Abstract
Hypercholesterolemia is correlated with cardiovascular diseases. The search for effective alternatives for lipid-lowering drugs is continuous. We investigated the hypocholesterolemic activity of Bassia muricata methanolic extract (BMME) in a model of hyperlipidemia. B. muricata was extracted with methanol. Male rats were randomly divided into six groups: normal control group (G1) was fed normal diet, negative control group (G2) was fed high cholesterol and fat diet (HCFD), positive control group (G3) was fed HCFD and treated with atorvastatin (20 mg/kg), a fourth, fifth and sixth groups (G4, G5, and G6) were fed HCFD and treated with 10, 30 and 100 mg/Kg of BMME, respectively. All rat groups received, for 4 weeks, the appropriate daily dose after initial two weeks of feeding normal diet or HCFD. Body weight, lipid profile, serum glucose, liver enzymes were measured weekly. HCFD caused an increased total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and glucose, decreased triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C), and blunted the normal gain of body weight. BMME doses restored the normal gain of body weight, caused significant decrease in serum TC, LDL-C, and increased HDL-C when compared to G2. 10 mg/kg and 30 mg/kg of BMME failed to induce any change in alkaline phosphatase whereas 100 mg/Kg of BMME caused a significant increase in alanine transaminase. 10 mg/kg and 30 mg/kg of BMME significantly decreased serum glucose whereas 100 mg/kg BMME significantly increased it. BMME had significant hypocholesterolemic effect and 100 mg/kg BMME increased alanine transaminase, TG and glucose in rats.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypercholesterolemia is characterized by increased serum total cholesterol, due to elevated levels of lipoproteins such as low-density lipoprotein cholesterol (LDL-C) and decreased concentrations of high-density lipoprotein cholesterol (HDL-C) [1]. It is considered a major risk factor in the development of cardiovascular diseases, leading to major health problems and may be death [2]. Many people could not successfully control their blood cholesterol by altering their lifestyles (e.g., smoking cessation, limiting alcohol consumption, increasing physical activity or controlling their diet) [3]. Drugs marketed to reduce hyperlipidemia include statins, ezetimibe, bile resins, fibrates and nicotinic acid [4]. Statins are the first-line drugs for LDL reduction since they inhibit the rate-limiting enzyme 3-hydroxy-3-methylglutaryl coenzyme-A reductase in the metabolic pathway of cholesterol biosynthesis, reduce the downstream by-products (e.g., mevalonate), and lead to up-regulation of LDL receptors, thus lowering the incidence of cardiovascular events [5]. The use of statin drugs is complicated by adverse effects such as myopathy, myalgia and myositis [6], elevated profile of hepatic enzymes [7], increased risk of memory impairment or loss [8] and can even be fatal as caused by rhabdomyolysis [9].
On the other hand, plants are indispensable source of chemical compounds that can be used for pharmaceutical purposes. Yet, researchers in the field encounter many challenges while obtaining the potential natural products. These challenges have been reviewed extensively by Atanasov, et al. [10]. In Jordan, about 363 medicinal plants are used by traditional therapists and all these have many pharmacological applications [11]. Bassia muricata, an annual plant that belongs to the Chenopodiaceae family, is widely distributed in the Arabian countries, and is considered an important plant in traditional medicine to be used for analgesia, and as antipyretic, anti-inflammatory, antispasmodic as well as diuretic [12]. It was also reported to treat kidney problems, rheumatic diseases, and to lower blood pressure [13, 14]. Moreover, different biological activities including antioxidant, antibacterial [15] and insecticidal activities were also reported for this species [16].
Chemically, B. muricata has been shown to contain triterpenoidal saponins, tannins, sterols, phenolics (coumarin, p-coumaric acid, gallic acid, p-catechuic acid, salicylic acid, ferulic acid), flavan-3-ol(catechum), flavonoids (kaempferol, kaempferol-3-O-α-L-rhamnoside, rutin, myricetin, qurcetin-3-O-α-L-rhamnoside, qurcetin-3-O-β-D-glucoside, 3′-methylquerctein, 3-O-[α-L-arabinopyranosyl-(1→2)-L-α-arabinopyranosyl)]-3′-methylquercetin), flavonoid glycosides (quercetin-3-O-sophoroside, quercetin-3,7-O-β-diglucopyranoside) and two acetylated flavonoid glycosides (caffeoyl and feruloyl) [14, 16, 17]. Thus, quercetin had the maximum value among flavonoid compounds in B. muricata [17]. The essential oil of the aerial parts of B. muricata was characterized by its high content of terpenoids like sesquiterpenes (58.2%), monoterpenes (9.8%) and diterpenes (1.2%) as well as significant amounts of aromatic compounds (21.9%) and 5 hydrocarbons (8.9%) [18]. Flavonoids have a wide spectrum of biological activities, including hypolipidemic and antioxidant properties [19]. In this study, we used methanol to extract the flavonoid-rich fraction of the whole plant material of B. muricata and we investigated the potential hypolipidemic effect of B. muricata methanolic extract (BMME) on rats fed a high cholesterol and fat diet.
2 Material and method
2.1 Plant material collection
Supplementary Fig. 1 shows the whole plant material (leaves, stem, flowers, fruits and roots) of B. muricata which was collected from Wadi Araba on February 2021 (0,731,569 E, 3,418,064 N), altitude (−59 m). The plant was authentically identified by a plant taxonomist. Voucher specimens were deposited at the Herbarium of the Department of Biological Sciences and at the Royal Society for the Conservation of Nature (Reference Nos. 137171 and 30,299, respectively).
2.2 Preparation and extraction procedures
The leaves, stems, flowers, and fruits of B. muricata were dried at 23 °C for 7 weeks and ground into a powder using electric mill (Ambar group l.l.c, Beirut- Leabnon).
The powder (3 kg) was infused in 20 L of methanol for one week to obtain BMME. The process was repeated couple of times on the same plant sample. BMME was collected and filtered using Whatman filter paper No. 3, then concentrated using a Heidolph rotary evaporator (Laborota 4001, Heidolph, Schwabach, Germany) at a temperature of 45 °C for 48 h.The stock extract was refrigerated and protected from light until used.
2.3 Determination of total phenol content (TPC)
Folin-Ciocalteu method was used to calculate the TPC of BMME [20]. In brief, 500 µL of BMME or gallic acid standard at different concentrations (10, 20, 50, 100, 150, 200, 250 and 300 µg/mL; Supplementary Fig. 2A) were mixed with 2.5 mL of diluted Folin-Ciocalteu reagent, then 2.5 mL of Na2CO3 solution was added, vortexed for a minute, then incubated for 20 min at 25°C and the absorbance was read at 760 nm using a spectrophotometer (Biotech Engineering Management CO. Ltd, U.K.) against the blank. TPC was reported as milligrams of gallic acid equivalent (GAE/g of extract) according to Shewale and Rathod [21].
2.4 Determination of total flavonoid content (TFC)
TFC of BMME was measured [22]. In brief, 500 µL of BMME or quercetin standard at different concentrations (10, 20, 50, 100, 150, 200, 250 and 300 µg/mL; Supplementary Fig. 2B) were mixed with 1.5 mL of distilled water, then 100 µL of 1 M sodium acetate was added and mixed. 100 µL of 10% aluminum chloride solution was added, diluted by addition of 2.8 mL of distilled water and vortexed for a minute. The reaction continued by incubation at room temperature for 30 min in the dark. The absorbance of the solution was read at 415 nm using a spectrophotometer against the blank. TFC was expressed as mg quercetin (QE/g of extract) according to the following equation [22].
where TFC: Total flavonoid content (mg QE/g extract).
C: Concentration of quercetin established from the calibration curve (mg/mL).
V: Volume of extract solution (mL).
M: Mass of the extract (g).
2.5 The high cholesterol and fat diet (HCFD)
The HCFD is composed of a standard rat chow (ND) to which we added 2% pure cholesterol powder, 1% cholic acid, 20% fat (animal source) and 2% corn oil [23]. The components were added gradually to the normal diet (ND), fully mixed until homogenous, formed into a dough with the addition of 1L distilled water, rolled and cut into small pellets which was allowed to dry for 2–3 days at room temperature [24]. The HCFD was prepared weekly and was stored at −20 °C until use to protect against oxidation [25]. Two types of rat diets were analyzed in the Feed Laboratory at the School of Agriculture, The University of Jordan (Table 1).
2.6 Animals
Adult male Sprague Dawley (SD) rats (n = 64) weighing 150–200 gm were used for the experiments. Rats were kept for one week to adapt to the laboratory environment, each four rats were housed in one cage. Rats were then divided randomly into six groups:
-
Group 1 (G1), a normal control group, included 10 rats that were fed a ND and treated with 0.9% NaCl solution.
-
Group 2 (G2), a negative control group, included 10 rats that served as a hypercholesterolemic control and were fed HCFD and treated with 0.9% NaCl solution.
-
Group 3 (G3) served as positive control and included 11 rats that were fed HCFD and treated with the reference hypolipidemic drug atorvastatin (20 mg/kg b.w.).
-
Group 4 (G4) included 11 rats that were fed HCFD and treated with 10 mg/kg b.w. of BMME.
-
Group 5 (G5) included11 rats that were fed HCFD and treated with 30 mg/kg b.w. of BMME.
-
Group 6 (G6) included 11 rats that were fed HCFD and treated with 100 mg/kg b.w. of BMME.
All rats received the proper dose (dissolved in 0.9% NaCl solution) daily in a volume of 1 mL orally during the last four weeks of the experiment according to the protocol shown in Supplementary Fig. 3. The choice of doses was determined based on a preliminary experiments in our laboratory and were chosen to distribute evenly on a logathimic scale.
2.7 Blood sample collection
Animals were fasted overnight. Blood was drawn from the retro-orbital plexus and collected in plain capillary tubes at 0, 2, 3, 4, 5 and 6 weeks. Blood was allowed to clot for 1 h. at room temperature, then centrifuged at a speed of 3000 rpm for 10 min (Heraeus Labofuge, model I HC122, Germany). Serum was separated and stored in 1 mL Eppendorf tubes (Citotest Labware Manufacturing Co., Ltd, China) at −20 °C for measurement of biochemical parameters [26].
2.8 Biochemical analyses
2.8.1 Estimation of lipid profile
Serum total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) levels were measured enzymatically using available assay kits. In brief, enzymatic colorimetric determination of TC was performed following the routine reactions of cholesterol esterase, cholesterol oxidase, and peroxidase according to the manufacturer’s instructions (Agappe, Switzerland). The red quinone product was measured by a spectrophotometer (UV 190–1000 nm-scanning, Biotech Engineering Management Co. Ltd, UK) at a wavelength of 505 nm. Similarly, the colored product from TG reactions was measured at 546 nm, and that of HDL was measured at 505 nm after precipitation of chylomicrons, VLDL, and LDL-C with phototungstic acid and magnesium ions followed by centrifugation. Low-density lipoprotein cholesterol (LDL-C) was calculated using the equations LDL = TC- (HDL + VLDL), whereas VLDL was calculated as TG/5 [27].
2.8.2 Liver functions test
Activities of alanine transaminase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were measured enzymatically in the serum sample using assay kits according to their manufacturers’ instructions (Agappe, Switzerland). The colored products resulting from these routine reactions were measured at wavelengths of 340 nm for ALT and AST, and at 405 nm for ALP.
2.8.3 Serum glucose measurement
Glucose level in serum was determined using glucose assay kit following the routine glucose oxidase and peroxidase reactions according to the manufacturer’s instructions (Human Diagnostic Worldwide, Germany). The colored prduct was measured spectrophotometrically (UV 190–1000 nm-scanning, Biotech Engineering Management Co. Ltd, UK) at a wavelength of 500 nm.
2.8.4 Body weight
Rats were weighed before the experiment once a week till the end of the experiment.
2.8.5 Ethical approval
Guidelines for the care and use of animals at The University of Jordan were followed. All experimental protocols were approved by the Graduate Studies and Research Committee of the School of Science at the University of Jordan, Amman, Jordan. An Institutional Review Board (IRB) approval (#113/2021, dated 23/11/2021) was obtained.
2.9 Statistical analysis
Data are presented as means ± SEM. Statistical analysis was performed using GraphPad Prism software version 9.1.2 and differences were screened by one-way ANOVA followed by Fisher’s LSD test. Differences were considered significant when P < 0.05.
3 Results
3.1 Plant extract yield
The yield of methanolic extract of B. muricata dry weights was calculated [28] as:
BMME gave a yield of 13.6% and was of tarry color.
3.2 Total phenolic and total flavonoid content
TPC was 199.2 ± 2.0 mg GAE/g extract and TFC was 159.6 ± 1.6 mg QE/g extract.
3.3 Effect of BMME on body weight
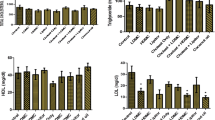
Figure 1A shows changes in body weight of rat groups over a period of 6 weeks. All animal groups started with approximately similar body weight. Groups 2–6 that were fed HFCD, however, did not gain weight at the same rate as rats fed the normal diet (p < 0.001). HCFD consumption for six weeks decreased the gain (p < 0.001) in body weight begining with week 1 as compared to group 1 that was fed the normal diet. Groups 4–6 that received different doses of BMME regained weight only by the sixth week. The increase in weight in these latter groups was significant when compared to that in group 2. Although group 3 that received atorvastatin gained some weight but their weight was still significantly lower than that of the normal control group (group 1) and it was not different from that of group 2.
Effect of BMME on body weight (A), and serum lipid profile (B–F). Group 1 (G1), rats were fed ND and treated with normal saline; Group 2 (G2), negative control, rats were fed HCFD and treated with normal saline; Group 3 (G3), positive control, rats were fed HCFD and treated with 20 mg/kg b.w. of atorvastatin; Group 4 (G4), rats were fed HCFD and treated with 10 mg/kg b.w. of BMME; Group 5 (G5), rats were fed HCFD and treated with 30 mg/kg b.w. of BMME; Group 6 (G6), rats were fed HCFD and treated with 100 mg/kg b.w. of BMME. Data for the first week were not collected. Data are expressed as means ± S.E.M. and are analyzed by one-way ANOVA followed by Fisher’s LSD test. *Significant when compared to the normal control group; ϯ significant when compared to the negative control group (G2)
3.4 Effect of BMME on hypercholesterolemia rats
Figure 1B–F shows the effect of three doses of BMME (10, 30 and 100 mg/kg) on serum lipid components of hypercholesterolemic rats. TC and LDL were of similar values (p > 0.05) in all experimental animals at the start of the experiment (week 0). After two weeks, groups 2–6 that received an HCFD showed significantly (p < 0.001) higher values of TC and LDL compared to group 1 that was fed the normal diet. TC and LDL exhibited significant decrease by the fifth week (p < 0.001, p < 0.0001, respectively) in the animal groups that received BMME (groups 4–6), and in group 3 that received atorvastatin, by the fourth week (Fig. 1B, C) when compared to group 2. In Fig. 1D, E, and F, the levels of HDL, TG and VLDL were statistically similar (p > 0.05) in all experimental groups at the start of the experiment. Two weeks of HCFD consumption, the levels of HDL, TG and VLDL decreased significantly in groups (2–6) (p < 0.001, p < 0.0001, p < 0.0001, respectively) begining with week 2, compared to G1. In week 5, HDL increased gradually in the animal groups that received BMME (groups 4–6), and the increase was significant in week 6. By the sixth week (the fourth week of BMME administration), TG and VLDL values started to increase significantly (p < 0.04) in group 6 that received 100 mg/kg of BMME.
3.5 Effect of BMME on serum glucose
Figure 2 shows serum glucose levels in animal groups over the six-week period of experiment. On day zero, serum glucose values were within the normal range for all groups and no significant differences were detected. HCFD consumption for six weeks caused serum glucose level to increase gradually beginning with week 3 compared to the group fed the normal diet (G1). By the sixth week, glucose began to fall significantly in the animal groups that received the smaller doses of BMME (groups 4 and 5), as well as in group 3 that received atorvastatin. Interestingly, glucose level increased significantly (p < 0.0001) in group 6 that received 100 mg/kg of BMME during the sixth week.
Effect of BMME on serum glucose. Group 1 (G1), rats were fed ND and treated with normal saline; Group 2 (G2), negative control, rats were fed HCFD and treated with normal saline; Group 3 (G3), positive control, rats were fed HCFD and treated with 20 mg/kg b.w. of atorvastatin; Group 4 (G4), rats were fed HCFD and treated with 10 mg/kg b.w. of BMME; Group 5 (G5), rats were fed HCFD and treated with 30 mg/kg b.w. of BMME; Group 6 (G6), rats were fed HCFD and treated with 100 mg/kg b.w. of BMME. Data for the first week were not collected. Data are expressed as means ± S.E.M. and are analyzed by one-way ANOVA followed by Fisher’s LSD test. Data are expressed as means ± S.E.M. and are analyzed by one-way ANOVA followed by Fisher’s LSD test. *Significant when compared to the normal control group; ϯ significant when compared to the negative control group
3.6 Effect of BMME on the activity of hepatic marker enzymes
Figure 3 shows the activity of hepatic marker enzymes in week 6 of the experiment. HCFD consumption for six weeks significantly increased (p < 0.0001) ALT and ALP activity in rat groups fed a HFCD compared to the normal diet group (G1). For AST, a significant increase was observed in G2, G4 and G6 whereas G3 and G5 showed an increase although it did not reach the statistical level (P values for the 2 groups were 0.1613 and 0.0794, respectively). BMME did not induce any significant decrease in the levels of ALT, AST, or ALP levels. In fact, the highest concentration of BMME caused a significant increase in ALT (Fig. 3A).
Effect of BMME on hepatic marker enzymes by the end of the sixth week of the experiment. A serum alanine transamines (U/L), B serum aspartate aminotransferase (U/L), C serum alkaline phosphatase (U/L). Group 1 (G1), rats were fed ND and treated with normal saline; Group 2 (G2), negative control, rats were fed HCFD and treated with normal saline; Group 3 (G3), positive control, rats were fed HCFD and treated with 20 mg/kg b.w. of atorvastatin; Group 4 (G4), rats were fed HCFD and treated with 10 mg/kg b.w. of BMME; Group 5 (G5), rats were fed HCFD and treated with 30 mg/kg b.w. of BMME; Group 6 (G6), rats were fed HCFD and treated with 100 mg/kg b.w. of BMME. Data are expressed as means ± S.E.M. and are analyzed by one-way ANOVA followed by Fisher’s LSD test. *Significant when compared to the normal control group (G1); ϯ significant when compared to the negative control group (G2)
4 Discussion
HCFD-fed hyperlipidemic rat model was reported as a reasonably good model for testing antihyperlipidemic drugs in vivo [23, 29]. Inducing hyperlipidemia in rats requires HCFD because rats are generally hypo-responsive to dietary cholesterol [30]. The diet must also contain cholic acid to induce hypercholesterolemia without which HCFD would not be effective in increasing the total cholesterol in rats [30]. Cholic acid enhances cholesterol absorption and reduces cholesterol excretion because of its emulsifying property. It also exerts an inhibitory action on hepatic cholesterol 7-α hydroxylase activity, an important enzyme for the regulation of cholesterol and synthesis of bile acid [31].
Generally, HCFD is a low carbohydrate and a high fat diet and is known as ketogenic diet [32]. In the present work, a remarkable decrease in the rate of body weight gain was observed in hypercholesterolemic rats relative to the normal control rats that consumed normal diet. This could be attributed to the low carbohydrate and the high fat in HCFD and to the energy expenditure increase which resulted in weight loss [32]. Alternatively, it could be due to anorexia which may lead to reduction in food intake [33]. The present data show that BMME administration (10, 30 and 100 mg/kg b.w.) to rats fed HCFD could significantly restore body weight gain pattern (15.9%, 12.7% and 11.4%, respectively) to near normal during the treatment period of 4 weeks. This increase in body weight gain may be associated with enhancement of lipid profile and antioxidant system.
In the current study, HCFD caused increased TC and LDL with accompanying decrease in TG and HDL-C, demonstrating that the model of hypercholesterolemia in rats was established successfully. Previous studies in our laboratory using the same feeding protocol showed an increase in both TC and LDL-C and a drop in both HDL-C and TG [34]. Our results demonstrate that BMME given orally to hypercholesterolemic rats daily for 4 weeks affected significantly lipid profile of these animals. All doses of BMME (10, 30, and 100 mg/kg) significantly decreased serum TC (58.0%, 51.8% and 51.3%, respectively) and LDL-C (69.2%, 54.5% and 62.8%, respectively). This decrease was of similar magnitude to that induced by the reference hypocholesterolemic drug atorvastatin (62.7% for TC and 78.5% for LDL-C). So, the present experiments confirmed the hypocholesterolemic activity of BMME. On the other hand, all doses of BMME (10, 30 and 100 mg/kg) significantly increased HDL level (47.7%, 41.8% and 45.9%, respectively). In epidemiological studies, HDL-C levels have an inverse relationship to the risk of atherosclerotic cardiovascular disease. It has been assumed that this reflects the protective function of HDL, which includes its ability to promote cholesterol efflux [35].
The total flavonoids content of B. muricata methanolic extract was 159.6 ± 1.6 mg QE/g extract. This was confirmed by the data from aluminum chloride colorimetric assay. This assay demonstrated that BMME is rich in flavonoids. Comparing total flavonoids content of B. muricata analysed in this study with B. muricata from other countries shows that B. muricata grown in Jordan is richer with flavonoids. For example, according to the study of Mohammedi, et al. [36] of B. muricata from Algeria, total flavonoids content was found to be 68.85 ± 1.5 mg quercetin equivalents (QE/g) [36]. The difference in flavonoid content may be due to the geographical location and the climate of the plant habitat, as there are many plants in Jordan that are rich in flavonoids such as Rosmarinus officinalis (481.99 ± 10.30 mg QE/100 g), Artemisia herba-alba (402.52 ± 1.13 mg QE/100 g), Olea europaea (344.68 ± 3.91 mg QE/100 g), Teucrium polium (205.95 ± 1.81 mg QE/100 g), Cleome arabica (173.50 ± 2.00) mg QE/g), Hypericum triquetrifolium (171 mg rutin/g) [37,38,39].
In animal models, using rats, mice and rabbits, many experimental studies demonstrated that flavonoids reduced TC and increased HDL [40]. These studies and others demonstrated that flavonoids prevented the occurrence and development of cardiovascular diseases through multiple mechanisms and different pathways, such as inhibition of oxidative stress, platelet aggregation, reduction of inflammation and improvement of lipid metabolism disorders [41]. The flavonoids’ anti-hyperlipidemic activity may be attributed to their action in lowering lipogenesis and increasing degradation of cholesterol [42]. Previous studies reported that flavonoids, like quercetin, reduced lipid activities in animals. They reduced the expression of SREBP-1 transcription factor as well as of its lipogenic target genes and inhibited acetyl-CoA carboxylase activity, which catalyses the first committed step in fatty acid biosynthesis [43, 44]. Quercetin also inhibited and prevented absorption of cholesterol from the intestine and/or its liver synthesis and improved cholesterol excretion into faeces [45].
Our observations showed that TG level in groups fed HCFD for 6 weeks was lower than that of the group fed the normal diet. This could be explained as due to the fact that the normal diet contains a higher amount of carbohydrate (33% more) compared to HCFD (Table 1). The currently available literature shows that low-carbohydrate diets acutely reduce circulating triglyceride levels [46]. Moreover, the low- carbohydrate diets were reported to be associated with significantly reduced triglyceride levels, body mass index and body weight [47]. Consistently, previous studies showed that animals fed a high carbohydrate diet exhibited hypertriglyceridemia [48], whereas TG level significantly decreased in animals that consumed low carbohydrate diet [46]. As VLDL-C is calculated as one-fifth of triglyceride level, the low levels of triglycerides are associated with decreased VLDL [49]. However, TG were not affected by 10 and 30 mg/kg of BMME in the present experiments whereas 100 mg/kg of BMME reversed the effect of HCFD and increased TG levels in serum by 28.5%.
Atorvastatin is a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, inhibiting cholesterol synthesis and reducing serum cholesterol levels [50]. In the present work, a dose of 20 mg/kg of atorvastatin caused 62.7% decrease in serum TC in HCFD-fed rats. Likewise, atorvastatin reduced serum LDL-C (78.5%) when compared to the negative control group (G2). Atorvastatin also increased HDL-C in serum (67.8%) in the present experiments in consistence with other studies that found beneficial effect for atorvastatin in increasing HDL-C and a reduction of cardiovascular risk [51].
In the current study, HCFD increased serum glucose level in rats. Such a ketogenic diet induced hepatic insulin resistance, and type 2 diabetes [52]. Kosinski and Jornayvaz [53], reported that a ketogenic diet impaired insulin ability to suppress endogenous glucose production, confirming insulin resistance in the liver. Such diet was found to cause severe hepatic insulin resistance, and this was explained as due to an increased hepatic diacylglycerol content. Finally, insulin resistance was also attributed to a decreased insulin-stimulated whole-body glucose disposal [32]. The current study showed that intragastric administration of 10 mg/kg and 30 mg/kg of BMME caused significant decline in the levels of blood glucose by the sixth week. This result may be due to the action of plant’s saponins and flavonoids that resulted in reduced glucose in rats in consistence with the findings of others [54]. Meliani et al. [55] reported a hypoglycaemic effect of Berberis vulgaris in normal group of rats and in streptozotocin-induced diabetic rats and attributed that to insulin release stimulation via saponins which have multiple sites of actions on beta cells of pancreas.
The highest dose (100 mg/kg) of BMME used in G6 had untoward effect on blood glucose since it caused significant increase in blood glucose (28.8%) compared to group 2 (G2). The reason for this effect remains to be explained but the concrete effects may depend on factors like flavonoid types, concentration in the extract, and the administered dose. In previous studies, a high dose such as 100 μmol/L of genistein was found to inhibit insulin secretion in rats [56], causing apoptosis in pancreatic islets and in beta-cell line, [\*MERGEFORMAT 0] and leading to increased blood glucose. Several flavonoids such as procyanidins, genistein, gallic acid and quercetin modulated cell proliferation [57]. BMME is rich in flavonoids which we found to equal 159.6 ± 1.6 mg QE/g extract. Accordingly, the high content of quercetin in the dose of 100 mg/kg may have an inhibitory effect on beta cells in the pancreas, resulting in increased level of glucose in rats.
The amount of literature on plants that have insulin release inhibitory effect and/or hyperglycaemic effects is relatively small [58]. Nevertheless, it is possible to cite some reports that reported a hyperglycaemic effect for some medicinal plants. The majority of these reports attributed the hyperglycaemic effects as due to inhibition of insulin release [58]. For example, previous studies indicated that Artocarpus communis root bark aqueous extract induced hyperglycaemia in animals and caused significant molecular and biochemical changes in the endocrine pancreas [59]. On the other hand, the volatile oil of Rosmarinus officinalis had hyperglycaemic and calcium antagonistic effects and it inhibited insulin release [60].
A ketogenic diet caused hepatic inflammation, hepatic steatosis, lipid accumulation and oxidative stress in rats liver [61]. Biological markers for fatty liver disease such as AST, ALT and ALP were increased by such a diet [62]. This is consistent with the finding of the present experiment where the ketogenic diet caused increased liver enzymes. In the present study, BMME did not decrease the high levels of liver enzymes, suggesting that BMME did not offer hepatoprotection in the current setting. To the contrary, 100 mg/kg of BMME actually increased serum ALT effectively but there was no effect on AST and ALP. The increase in serum ALT levels, a more sensitive marker than other liver enzymes for liver damage and hepatic insulin sensitivity, was associated with consequent development of type 2 diabetes [63].
In general, this study shows that the hypolipidemic effect produced by BMME was not dose-dependent since a dose of 10 mg/kg was more effective than the dose of 100 mg/kg of BMME (e.g., in glucose, TG and VLDL parameters). A possible reason may be attributed to the toxicity associated with the higher dose of BMME. This dose-independent activity of BMME could be attributed to the “hormesis” dose–response phenomenon, a term introduced by Goldman [64], meaning “the beneficial effect of a low-level exposure to an agent that is harmful at high levels”. In conclusion, BMME had significant hypocholesterolemic effect and the highest dose (100 mg/kg) had negative effect on ALT liver enzyme, TG level and glucose level in rats.
References
Flowers MT, Groen AK, Oler AT, Keller MP, Choi Y, Schueler KL, Attie AD (2006) Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diets. J Lipid Res 47(12):2668–2680. https://doi.org/10.1194/jlr.M600203-JLR200
Leuschner M (2022) Treatment of hypercholesterolaemia to improve cardiovascular disease risk in South Africa. South Afr Gen Pract 3(1):10–14. https://doi.org/10.36303/SAGP.2022.3.1.0116
Kabiri N, Asgary S, Setork M (2011) Lipid lowering by hydroalcoholic extracts of AmaranthusCaudatus L. induces regression of rabbit’s atherosclerotic lesions. Lipids Health Dis 10(1):1–8. https://doi.org/10.1186/1476-511X-10-8
Beshir SA, Hussain N, Elnor AA, Said AS (2021) Umbrella review on non-statin lipid-lowering therapy. J Cardiovasc Pharmacol 26(5):437–452. https://doi.org/10.1177/10742484211002943
Chan MLY, Shiu SWM, Cheung CL, Leung AYH, Tan KCB (2022) Effects of statins on the inducible degrader of low-density lipoprotein receptor in familial hypercholesterolemia. Endocr Connect. https://doi.org/10.1530/EC-22-0019
Moosmann B, Behl C (2004) Selenoprotein synthesis and side-effects of statins. Lancet 363(9412):892–894. https://doi.org/10.1016/S0140-6736(04)15739-5
Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH (2007) Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis and cancer: Insights from large randomized statin trials. J Am Coll Cardiol 50(5):409–418. https://doi.org/10.1016/j.jacc.2007.02.073
Strom BL, Schinnar R, Karlawish J, Hennessy S, Teal V, Bilker WB (2015) Statin therapy and risk of acute memory impairment. JAMA Intern Med 175(8):1399–1405. https://doi.org/10.1001/jamaniternmed.2015.2092
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Evans S (2016) Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388(10059):2532–2561. https://doi.org/10.1016/S0140-6736(16)31357-5
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Rollinger JM (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33(8):1582–1614. https://doi.org/10.1016/j.biotechadv.2015.08.001
Alsarayreh AZA, Attalah Oran S, Shakhanbeh JM (2022) In vitro and in vivo wound healing activities of Globularia arabica leaf methanolic extract in diabetic rats. J Cosmet. https://doi.org/10.1111/jocd.14882
EL-khatiba AS, Khaleel AE, (1995) Evaluation of some pharmacological properties of different extract of Bauhinia racemosa leaf and Bassia muricata whole plant. Bull Fac Pharm 33:59–65
Al-Yahya MA, Al-Meshal IA, Mosa JS, Al-Badr A, Tariq M (1990) Saudi plants: a phytochemical and biological approach King Abdulaziz City for science and technology. King Saud University Press, King Abdulaziz City
Kamel MS, Mohamed KM, Hassanean HA, Ohtani K, Kasai R, Yamasaki K (2001) Acylated flavonoid glycosides from Bassia muricata. Phytochemistry 57(8):1259–1262. https://doi.org/10.1016/S0031-9422(01)00240-0
Chemsa AE, Derdouri S, Labbi Z, Acila S, Amara DG, Chouikh A, Zellagui A (2016) Total phenolic and total flavonoid contents of different solvent extracts of Bassia muricata (L.) Asch. and evaluation of antibacterial and antioxidant activities. J Chem Pharm 8(4):1317–1321
El-Sayed NH, Mogahed MI, Haron AA, Mabry TJ (1998) Flavonoids and other constituents from Bassia muricata and their insecticidal activities. Rev Latinoam Quím 26(3):81–85
Abozeed AE (2014) Comparative study on the active chemical constituents of some chenopodiaceae plants. Bull Fac Agric 65:68–77
Abd-El Gawad A, El Gendy AEN, El-Amier Y, Gaara A, Omer E, Al-Rowaily S, El shamy A, (2020) Essential oil of Bassia muricata: chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J Biol Sci 27(7):1900–1906. https://doi.org/10.1016/j.sjbs.2020.04.018
Karak P (2019) Biological activities of flavonoids: an overview. Int J Pharm Sci Rev Res 10(4):1567–1574. https://doi.org/10.13040/IJPSR.0975-8232.10(4).1567-74
Islam MN, Kabir MSH, Kader SMA, Hasan M, Samrat EK, Habib IB, Rahman MM (2016) Total phenol, total flavonoid content and antioxidant potential of methanol extract of Boehmeria platyphylla D Don leaves. World J Pharm Res 5(5):334–344
Shewale S, Rathod VK (2018) Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep Biochem Biotechnol 48(4):312–320. https://doi.org/10.1080/10826068.2018.1431784
Tristantini D, Amalia R (2019) Quercetin concentration and total flavonoid content of anti-atherosclerotic herbs using aluminum chloride colorimetric assay. Am Inst Phys Conf Proc 2193(1):030012. https://doi.org/10.1063/1.5139349
Sampathkumar M, Kasetti B, Nabi S, Sudarshan P, Swapna S, Apparao C (2011) Antihyperlipidemic and antiatherogenic activities of Terminalia pallida Linn fruits in high fat diet-induced hyperlipidemic rats. J Pharm Bioallied Sci 3(3):449–452
Nishina PM, Verstuyft J, Paigen B (1990) Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res 31:859–869. https://doi.org/10.1016/S0022-2275(20)42785-3
Harb AA, Bustanji YK, Abdalla SS (2018) Hypocholesterolemic effect of β-caryophyllene in rats fed cholesterol and fat enriched diet. J Clin Biochem Nutr 62(3):230–237. https://doi.org/10.3164/jcbn.17-3
Shad MA, Iqbal T, Shah MH, Mahmood RK, Tayyab M (2003) Vegetable oils and dyslipidemia. Pak J Med Sci 19:45–51
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502. https://doi.org/10.1093/clinchem/18.6.499
D’Auria M, Mecca M, Bruno MR, Todaro L (2021) Extraction methods and their influence on yield when extracting thermo-vacuum-modified chestnut wood. Forest 12(1):73. https://doi.org/10.3390/f12010073
Pande VV, Dubey S (2009) Antihyperlipidemic activity of Sphaeranthus indicus on atherogenic diet induced hyperlipidemia in rats. Int J Green Pharm 3(2):159–161
Moghadasian MH (2002) Experimental atherosclerosis: a historical overview. Life Sci 70(8):855–865. https://doi.org/10.1016/S0024-3205(01)01479-5
Chinellato A, Ragazzi E, Petrelli L, Paro M, Mironov A, Aliev G (1994) Effect of cholesterol-supplemented diet in heritable hyperlipidemic Yoshida rats: functional and morphological characterization of thoracic aorta. Atherosclerosis 106(1):51–63. https://doi.org/10.1016/0021-9150(94)90082-5
Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Shulman GI (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol 299(5):808–815. https://doi.org/10.1152/ajpendo.00361.2010
Ji G, Zhao X, Leng L, Liu P, Jiang Z (2011) Comparison of dietary control and atorvastatin on high fat diet induced hepatic steatosis and hyperlipidemia in rats. Lipids Health Dis 10(1):1–10. https://doi.org/10.1186/1476-511X-10-23
Harb AA, Bustanji YK, Almasri IM, Abdalla SS (2019) Eugenol reduces LDL cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating TRPV1 receptor. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-50352-4
Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA (2014) High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res 114(1):205–213. https://doi.org/10.1161/circresaha.114.300760
Mohammedi H, Idjeri-Mecherara S, Menaceur F, Hassani A (2019) The effect of solvents and extraction procedure on the recovery of phenolic compounds and the antioxidant capacity of Algerian Bassia muricata L. extracts. Chem J Mold 14(2):79–89. https://doi.org/10.19261/cjm.2019.637
Bilto YY, Alabdallat NG, Salim M (2015) Antioxidant properties of twelve selected medicinal plants commonly used in Jordan. Br J Pharm Res 6(2):121–130. https://doi.org/10.9734/BJPR/2015/16136
Al-Kharabsheh S, Al-Dabbas M, Ghazzawi H, Zatimeh A, Abulaila K (2017) Antioxidant activity and α-amylase inhibitory effect of selected medicinal plants grown in Jordan: an in-vitro study. J Arab Soc Med Res 12(1):19. https://doi.org/10.4103/jasmr.jasmr_18_16
Alkhatib RQ, Almasarweh AB, Abdo NM, Mayyas AS, Al-Qudah MA, Abu-Orabi ST (2022) Chromatographic analysis (LC-MS and GC-MS), antioxidant activity, antibacterial activity, total phenol, and total flavonoid determination of Cleome arabica L. growing in Jordan. Int J Food Prop 25(1):1920–1933. https://doi.org/10.1080/10942912.2022.2115063
Peluso I, Palmery M, Serafini M (2015) Association of flavonoid-rich foods and statins in the management of hypercholesterolemia: a dangerous or helpful combination? Curr Drug Metab 16(9):833–846. https://doi.org/10.2174/1389200216666151015113828
Deng Y, Tu Y, Lao S, Wu M, Yin H, Wan, L, Liao W (2021) The role and mechanism of citrus flavonoids in cardiovascular diseases prevention and treatment. Crit Rev Food Sci Nut 1–24. https://doi.org/10.1080/10408398.2021.1915745
Koshy AS, Anila L, Vijayalakshmi NR (2001) Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem 72(3):289–294. https://doi.org/10.1016/S0308-8146(00)00225-9
Baghdadi HH (2014) Antioxidant potential of quercetin: remarkable protection against hypercholesterolemia in rats. J Adv Med Res 4(25):4382–4391. https://doi.org/10.9734/BJMMR/2014/11126
Gnoni A, Di Chiara SB, Giannotti L, Gnoni GV, Siculella L, Damiano F (2022) Quercetin reduces lipid accumulation in a cell model of NAFLD by inhibiting de novo fatty acid synthesis through the acetyl-CoA carboxylase 1/AMPK/PP2A axis. Int J Mol Sci 23(3):1044. https://doi.org/10.3390/ijms23031044
Dabeek WM, Marra MV (2019) Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutr 11(10):2288. https://doi.org/10.3390/nu11102288
Adam-Perrot A, Clifton P, Brouns F (2006) Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 7(1):49–58. https://doi.org/10.1111/j.1467-789X.2006.00222.x
Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Worm N (2015) Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31(1):1–13. https://doi.org/10.1016/j.nut.2014.06.011
Parks EJ (2001) Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr 131(10):2772–2774. https://doi.org/10.1093/jn/131.10.2772S
Berry EM, Hirsch J, Most J, McNamara DJ, Thornton J (1986) The relationship of dietary fat to plasma lipid levels as studied by factor analysis of adipose tissue fatty acid composition in a free-living population of middle-aged American men. Am J Clin Nutr 44(2):220–231. https://doi.org/10.1093/ajcn/44.2.220
Lea AP, McTavish D (1997) Atorvastatin. Drugs 53(5):828–847. https://doi.org/10.2165/00003495-199753050-00011
Asztalos BF, Horvath KV, Mc-Namara JR, Roheim PS, Rubinstein JJ, Schaefer EJ (2002) Effects of atorvastatin on the HDL subpopulation profile of coronary heart disease patients. J Lipid Res 43(10):1701–1707. https://doi.org/10.1194/jlr.M200037-JLR200
Van Stee MF, de Graaf AA, Groen AK (2018) Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc Diabetol 17(1):1–22. https://doi.org/10.1186/s12933-018-0738-4
Kosinski C, Jornayvaz FR (2017) Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients 9(5):1–16. https://doi.org/10.3390/nu9050517
Vessal M, Hemmati M, Vasei M (2003) Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol Part C Toxicol Appl Pharmacol 135(3):357–364. https://doi.org/10.1016/S1532-0456(03)00140-6
Meliani N, Dib MEA, Allali H, Tabti B (2011) Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 1(6):468–471. https://doi.org/10.1016/S2221-1691(11)60102-0
Pinent M, Castell A, Baiges I, Montagut G, Arola L, Ardévol A (2008) Bioactivity of flavonoids on insulin-secreting cells. Compr Rev Food Sci Food Saf 7(4):299–308. https://doi.org/10.1111/j.1541-4337.2008.00048.x
Elliott J, Scarpello J, Morgan N (2002) Differential effects of genistein on apoptosis induced by fluoride and pertussis toxin in human and rat pancreatic islets and RINm5F cells. J Endocrinol 172(1):137–143
Al-Hader AA, Hasan ZA, Aqel MB (1994) Hyperglycaemic and insulin release inhibitory effects of Rosmarinus officinalis. J Ethnopharmacol 43(3):217–221. https://doi.org/10.1016/0378-8741(94)90046-9
Adewole SO, Ojewole JA (2007) Hyperglycaemic effect of Artocarpus communisforst (moraceae) root bark aqueous extract in Wistar rats: cardiovascular topic. Cardiovasc J Afr 18(4):221–227
Aqel MB (1991) Relaxant effect of the volatile oil of Romarinus officinalis on tracheal smooth muscle. J Ethnopharmacol 33(1–2):57–62. https://doi.org/10.1016/0378-8741(91)90161-6
Otunola GA, Oloyede OB, Oladiji AT, Afolayan AA (2010) Effects of diet-induced hypercholesterolemia on the lipid profile and some enzyme activities in female Wistar rats. Afr J Biochem Res 4(6):149–154. https://doi.org/10.5897/AJBR.9000099
Anekwe CV, Chandrasekaran P, Stanford FC (2020) Ketogenic diet-induced elevated cholesterol, elevated liver enzymes and potential non-alcoholic fatty liver disease. Cureus 12(1):1–10. https://doi.org/10.7759/cureus.6605
Mandal A, Bhattarai B, Kafle P, Khalid M, Jonnadula SK, Lamicchane J, Gayam V (2018) Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus 10(11):1–9. https://doi.org/10.7759/cureus.3626
Goldman M (1996) Cancer risk of low-level exposure. Science 271(5257):1821–1822. https://doi.org/10.1126/science.271.5257.1821
Funding
The funding was provided by The University of Jordan, (grant # 2001/23369), Amal Al-Obeidyeen
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Obeidyeen, A., Zarga, M. & Abdalla, S. The hypocholesterolemic effect of methanolic extract of Bassia muricata l. on hypercholesterolemic rats. SN Appl. Sci. 5, 101 (2023). https://doi.org/10.1007/s42452-023-05320-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05320-z