Abstract
The increment in the usage of automobiles is resulting in increased greenhouse gases (GHG) emissions continuously and there is a substantial need to reduce them effectively. The present research work investigates the emission behavior of waste cooking oil biodiesel doped with CuO nanoparticles during testing in Compression Ignition (CI) engines. This investigation is based on the effective emission reduction analysis emitted by diesel fuel during experimentation on CI engines. It suggests a cost effective modification of biodiesel as a fuel prepared from waste cooking oil (WCO) by a novel hydrodynamic cavitation technique which includes the hydrodynamic cavitation reaction mixture composed of 1.28 L of methanol and 10 g KOH and 5 L of preheated WCO at 45 °C in the cavitation reactor for 40 min. These reactants are synthesized utilizing the principle of cavitation and the final manufactured esterified oil is authenticated with ASTM Standard property measurement for suitability check. In the research work, two different investigations are carried out. In the first one, WCO biodiesel-diesel blends of 0, 30, and 100% (B0, B30, B100) ratio are prepared and the emission characteristics have investigated at 1500 rpm constant speed with varying load and indicated mean effective pressure (IMEP). In the second investigation, the emission suitable blend B30 is doped with CuO nanoparticles, kee** other parameters as per the previous setup, the emission characteristics investigated for the second one. For precise results, more experimental trials are needed to achieve this decrease in the emission of harmful gases. Using an amalgamation of L9 Taguchi and response surface methodology (RSM) the maximum emission control with a minimum number of experimental trials is achieved. The first investigation includes the predefined predictors as A (blend), B (load), and C (IMEP), where blends (0 ≤ A ≤ 100%), load (0 ≤ B ≤ 12 kg), IMEP (3.5 ≤ C ≤ 7.5 bar) are controllable features. Optimization process resulted into a minimum emission of CO, CO2, and NOx by appertaining the condemnatory merger of inputs such as blend B0 (Diesel), load 12 kg, and IMEP 3.48 bar in the first investigation, which has resulted into 0.08 ppm CO, 0.6 ppm CO2 and 30 ppm NOx emission. Taguchi analysis-based second experimental investigation includes the predefined predictors as A (CuO), B (load), and C (IMEP), including nanoparticles CuO in blend B30, and the prognosticated results of optimization are 0.03 ppm CO, 0.3 ppm CO2 and 21 ppm NOx emission. In current investigation, the percentage reduction is found to be 92.3%, 94.82%, and 96% compared to the emission of diesel in CO, CO2 and NOx gases, respectively. The coefficient of determination is almost equal to 1, which reveals the chosen optimization technique is very accurate in prediction. The investigation has provided suitable minimum emission characteristics in a cost-effective way.
Article highlights
-
The present investigation explores the harmful gases emission reduction analysis of biodiesel by varying the load, IMEP and proportion of CuO nanoparticles on CI engine testing.
-
Modification of fuel with nanotechnology can be a cost-effective option instead of costlier engine modification.
-
Lesser emission is obtained with WCO biodiesel blend B30 by adding nanoparticles CuO in it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the present scenario, both developed and develo** countries are having substantial energy demand. Functioning of both industrial as well as automobile sectors is dominated by fossil fuels [1]. However, improper combustion of fossil fuels leads to considerable environmental pollution [2], affecting the health of people in the developed nations adversely [3]. Available emission curtailing techniques such as pretreatment of fuel sources, additives, and modification of engines are costly. It is the reason behind the increasing significance of research work based on alternative fuels. The key benefits of biodiesel are efficiency improvement, sustainability, and reduction in harmful gases. On top of that, the limited reservoirs of fossil fuels will not be adequate to meet world-wide energy consumption, while biodiesel can be a great substitute [4]. It is more environment friendly, oxygenated, sulphur-free, biodegradable, releasing lower emissions, more prominent among biofuels [5, 6]. Apart from this, there are certain disadvantages of biodiesel usage, such as viscos nature, denser than fossil fuels, higher modulus of elasticity, lower heating value, unequal atomization, and higher level of NOX emission [7, 8], which need to be tackled. Nanoparticles are found to be one of the most effective solutions among other additives improving the overall engine performance. They are enhancing engine performance parameters and reducing emission parameters dramatically [9].
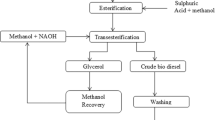
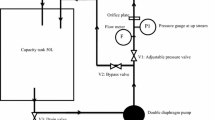
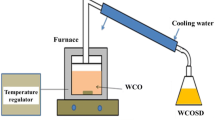
Conventional methods of biodiesel production are having many drawbacks [10]. Some examples are pyrolysis; high temperatures should be maintained. So, the materials used for the experiments are expensive. Moreover, the purity of biodiesel is low [11]. For micro-emulsion and dilution, the product is not volatile and would’t have enough stability to form a perfect product [12]. For transesterification, biodiesel prepared by this method must be washed and neutralized for further use. There is a risk of side reactions, also it is difficult to separate the products obtained from transesterification [13]. It is also time consuming and has an impurity mixture of biodiesel [14]. Some of the drawbacks can be removed by using a costlier catalyst, but it is necessary to find an economical solution for the production of biodiesel. For feedstocks like waste cooking oil, which has a high acid value containing free fatty acids (FFA) and water, a routine transesterification procedure is ineffective. Traditional methods could not work as a sustainable solution. Moreover, the sustainability of biodiesel is largely affected by the methodology of production. In addition, traditional biodiesel production methods need reconsideration and modification for effective usage. To conquer the loopholes of traditional methods, research work is going in this direction extensively. Exploring a new methodology for biodiesel production is becoming necessary to minimize energy losses and increase efficiency. The most important step in the preparation of biodiesel is the mixing of raw materials and catalyst with solvent. In new trends of preparation of biodiesel, various reactors are developed for intensification of chemical reaction making it quicker and cheaper [15]. The study indicates that the increased production capacity can be achieved by shortening the time for separation and purification. To conquer the loopholes of traditional methods, a novel patented device based on the principle of hydrodynamic cavitation, is used for biodiesel production in the current investigations. An acid transesterification reaction almost takes 2–8 h whereas base transesterification reaction using two-step process takes 60–120 min. In addition, the current methodology is very time effective, as using the current methodology, the transesterification reaction time is reduced up to 40 min only.
After preparation, another important aspect for biodiesel preparation is the selection of feedstock [16, 17]. Determination of the cost of biodiesel depends on the selection of feedstock. Compared to consumable and non-consumable raw materials, waste cooking oil (WCO) is a potential substitute and far cheaper. In the current research studies, WCO is used as a feedstock for the preparation of biodiesel.
Nanotechnology has been playing a very crucial role by providing efficient solutions in the form of different nanoparticles such as in the case of cost economic karanja and jatropha oils, lithium impregnated solid catalyst is used for enhancement in properties [18]. By adding carbon coated aluminum nanoparticles, it shows the improving performance of the engine by reducing BSFC and NOx emission [2.6 Correlative exploration of emissions of blends consisting diesel and WCO biodiesel without CuO nanodopes Harmful emissions of CO of WCO biodiesel blend and diesel fuel are recorded using AVL DIGAS (444N) 5 Gas Analyzer. In this experimental study, it was found that the WCO biodiesel blend B30 testing resulted in lesser CO emission compared to diesel with CI engine testing with compression ratio 18 and constant speed of 1500 rpm, varying the load from 0–6 to 12 kg, blend 0–30 to 100. CO emission of B100 blend is found to be 69.23% lower than diesel, whereas CO emission of B30 were 61.54% lower than pure diesel. It is commonly observed that in the case of diesel, the CO2 emissions are maximum, as compared to blends of biodiesel [38, 39]. As diesel fuels have the maximum calorific value as compared to biodiesel blends, so the combustion of diesel fuel gives the maximum generation of carbon dioxide in the engine exhaust. In this experimental study, it was found that the blend B0 (diesel) testing resulted in lesser CO2 emission compared to biodiesel with CI engine testing with compression ratio 18 and constant speed of 1500 rpm, varying the load from 0–6 to 12 kg, blend 0–30 to 100. CO2 emission of B100 is 68.96% and B30 are 51.72% lower than diesel, respectively. During testing WCO biodiesel was added in blending with diesel, the carbon to hydrogen ratio of the mixture was increased. This is the main reason behind the increase in CO2 emissions for the B30 blend of biodiesel. In the case of nitrogen gas is a stable component at normal aerial condition [40]. But the higher temperature generated during the combustion process supports the formation of nitrogen oxide due to the breakage of nitrogen molecules. The NOx is maximum in case of diesel as compared to biodiesel blends due to the higher rate of combustion process. High rate of combustion process of injected fuel particles shows the phenomenon of maximum combustion temperature as compared to biodiesel blend B30. Moreover, the load is having a significant impact on NOx emissions. As the load increases, NOx emission was found to increase in all readings. NOx emissions of B100 are 66.51% and B30 is 48.16% lower than pure diesel, respectively. The average diameter 32 nm ± 12.56 nm is obtained as shown in Fig. 2 a, revealing the scanning electron microscopy (SEM) image of CuO nanoparticles under biological synthesis circumstances. Figure 2b reveals the confirmation of the diameter of CuO nanoparticles under ImageJ software, where x axis is representing the diameter of CuO nanoparticles and y axis is representing the frequency distribution of CuO nanoparticles’ diameter. In biosynthesis Magnolia Kobus leaf extract is utilized as a biological source. It is treated with aqueous solution of CuSO4.5H2O. Finally, CuO nanoparticles are synthesized through SEM. Correlative exploration of emissions of blends consisting diesel and WCO biodiesel with CuO nanodopes. In further testing of emission reduction, the CuO nanoparticles do** is varied as 50–100-150 ppm. From the nanoparticles do** results, it is concluded that 100 ppm is given almost 0% CO emission compared to 50 ppm and even 150 ppm. Out of three different additions of CuO nanoparticles in B30 blend such as blend B30 + CuO (50 ppm), blend B30 + CuO (100 ppm) and blend B30 + CuO (150 ppm), CO emissions of blend B30 + CuO (50 ppm) and blend B30 + CuO (100 ppm) were found to be equally lowest, which is 92.30% lower than diesel. The blend B30 doped with CuO nanoparticles in different ppm resulted in the effective CO emission reduction. Figure 3 which is a graphical representation with load parameter in kg on x axis and CO emission in terms of % vol. on y axis, demonstrate the effect of load and CuO nanoparticles do** on CO emission variation of diesel and biodiesel blends. On the condition of CO2 emission, 50 ppm CuO nanoparticles do** result [41] were giving almost 0% CO2 emission compared to 100 ppm and even 150 ppm do**. Out of three different additions of CuO nanoparticles in B30 blend such as blend B30 + CuO (50 ppm), B30 + CuO (100 ppm), and blend B30 + CuO (150 ppm), CO2 emissions of blend B30 + CuO (50 ppm) and blend B30 + CuO (100 ppm) are found to be equally lowest, which is 94.83% lower than the diesel. Figure 4, which is a graphical representation with load parameter in kg on x axis and CO2 emission in terms of % vol. on y axis, demonstrates the effect of load and CuO nanoparticles do** in different proportions on CO2 emission variations. In the emission case of NOx, blend B30 blend of WCO biodiesel added with CuO nanoparticles resulted in a decrease in NOx as the load on the engine increases compared to WCO biodiesel blend without nanoparticles [42, 43]. From CuO nanoparticles do** results, it was concluded that 50 ppm was given almost 0% NOx emission compared to 100 ppm and even 150 ppm. Out of three different additions of CuO nanoparticles in B30 blend such as blend B30 + CuO (50 ppm), blend B30 + CuO (100 ppm) and blend B30 + CuO (150 ppm), NOx emissions of blend B30 + CuO (50 ppm) was found to be equally lowest, which was 96% lower than pure diesel. Figure 5 which is a graphical representation with load parameter in kg on x axis and NOx emission in terms of % vol. on y axis, demonstrate the effect of load and CuO nanoparticles do** in different proportions on NOx emission variation of diesel and biodiesel blends The response surface methodology (RSM) is utilized as an effective tool using the data obtained in the experimental investigation as shown in Table 3A and B to fit the polynomial equation generated by using Minitab 2019 [44]. Figure 6 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of the blend (B0, B30, and B100, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg), and IMEP (3.5–7.5 bar with variation of 2 bar) on x axis and means of CO emission variation on y axis. As per the results, diesel (B0) is having the highest emission of CO, it reduces exponentially in blend B30 but after that it has started increasing again when the biodiesel percentage in diesel has increased. Similarly, in 0 kg and 12 kg load CO emission is very high, whereas at half load (6 kg) CO emission is lowest. Figure 7 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of CuO do** (50 -150 ppm with variation of 50 ppm, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg, where low level is represented by 1, medium by 2 and high by 3), and IMEP (3.5–7.5 bar with variation of 2 bar, where low level is represented by 1, medium by 2 and high by 3) on x axis and means of CO emission variation on y axis. For CuO do** (Fig. 7) between 50 and 100 ppm, CO emission is very less, almost constant, whereas it is going on increasing exponentially with the increment of the nanoparticles. Variations of load are showing almost similar variation in both cases (Figs. 6 and 7). However, CO emission is much lower in CuO nanoparticles case compared to blends. In the case of IMEP, almost inverse graphs are obtained in the case of blends and nanoparticles. Figure 8 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of blends (B0, B30, and B100, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg), and IMEP (3.5–7.5 bar with variation of 2 bar) on x axis and means of CO2 emission variation on y axis. In this Figure, it was shown that diesel (B0) was having the highest emission of CO2, it reduces with inducing more proportion of biodiesel. Whereas in 0 kg load CO2 emission was the lowest and it was almost proportional to the load parameter. In the case of IMEP, after 5.45 bar CO2 emission got increased. Figure 9 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of CuO do** (50–150 ppm with variation of 50 ppm, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg, where low level is represented by 1, medium by 2 and high by 3), and IMEP (3.5 -7.5 bar with variation of 2 bar, where low level is represented by 1, medium by 2 and high by 3) on x axis and means of CO2 emission variation on y axis. Figure 9 which is representing the case of CuO nanoparticles between 50 and 100 ppm, CO2 emission was very less, almost constant whereas they were going on increasing exponentially with the increment of the nanoparticles. Figure 10 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of the blend (B0, B30, and B100, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg), and IMEP (3.5–7.5 bar with variation of 2 bar) on x axis and means of NOx emission variation on y axis. In Fig. 10, it was shown that diesel (B0) was having the highest emission of NOx, and it reduced with inducing more proportion of biodiesel. Whereas in 0 kg load, NOx emission was the lowest and it was almost proportional to the load parameter. In the case of IMEP after 5.45 bar NOx emission increased. Figure 11 is a graphical representation of the main effect plots for each determined parameter highlighting the variation of CuO do** (50 -150 ppm with variation of 50 ppm, where low level is represented by 1, medium by 2, and high by 3), load (0–12 kg with variation of 6 kg, where low level is represented by 1, medium by 2 and high by 3), and IMEP (3.5 -7.5 bar with variation of 2 bar, where low level is represented by 1, medium by 2 and high by 3) on x axis and means of NOx emission variation on y axis. Figure 11, which is representing the case of CuO do** between 50 and 100 ppm, NOx emission was very less, almost constant whereas they were going on increasing exponentially with the increment of the nanoparticles. The response model which is fitted in the equations is as described. CO, CO2 and NOx with and without nanoparticles, are the response factors. Regression Eqs. (1–6) are as follows: The design matrices are described in Tables 2A, B and 3A, B. The optimization was required for minimal condition emission of CO, CO2, and NOx. Based on Tables 5, 6, and 7, the signal-to-noise ratio plots are shown in Figs. 6, 7, 8, 9, 10, and 11. With the help of these plots, nature of the responses is governed by the variation of crucial parameters. Investigation with Taguchi for CO versus blend, load (kg), IMEP (bar) and CO versus CuO, load (kg), IMEP (bar): with and without nanoparticles is shown in Table 5. Investigation with Taguchi for CO2 versus blend, load (kg), IMEP (bar) and CO2 versus CuO, load (kg), IMEP (bar): with and without nanoparticles is shown in Table 6. Taguchi Analysis for NOx versus blend, load (kg), IMEP (bar) and NOx versus CuO, load (kg), IMEP (bar) with and without nanoparticles is shown in Table 7. In the Pareto chart (Fig. 12), for CO emission without nanoparticles, which is a graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (blend), B (load), and C (IMEP). From the influencing parameters in Pareto chart, the sequence A > C > B was considered as the outcome of predefined predictors for regression analysis of CO versus Blend, Load (kg), and IMEP (bar). The observation showed that A (blend) is the highest influence factor on CO emission. From the influencing parameters in the previous Pareto chart (Fig. 12), in the next Pareto chart (Fig. 13) for CO emission from nanoparticles, which is a graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (CuO), B (load), and C (IMEP). From the influencing parameters in this Pareto chart, the sequence A > B > C was considered as the outcome of predefined predictors for regression analysis of CO versus CuO nanoparticles, Load (kg), and IMEP (bar), where A represents CuO mixed blend B30 kee** the other influencing parameters the same. The observation showed that A (CuO) is the highest influence factor on CO emission. The coefficient of determination is almost equal to 1, which reveals the chosen optimization technique is very accurate in prediction. Residual plot is a category of scatter plots representing the input of data or independent variables on the x axis and residuals on the y axis. In this four-in-one residual plot (Figs. 14, 18, and 22) for CO, CO2, and NOx emissions without nanoparticles, also in the residual plots for CO, CO2, and NOx emissions with nanoparticles (Figs. 15, 19 and 23) the residuals were reaching almost to a linear nature in normal probability plot. In addition, an approximate symmetric nature of the histogram indicated that the residuals were normally distributed. Residuals were possessing constant variance as they were scattered randomly around zero in residual versus fitted values. Residuals exhibited no clear pattern in the residual versus order plot (there is no undesirable effect). In the Pareto chart (Fig. 16), for CO2 emission without nanoparticles, which is a graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (blend), B (load), and C (IMEP). From the influencing parameters in Pareto chart, the sequence B > A > C was considered as the outcome of the predefined predictors for regression analysis of CO2 versus Blend, Load (kg), and IMEP (bar). The observation showed that B (load) is the highest influence factor on CO2 emission. In the Pareto chart (Fig. 17) for CO2 emission from nanoparticles, which is a graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (CuO), B (load), and C (IMEP). From the influencing parameters in this Pareto chart, the sequence A > B > C was considered as the outcome of a predefined predictor for regression analysis, where A represents CuO mixed blend B30 kee** the other influencing parameters the same. The observation showed that A (CuO) is the highest influence factor on CO2 emission. In the Pareto chart (Fig. 20), for NOx emission without nanoparticles, which is a graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (blend), B (load), and C (IMEP). From the influencing parameters in Pareto chart, the sequence B > A > C was considered as the outcome of the predefined predictors for regression analysis of NOx versus Blend, Load (kg), and IMEP (bar). The observation showed that B (load) is the highest influence factor on NOx emission. In the Pareto chart (Fig. 21), NOx emission from nanoparticles, which is graphical representation of the standardization effect on the x axis and the predefined predictor effect on y axis, the predefined predictors were A (CuO), B (load), and C (IMEP). From the influencing parameters in this Pareto chart, the sequence A > B > C was considered as the outcome of a predefined predictor for regression analysis of NOx, where A represents CuO mixed blend B30, kee** the other influencing parameters the same. The observation showed that A (CuO) is the highest influence factor on NOx emission. As per the observation from Pareto chart for CO emission without nanoparticles, sequence, A > C > B was considered for regression analysis of CO versus Blend, Load (kg), and IMEP (bar). A (blend) was the highest influential factor before C (IMEP). In Figs. 24 and 25, the contour plot and surface plot of CO are shown without CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the blend variation, and y axis is representing IMEP in the bar, and z axis is representing the variation of CO as the combined outcome of variation of IMEP and blend, highlighting the 2D and 3D response contour plot and surface plot of CO, plotted using response surface methodology approach. From both experimental as well as RSM approach, Minimum CO emission is obtained at 6.28 kg of blend (B30) and IMEP (5.75 bar). As per the observation from Pareto chart for CO emission from nanoparticles, the sequence A > B > C was obtained for regression analysis. A (CuO nanoparticles) was the highest influence factor. In Figs. 26 and 27, the contour plot and surface plot of CO are shown for CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the variation of CuO nanoparticles, and y axis is representing the load variation, and z axis is representing the variation of CO as combined outcome of variation of CuO nanoparticles and load, highlighting the 2D and 3D response contour plot and surface plot of CO, plotted using response surface methodology approach. From both experimental as well as RSM approach, the minimum CO emission from nanoparticles is obtained at the lowest do** of nanoparticles (50 ppm) in the blend B30 even at the highest load 12.17 kg. As per the observation from Pareto chart for CO2 emission without nanoparticles, B (load) was the highest influence factor, after that blend was the second highest. In Figs. 28 and 29, the contour plot and surface plot of CO2 are shown without CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the blend variation, and y axis is representing the load in kg, and z axis is representing the variation of CO2 as combined outcome of variation of load and blend, highlighting the 2D and 3D response contour plot and surface plot of CO2, plotted using response surface methodology approach. From both experimental as well as RSM approach, minimum CO2 emission without nanoparticles is obtained at 0 kg load and 3.48 bar IMEP for B0 (diesel), B30 and B100 blends. As per the observation from Pareto chart for CO2 emission from nanoparticles, A (CuO) was the highest influence factor, after that load was the second highest. In Figs. 30 and 31, the contour plot and surface plot of CO2 are shown on CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the variation of CuO nanoparticles, and y axis is representing the load variation, and z axis is representing the variation of CO2 as combined outcome of variation of CuO nanoparticles and load, highlighting the 2D and 3D response contour plot and surface plot of CO2, plotted using response surface methodology approach. From both experimental as well as RSM approach, the minimum CO2 emission from nanoparticles is obtained at the lowest do** of nanoparticles (50 ppm) in the blend B30 even at the highest load 12.17 kg. As per the observation from Pareto chart for NOx emission without nanoparticles, B (load) was the highest influence factor, after that blend was the second highest. In Figs. 32 and 33, the contour plot and surface plot of NOx are shown without CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the blend variation, and y axis is representing the load in the kg, and z axis is representing the variation of NOx as combined outcome of variation of load and blend, highlighting the 2D and 3D response contour plot and surface plot of NOx, plotted using the response surface methodology perspective. From both experimental as well as RSM approach, minimum NOx emission without nanoparticles is obtained at 0 kg load and 3.48 bar IMEP for B0 (diesel), B30 and B100 blends. As per the observation from Pareto chart for NOx emission from nanoparticles, A (CuO) was the highest influence factor after that load was the second highest. In Figs. 34 and 35, the contour plot and surface plot of NOx are shown with CuO nanoparticles, respectively, which are the graphical representations of predefined parameters, where x axis is representing the variation of CuO nanoparticles, and y axis is representing the load variation, and z axis is representing the variation of NOx as combined outcome of variation of CuO nanoparticles and load, highlighting the 2D and 3D response contour plot and surface plot of NOx, plotted using response surface methodology approach. From both experimental as well as RSM approach, minimum NOx emission from nanoparticles is obtained at the lowest do** of nanoparticles (50 ppm) in the blend B30 even at the highest load 12.17 kg. In the present investigation, economical techniques are discussed for the reduction in harmful GHG emissions due to automobiles. Newly designed device based on hydrodynamic cavitation principal is used for the preparation of biodiesel conquering the flaws of traditional methods of biodiesel preparation. WCO biodiesel used in the current work can be a cost-effective substitute for diesel. Giancarlo Chiatti et al. [45] also agreed that WCO biodiesel can be a sustainable source of energy in their study of impact of WCO biodiesel on particle size distribution. In the experimental analysis during testing WCO biodiesel blend as shown in Table 2A, it is found that CO emissions of diesel fuel are higher than WCO biodiesel blends, and CO2 emissions are found to increase as the blend proportion of WCO biodiesel blend is increasing. Lai Fatt Chuah et al. [46] also stated the same pattern in the emissions of CO and CO2 during the comparative analysis of biodiesel and diesel. P. Karthikeyan et al. [47] also stated on the addition of nanoparticles, the reduction in NOx emission in biodiesel blends compared to diesel. In the current investigation, for better emission reduction results, the enhancement is done in WCO biodiesel blend with CuO nanoparticles which is hel** in order to achieve the complete combustion and improving the quality of combustion process reducing CO emission in comparison to diesel fuel. Moreover, speed of the combustion process, in the case of CuO nanoparticles doped WCO blend B30 is improved due to the high surface to volume ratio of CuO nanoparticles resulting in good atomization and rapid evaporation of fuel. Rapid evaporation might be the main reason behind the decreasing emission of CO2 in the engine exhaust compared to B30 blend of WCO biodiesel without nanoparticles. In addition to that, a good catalytic reaction due to CuO nanoparticles in the B30 blend of WCO biodiesel increases the heat transfer rate in the combustion chamber, which means the combustion temperature in the cylinder is less. Lower combustion temperature does not support the formation of NOx in the cylinder. In the case of B30 blend of WCO biodiesel added with CuO nanoparticles, there is a decrease in the NOx as the load on the engine increases compared to WCO biodiesel blend without nanoparticles. Silambarasan Rajendran [48] also agreed that effective mitigation of the emission of NOx is possible with effective antioxidant additives in biodiesel blends. According to the research study of Sukumar S. et al. [49], CuO is exhibiting good antioxidant properties. Moreover, CuO nanoparticles have a tendency to improve oxidation stability, this might be one of the reasons behind preventing oxidation and reducing NOx emission in the combustion process [50]. Moreover, the load is having significant impact on NOx emission. As the load increases, NOx emission is found to increase in all readings.2.7 Synthesis of CuO nanoparticles
2.8 Analysis of L9 Taguchi Optimization
2.9 Key findings
3 Conclusion
Emission of GHG gases such as CO, CO2 and NOx by using diesel as a fuel in four-stroke single-cylinder CI engines is found to be very high. Available pollution control devices are costly. WCO biodiesel can be one of the cost effective and sustainable solutions. In addition to that, the loopholes of traditional methods are conquered by a novel patented device based on the principle of hydrodynamic cavitation, used for biodiesel production in the current investigation. Two different experimental investigations are carried out using WCO biodiesel as fuel in CI engine. In the first one, blend, load, IMEP are considered as independent parameters, in the second one the blend B30 from the previous case has kept constant and is mixed with CuO nanoparticles which became a new independent parameter in the second case, kee** the other two parameters (load, IMEP) similar to the first one, at three levels. By varying these parameters, after application of the L9 Taguchi approach, a minimum emission of CO, CO2, and NOx is achieved. Optimized results as per Taguchi approach are as follows:
-
For the first investigation are 0.08 ppm CO, 0.6 ppm CO2 and 30 ppm NOx emission by appertaining the condemnatory merger of inputs such as blend (B0-Diesel), load (12.24 kg) and IMEP (3.48 bar) as shown in Fig. 36.
-
Effective CuO nanoparticles do**: after do** CuO nanoparticles, percentage reduction is found to be 92.3%, 94.82%, and 96% compared to the emission of diesel in CO, CO2 and NOx gases respectively.
-
Taguchi analysis based second experimental investigation on nanoparticles CuO in blend B30, the prognosticated results of optimization were 0.03 ppm CO, 0.3 ppm CO2 and 21 ppm NOx emission, by appertaining the condemnatory merger of inputs such as CuO nanoparticles (50 ppm), load (0.06 kg) and IMEP (3.48 bar) as shown in Fig. 37.
-
It can be studied further by adding a combination of effective nanoparticle does for effective combustion reduction as well as performance improvement.
-
Current experimental investigation has proved that the blending process of pure diesel and hydrodynamic cavitation based WCO biodiesel specifically with B30 blend has reduced CO and CO2 emissions.
Further conduction of the experiments utilizing the prognosticated results of optimization and outcome results were substantiated under the 3% error to prove the efficacy of the present investigation. Few limitations of the current research investigation are for the collection of waste cooking oil, a proper system has to be developed where direct collection provision will be there for food supply chains, various packaged food industries, big as well as small hotels, mess and even households. In this new methodology of biodiesel production pretreatment of waste cooking oil such as preheating and filtering are compulsory. Moreover, the experimental study is not able to reduce the emission of NOx gas by adding nanoparticles at 150 ppm. In the current investigation, WCO biodiesel is prepared by one of the cost effective and sustainable technologies. This biodiesel production technology can be further tested and used for various edible and non-edible feedstocks. Testing of the prepared various biodiesel blends could be done with multiple nanoparticles do** for more productive results. The results of the current investigation are concluded that enhanced WCO biodiesel blends doped with CuO nanoparticles are an environmentally friendly workable solutions to automobile industry. Present optimization work will be inscribing the problems of air pollution and providing an effective solution of reduction in emissions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- GHG:
-
Greenhouse gases
- CI engine:
-
Compression ignition engine.
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- NOx:
-
Nitrogen oxide
- CI engine:
-
Compression ignition engine
- VCR:
-
Variable compression ratio
- WCOBD:
-
Waste cooking oil biodiesel
- SEM:
-
Scanning electron microscope
- B0:
-
0 V. % biodiesel, 100 v. % diesel
- B20:
-
20 V. % biodiesel, 80 v.% diesel
- B30:
-
30 V. % biodiesel, 70 v. % diesel
- B40:
-
40 V. % biodiesel, 60 v. % diesel
- B50:
-
50 V. % biodiesel, 50 v. % diesel
- B75:
-
75 V. % biodiesel, 25 v. % diesel
- B100:
-
100 V. % biodiesel, 0 v. % diesel,
- BP:
-
Brake power
- WCO:
-
Waste cooking oil
- Cv:
-
Calorific value of fuel
- XRD:
-
X-ray diffraction analysis
- IMEP :
-
Indicated mean effective pressure
- KOH:
-
Potassium hydroxide
- CuO:
-
Copper oxide
- BTHE:
-
Brake thermal efficiency
- nano:
-
Nanoparticles
- RSM:
-
Response surface methodology
References
Yang J, Yu Y, Ma T, Zhang C, Wang Q (2021) Evolution of energy and metal demand driven by industrial revolutions and its trend analysis. Chinese J Population Resources Environ 19(3):256–264. https://doi.org/10.1016/j.cjpre.2021.12.028
Bokhari A, Yusup S, Chuah L, Kamil R (2016) Relative efficiency of esterified rubber seed oil in a hydrodynamic cavitation reactor and purification via distillation column. Chem Eng Trans 52:775–780. https://doi.org/10.3303/CET1652130
Chuah LF, Bokhari A, Asif S, Klemeš JJ, Dailin DJ, El Enshasy H, Yusof AHM (2022) A review of performance and emission characteristics of engine diesel fuelled by biodiesel. Chem Eng Trans. https://doi.org/10.3303/CET2294183
Chuah LF, Klemeš JJ, Bokhari A, Asif S, Cheng YW, Chong CC, Show PL (2022) Chapter 3 - A review of intensification technologies for biodiesel production. In: Gutiérrez-Antoni C, Israel F, Castro G (eds) Biofuels and Biorefining. Elsevier, Amsterdam, pp 87–116. https://doi.org/10.1016/B978-0-12-824117-2.00009-0
Huang MH, Huang Y, Cao JJ, Tao WQ (2021) Studys on mitigation of automobile exhaust pollution in an urban street canyon: Emission reduction and air cleaning street lamps. Build Environ 193:107651. https://doi.org/10.1016/j.buildenv.2021.107651
Bastawissi HAE, Elkelawy M, Panchal H, Sadasivuni KK (2019) Optimization of the multi-carburant dose as an energy source for the application of the HCCI engine. Fuel 253:15–24. https://doi.org/10.1016/j.fuel.2019.04.167
Elkelawy M, El Shenawy EA, Mohamed SA, Elarabi MM, Bastawissi HAE (2022) Impacts of using EGR and different DI-fuels on RCCI engine emissions, performance, and combustion characteristics. Energy Conv Manag X 15:100236. https://doi.org/10.1016/j.ecmx.2022.100236
Elkelawy M, El Hagar Alm-Eldin Bastawissi EA, Shenawy MM, Shams HP, Sadasivuni KK, Choudhary AK (2021) Influence of lean premixed ratio of PCCI-DI engines fueled by diesel/biodiesel blends on combustion, performance, and emission attributes; comparison study. Energy Conv Manag X 10:100066. https://doi.org/10.1016/j.ecmx.2020.100066
Elkelawy M, Etaiw SEDH, Ayad MI, Marie H, Dawood M, Panchal H, Bastawissi HAE (2021) An enhancement in the diesel engine performance, combustion, and emission attributes fueled by diesel biodiesel and 3D silver thiocyanate nanoparticles additive fuel blends. J Taiwan Inst Chem Eng 124:369–380. https://doi.org/10.1016/j.jtice.2021.02.019
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013) Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production characteristics, engine performance and emissions production. Energy Rev 18(C):211–245. https://doi.org/10.1016/j.rser.2012.10.013
Maher KD, Bressler DC (2006) Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals, 98(12): 2351–68. https://doi.org/10.1016/j.biortech.2006.10.025
Gashaw A, Teshita A (2014) Production of biodiesel from waste cooking oil and factors affecting its formation: A Review. Int J Renew Sustain Energy 3(5):92–98. https://doi.org/10.11648/j.ijrse.20140305.12
Meng YL, Tian SJ, Li SF, Wang BY, Zhang MH (2013) Transesterification of rapeseed oil for biodiesel production in trickle-bed reactors packed with heterogeneous Ca/Al composite oxide-based alkaline catalyst. Bioresource Technol 136:730–734. https://doi.org/10.1016/j.biortech.2013.03.081
Negm NA, Betiha MA, Alhumaimess MS, Hassan HM, Rabie AM (2019) Clean transesterification process for biodiesel production using heterogeneous polymer heteropoly acid nanocatalyst. J Cleaner Prod 238:117854. https://doi.org/10.1016/j.jclepro.2019.117854
Halwe-Pandharikar A, Deshmukh SJ, Kanu NJ (2022) Numerical investigation and experimental analysis of nanoparticles modified unique waste cooking oil biodiesel fueled C.I. engine using single zone thermodynamic model for sustainable development. AIP Adv 12:095218. https://doi.org/10.1063/5.0103308
El Shenawy EA, Elkelawy M, Bastawissi HAE, Shams MM, Panchal H, Sadasivuni K, Thakar N (2019) Investigation and performance analysis of water-diesel emulsion for improvement of performance and emission characteristics of partially premixed charge compression ignition (PPCCI) diesel engines. Sustain Energy Technol Assess 36:100546. https://doi.org/10.1016/j.seta.2019.100546
Elkelawy M, Etaiw SEdH, Bastawissi HAE et al (2022) WCO biodiesel production by heterogeneous catalyst and using cadmium (II)-based supramolecular coordination polymer additives to improve diesel/biodiesel fueled engine performance and emissions. J Therm Anal Calorim 147:6375–6391. https://doi.org/10.1007/s10973-021-10920-1
Kaur M, Ali A, Lithium-ion impregnated calcium oxide as a nano catalyst for biodiesel production from karanja and jatropha oils. Renew Energy, 36(11): 2866–2871.
Wua Q, **ea X, Wanga Y, Roskillya T (2018) Effect of carbon-coated aluminum nanoparticles as an additive to biodiesel-diesel blends on the performance and emission characteristics of diesel engine. Appl Energy 221:597–604. https://doi.org/10.1016/j.apenergy.2018.03.15
Elkelawy M, Etaiw SEDH, Bastawissi HAE, Ayad MI, Radwan AM, Dawood MM (2021) Diesel/ biodiesel /silver thiocyanate nanoparticles/hydrogen peroxide blends as a new fuel for enhancement of performance, combustion, and Emission characteristics of a diesel engine. Energy 216:119284. https://doi.org/10.1016/j.energy.2020.119284
Ullah Z, Bustam MA, Man Z (2015) Biodiesel production from waste cooking oil by acidic ionic liquid as a catalyst. Renew Energy 77:521–526. https://doi.org/10.1016/j.renene.2014.12.040
Kundu SK, Bhaumik A (2015) Pyrene-based porous organic polymers as an efficient catalytic support for the synthesis of biodiesel at room temperature. ACS Sustain Chem Eng 3:1715–1723. https://doi.org/10.1021/acssuschemeng.5b00238
Wan M, Daud WMA, Aroua MK (2009) Activity of solid catalysts for biodiesel production: a review. Fuel Process Technol 90(6):770–777. https://doi.org/10.1016/J.FUPROC.2009.03.010
Srinidhi C, Madhusudhan A, Channapattana SV (2019) Effect of NiO nanoparticles on performance and emission characteristics at various injection timings of biodiesel-diesel blends. Fuel 235:185–193. https://doi.org/10.1016/j.fuel.2018.07.067
Wardle KE (2015) Liquid–liquid mixing studies in annular centrifugal contactors comparing stationary mixing vane options. Solvent Extr Ion Exch 33:671–690. https://doi.org/10.1080/07366299.2015.1082835
Rastogi PM, Sharma A, Kumar N (2021) Effect of CuO nanoparticles concentration on the performance and emission characteristics of the diesel engine running on jojoba (Simmondsia Chinensis) biodiesel. Fuel 286(Part 1):119358. https://doi.org/10.1016/j.fuel.2020.119358
Elkelawy M, Yu-Sheng Z, El-Din HA, **g-zhou Y (2008) A comprehensive modeling study of natural gas (HCCI) engine combustion enhancement by using hydrogen addition, SAE Technical Paper 2008-01-1706, https://doi.org/10.4271/2008-01-1706.
Halwe AD, Deshmukh SJ, Kanu NJ, Gupta E, Tale RB (2021) Optimization of the novel hydrodynamic cavitation based waste cooking oil biodiesel production process parameters using integrated L9 Taguchi and RSM approach. Mater Today Proc 47:5934–5941. https://doi.org/10.1016/j.matpr.2021.04.484
Chuah LF, Aziz ARA, Yusup S et al (2015) Performance and emission of diesel engine fuelled by waste cooking oil methyl ester derived from palm olein using hydrodynamic cavitation. Clean Techn Environ Policy 17:2229–2241. https://doi.org/10.1007/s10098-015-0957-2
Kanu NJ, Guluwadi S, Pandey V, Suyambazhahan S (2021) Experimental investigation of emission characteristics of can-combustor using jatropha based bio-derived synthetic paraffinic kerosene. SmartScience. https://doi.org/10.1080/23080477.2021.1938503
Elkelawy M, Bastawissi H, Chandra Sekar S, Karuppasamy K et al (2018) Numerical and experimental investigation of ethyl alcohol as oxygenator on the combustion, performance, and emission characteristics of diesel/cotton seed oil blends in homogenous charge compression ignition engine, SAE Technical Paper 2018-01-1680. https://doi.org/10.4271/2018-01-1680.
Vates UK, Kanu NJ, Gupta E, Singh GK, Sharma BP, Pandey V (2021) Optimization of electro discharge critical process parameters in tungsten carbide drilling using L9 Taguchi approach. Mater Today Proc 47:3227–3234. https://doi.org/10.1016/j.matpr.2021.06.438
Putrasari Y, Nur A, Muharam A (2013) Performance and emission characteristics of a two cylinder di diesel engine fuelled with ethanol-diesel blends. Energy Proc 32:21–30. https://doi.org/10.1016/j.egypro.2013.05.004
Ali MH, Abdullah A, Yasin MM, Kamarulzaman MK (2020) Cyclic pressure variations in a small diesel engine fueled with biodiesel and antioxidant blends. Int J Automotive Mech Eng. 17:7851–7857. https://doi.org/10.15282/ijame.17.2.2020.04.0585
Ali OM, Rosli N, Mamat R, Adam A (2015) Comparison of the effect of different alcohol additives in blended fuel on cyclic variation in diesel engine. Energy Proc 75:2357–2362
Kanu NJ, Gupta E, Sutar V, Singh GK, Vates UK (2021) An insight into biofunctional curcumin/gelatin nanofibers. In: Kumar B (ed.) Nanofibers–synthesis, properties and applications, IntechOpen. https://doi.org/10.5772/intechopen.97113.
Panda D, Saharan VK, Manickam S (2020) Controlled hydrodynamic cavitation: a review of recent advances and perspectives for greener processing. Processes 8:220. https://doi.org/10.3390/pr8020220
Saravanamuthu M, Thulasiram R, Ramasamy D et al (2022) Optimization of engine parameters using NSGA II for the comprehensive reduction of emissions from VCR engines fuelled with ROME biodiesel. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-19752-4
Gurunathan B, Ravi A (2015) Process optimization and kinetics of biodiesel production from neem oil using copper doped zinc oxide heterogeneous nanocatalyst. Bioresource Technol 190:424–428. https://doi.org/10.1016/j.biortech.2015.04.101
Karthikeyan T, Alwarsamy T (2019) Effects of copper oxide nanoparticle addition in diesel and biodiesel blends on the performance and emission characteristics of a diesel engine. Int J Res Appl Sci Eng Technol. https://doi.org/10.22214/ijraset.2019.4028
Masera K, Hossain AK (2021) Modified selective non-catalytic reduction system to reduce NOx gas emission in biodiesel powered engines. Fuel 298:120826. https://doi.org/10.1016/j.fuel.2021.120826
Awang MSN, Mohd Zulkifli NW, Abbas MM, Amzar Zulkifli S, Kalam MA, Ahmad MH, Mohd Yusoff MNA, Mazlan M, Daud WMAW (2021) Effect of addition of palm oil biodiesel in waste plastic oil on diesel engine performance, emission, and lubricity. ACS Omega 6(33):21655–21675. https://doi.org/10.1021/acsomega.1c03073
Blinová L, Fiala J, Balog K (2014) Biodiesel production from waste cooking oil in laboratory scale. Appl Mech Mater. https://doi.org/10.4028/www.scientific.net/AMM.448-453.1656
Bayat A, Baghdadi M, Bidhendi GN (2018) Tailored magnetic nano-alumina as an efficient catalyst for transesterification of waste cooking oil: Optimization of biodiesel production using response surface methodology. Energy Conv Manag. 177:395–405. https://doi.org/10.1016/j.enconman.2018.09.086
Chiatti G, Chiavola O, Palmieri F (2016) Impact of waste cooking oil in biodiesel blends on particle size distribution of a city-car engine. J Energy Inst. https://doi.org/10.1016/j.joei.2016.11.009
Chuah LF, Aziz A, Suzana Y, Klemeš J, Bokhari A (2016) Waste cooking oil biodiesel via hydrodynamic cavitation with a diesel engine performance and greenhouse gas footprint reduction. Chem Eng Trans 50:301–306. https://doi.org/10.3303/CET1650051
Karthikeyan P, Viswanath G (2020) Effect of titanium oxide nanoparticles in tamanu biodiesel operated in a two-cylinder diesel engine. Mater Today Proc 22(Part 3):776–780. https://doi.org/10.1016/j.matpr.2019.10.138
Rajendran S (2020) Effect of antioxidant additives on oxides of nitrogen (NOx) emission reduction from annona biodiesel operated diesel engine. Renew Energy 148:1321–1326. https://doi.org/10.1016/j.renene.2019.10.104
Sukumar S, Rudrasenan A, Padmanabhan Nambiar D (2020) Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extracts and their applications. ACS Omega 5(2):1040–1051. https://doi.org/10.3390/en15031032
Lv J, Wang S, Meng B (2022) Review The effects of nano-additives added to diesel-biodiesel fuel blends on combustion and emission characteristics of diesel engine: a review. Energies 15:1032. https://doi.org/10.3390/en15031032
Funding
The Authors have not been funded in any way to carry out the research activities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halwe, A.D., Deshmukh, S.J., Kanu, N.J. et al. Optimization of combustion characteristics of novel hydrodynamic cavitation based waste cooking oil biodiesel fueled CI engine. SN Appl. Sci. 5, 65 (2023). https://doi.org/10.1007/s42452-023-05284-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05284-0