Tunchaiyaphum S, Eshtiaghi MN, Yoswathana N (2013) Extraction of bioactive compounds from mango peels using green technology. Int J Chem Eng Appl 4(4):194–198. https://doi.org/10.7763/IJCEA.2013.V4.293
Ansari MDZ, Sofi G, Hamiduddin Ahmad H, Basri R, Alam A (2021) Optimization of the whole extract of Zarawand Mudaharaj (Aristolochia rotunda L.) root by Response Surface Methodology (RSM). Cell Med 11(3):1–9. https://doi.org/10.5667/CellMed.2021.0015
Domínguez R, Zhang L, Rocchetti G, Lucini L, Pateiro M, Munekata PE, Lorenzo JM (2020) Elderberry (Sambucus nigra L.) as potential source of antioxidants Characterization, optimization of extraction parameters and bioactive properties. Food Chem 330:127266. https://doi.org/10.1016/j.foodchem.2020.127266
Mullauer FB, Kessler JH, Medema JP (2010) Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs 21(3):215–227. https://doi.org/10.1097/CAD.0b013e3283357c62
Kroes BV, Van den Berg AJJ, Van Ufford HQ, Van Dijk H, Labadie RP (1992) Anti-inflammatory activity of gallic acid. Planta Med 58(06):499–504. https://doi.org/10.1055/s-2006-961535
Lou Z, Wang H, Zhu S, Ma C, Wang Z (2011) Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 76(6):398–403. https://doi.org/10.1111/j.1750-3841.2011.02213.x
Mirsane S, Mirsane S (2017) Benefits of ellagic acid from grapes and pomegranates against colorectal cancer. Caspian J Intern Med 8(3):226–227. https://doi.org/10.22088/cjim.8.3.226
Galano A, Francisco Marquez M, Pérez-González A (2014) Ellagic acid: an unusually versatile protector against oxidative stress. Chem Res Toxicol 27(5):904–918. https://doi.org/10.1021/tx500065y
Semwal R, Joshi SK, Semwal RB, Semwal DK (2021) Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem Lett 46:119–128. https://doi.org/10.1016/j.phytol.2021.10.006
Lee CS, Chong MF, Binner E, Gomes R, Robinson J (2018) Techno-economic assessment of scale-up of bio-flocculant extraction and production by using okra as biomass feedstock. Chem Eng Res Des 132:358–369. https://doi.org/10.1016/j.cherd.2018.01.050
Mahmud N, Rosentrater KA (2019) Techno-economic analysis of low moisture anhydrous ammonia (LMAA) pretreatment for butanol production from oil palm frond. Biomass Convers Biorefin 12:1–15. https://doi.org/10.1007/s13399-019-00564-5
Funding
This present research received no grant either in the public, commercial or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Software, Writing—Original Draft, Writing—Review & Editing were by AO, OOE and AJA. Formal analysis, Supervision and data curation were by OAO, AOS, QT, TEA and MY. Project administration was by OIB and OAD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any relevant financial or non-financial interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adeyi, O., Adeyi, A.J., Oke, E.O. et al. Heat-assisted extraction of phenolic-rich bioactive antioxidants from Enantia chlorantha stem bark: multi-objective optimization, integrated process techno-economics and profitability risk assessment. SN Appl. Sci. 5, 153 (2023). https://doi.org/10.1007/s42452-023-05372-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05372-1