Abstract
Abstract
This article presents a cost-effective ultraviolet-ozone cleaner (UV/O3 Cleaner) for surface pre-treatment of substrates in the field of semiconductor technology. The cleaner consists of two chambers, the upper one contains the electronics, including the time counter. The lower chamber contains the two UV sterilisation lamps and a UV reflector of anodized aluminium, which confines the area of high Ozone concentration in the area of interest. The device is successfully used for surface cleaning and modification of different materials. To this end, the two important wavelengths 253.7 nm (excitation of organic residues) and 184.9 nm (production of ozone from the atmospheric environment as a strong oxidant) were first detected. The effectiveness of UV/O3 cleaning is demonstrated by improving the properties of indium tin oxide (ITO) for OLED fabrication. The contact angle of water to ITO could be reduced from 90° to 3° and for diiodomethane, it was reduced from 55° to 31° within the 10 min of irradiation. This greatly improved wettability for polar and non-polar liquids can increase the flexibility in further process control. In addition, an improvement in wettability is characterized by measuring the contact angles for titanium dioxide (TiO2) and polydimethylsiloxane (PDMS). The contact angle of water to TiO2 decreased from 70° to 10°, and that of diiodomethane to TiO2 from 54° to 31°. The wettability of PDMS was also greatly increased. Here, the contact angle of water was reduced from 109° to 24° and the contact angle to diiodomethane from 89° to 49°.
Article Highlights
-
We report a cost-effective dry-cleaning device for surface cleaning and modification based on ultraviolet-ozone irradiation.
-
Contact angle measurements show an increase of wettability for different materials due to surface modification.
-
The UVO3 pre-treatment improves layer formation and optoelectrical properties of OLEDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pre-treatment and cleaning of substrate surfaces have established themselves as an important step in device fabrication, particularly in the electronics, semiconductors industries, food processing, wastewater purification and many other fields [1,2,3]. Cleaning and modification of surfaces for removing particles and organic, molecular, metallic or microbiological contaminations can be achieved by a variety of different methods, whereby one can distinguish in particular between wet-chemical [2, 4, 5] and dry cleaning methods [3, 6, 7].
The cleaning method should always be specialized for the application. Various parameters such as substrate material, type and size of contamination and subsequent process steps are of great importance. Thus, partially organic and inorganic contamination can be treated differently. The production costs, environmental compatibility of the process and cleanliness of the substrate can be optimized by carefully selecting the cleaning method [8]. Thus, the cleaning methods were extended, specialized and improved, in particular by the advancing technologizing and miniaturization. As already mentioned, a distinction is made between wet chemical and dry-cleaning methods. Wet chemical cleaning generally involves dissolving contamination by using a solvent. These include the typical cleaning steps such as wipe cleaning, dip cleaning, spray cleaning as well as ultrasonic and megasonic cleaning [5, 9, 10]. The latest methods such as the various CO2-cleaning methods (solid CO2-dry ice pellets, CO2-snow cleaning, supercritical CO2 or liquid CO2), microbubble cleaning, water ice cleaning or ionic liquid cleaning can also be counted among the wet-chemical cleaning methods [11, 12]. Wet-chemical cleaning has some advantages, such as the usually low process and aperture costs, good selectivity and often short process times due to high etching rates. The usability under atmospheric conditions and the possibility to clean large substrates also make wet chemical cleaning a very frequently used method. Disadvantages resulting from the low effectiveness with small features such as pores or deep groove structures which are smaller than 1 μm, as they are often used in the electronics and semiconductor industry. In addition, solvents can be harmful, and toxic, causing safety problems and generating waste [8, 13, 14]. In addition to these solvent-based cleaning methods, there are also a variety of dry-cleaning methods. The best-known dry methods include mechanical cleaning, plasma cleaning, laser cleaning, dry ice cleaning, jet cleaning and UV/O3 cleaning processes [3, 15,16,17,18,19,20]. In general, dry-cleaning processes often have the advantage of safety and environmental friendliness. Hazardous or toxic aids are rarely used. In addition, many of these methods can clean the substrates at the molecular level and modify or etch the surface. The costs in the running process are also usually low, whereby the acquisition costs of the system can be very high. Nevertheless, there are advantages and disadvantages due to the specialization of the individual systems in the respective areas of application. A comparative selection of these is shown in Table 1 for laser, plasma and UV/O3-cleaning methods.
Laser cleaning must be emphasized here, since it enables the combination of the advantages for both types of cleaning (dry and wet) for instance by combining it with solvents as a wet cleaning method [26, 32, 33]. This is one of the reasons why laser cleaning is an established and widely used cleaning method. The applications of this versatile process ranges from surface cleaning and modification in the semiconductor industry [22,23,24,25], through the removal of entire layers [21, 26] to the restoration of historical works of art [26, 34]. Another big advantage of laser cleaning is the possibility to combine several cleaning steps in one system. For example, a targeted structuring of a substrate with simultaneous surface modification and result analysis via laser induced plasma spectroscopy (LIBS) can be carried out by the separate setting of the individual laser parameters [26]. This versatility can hardly be realized with other cleaning methods or only by additional steps such as applying a sacrificial layer for partial protection of the substrate. To be able to use the full scope of laser cleaning with all its facets, in addition to the high acquisition costs, well-trained personnel are needed for operation, because the use of incorrect parameters can have undesirable consequences for the substrate or even destroy it. A detailed overview of laser cleaning can be found in [26, 32, 35] The increase in wettability of different substrates shown in this work can also be achieved with laser cleaning or plasma treatment [22, 36, 37].s The UV/O3 treatment described here is intended to be a cost-effective alternative to remove organic contamination from purchasable pre-cleaned substrates (free from inorganic contaminations and large particles) and therefore produce ultra-pure surfaces and to modify their characteristics. A detailed summary of current methods can be found in “Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques, Volume 11, (2019)” [38].
Using the application example of organic semiconductor components, the necessary hole-injection layer (HIL) for example, poly(3,4-ethylene-dioxythiophene): poly(styrenesulfonate) (PEDOT: PSS) is typically prepared from a water-based solution [39,40,41]. Therefore, the surface must be clean and primed hydrophilic to allow subsequent coating processes. To do this, ultraviolet-ozone-treatment (UV/O3-treatment) is widely used due to its soft processing. Thereby, the immersion of the device or component into chemicals is no longer necessary and the treatment can be realised under atmospheric conditions. This method was originally developed to remove photoresist and organic residues from Silicon wafers in the semiconductor industry [29, 42]. Later it was used to clean and modify indium tin oxide films for OLED or OPV applications [17, 40, 43], to activate TiO2 layers before deposition of different metals [44, 45] as well as to enhance the wettability of PDMS for water based solutions [46,47,48,49].
In this article, the low-price UV/O3-cleaner is successfully used for cleaning and excitation of typical surfaces from semiconductor technology such as ITO or TiO2. To be used as a full-fledged alternative for cleaning devices, it must be demonstrated that the presented device is at least comparable to commercially available devices. For this purpose, the emitting spectra were measured. If the two critical wavelengths of 253.7 nm (excitation of organic residues) and 184.9 nm (production of ozone from the atmospheric environment as a strong oxidant) are present, an excitation of organic impurities can take place and the substrate surface can be modified by the deep UV radiation [29, 30]. In this work, this effect was demonstrated with 3 examples (PDMS, TiO2 and indium tin oxide) and the underlying mechanisms were discussed. Preliminary experiments showed the application of PDMS thin films on aluminium substrates and the usage in the pre-treatment of indium tin oxide (ITO) substrates for OLED application. The functionality was quantified through time-dependent experiments with solid, amorphous TiO2 and pure PDMS samples. Here, the contact angle of water and diiodomethane was measured in dependence on the exposure time and the free surface energy (SFE) and the fractional polar and dispersive parts were calculated. The positive effect of surface pre-treatment was also demonstrated using the OLED application example. The cleaning and activation of the ITO surface resulted in a significantly more homogeneous layer formation of the typically water-based PEDOT:PSS solution. Thus, a more uniform lighting behaviour was achieved and the efficiency was improved [1, 2, 43].
The following Section describes the sample preparation, the setup of the cleaning device and the measuring methods used for device and sample characterization. In Sect. 3 the results of the UV/O3 treatment are presented and discussed. Section 4 summarizes the results and classifies the applicability of the cleaning device. Finally, the cited literature is shown in Sect. 5.
2 Materials and methods
2.1 Preparation of the TiO2-surfaces
TiO2 layers were deposited on Silicon Wafers by plasma-enhanced atomic layer deposition (PEALD) with the ALD150 system from the company FHR, Germany. Due to the plasma support in the layer deposition, the layer growth was realized at low temperatures and a high-density layer was produced simultaneously. The low temperature enabled the fabrication of amorphous TiO2 layers [50].
Titanium tetrachloride was used as a precursor in combination with an oxygen plasma. The oxygen flow was set to 25 sccm and has been ionized at 300 W through an inductively coupled plasma source at radio frequency (13.56 MHz). The purging steps were realized with Argon gas. The initial chamber pressure was 4.5 × 10− 2 mbar and the substrates were heated to 120 °C [50].
The layers were analysed via spectroscopic Ellipsometry and XRD before the UV/O3-treatment was applied. The measurements showed that the produced TiO2 layer was completely amorphous and had a thickness of 30 nm. The thickness was determined under the use of the Tauc-Lorentz-Model.
2.2 Preparation of the PDMS stamps
The PDMS samples are fabricated by pouring the silicone rubber Elastosil RT 601 from Wacker, Germany, on a polished and cleaned flat silicon wafer. The silicon rubber is prepared by mixing the platinum catalysator (Elastosil RT 601 A) with the associated crosslinker (Elastosil RT 601 B) at a weight ratio of 9:1 (A:B) in a liquid solution. After stirring the mixture for 1 min, the solution was casted on the silicon wafer in an appropriate form. The gelling process was performed under a vacuum bell jar to remove air bubbles at room temperature (21 °C) for 24 h.
2.3 OLED fabrication
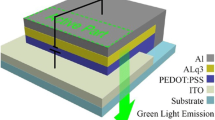
In order to be able to show a possible influence on the substrate surface in addition to the change in wettability, 2 OLEDs (prepared one with UV/O3 pre-treatment and one without). The used samples are ITO glass substrates with pixelated anodes (6 pixels). The substrates were properly cleaned with the wet chemical pre-treatment described above. One sample was treated for 20 min with UV/O3, the other was not. In a glove box, the hole injection layer PEDOT:PSS was applied by a spin coater (Lab Spin 6 TT from SUSS Microtec, Germany) at 5,000 rpm and annealed at 150 °C for 5 min. The active layer F8:F8BT (mixture ratio 19:1) was then applied at 2,000 rpm and annealed at 115 °C for 5 min. PEDOT:PSS was purchased from Clevios™, Heraeus, Germany. The ITO glass substrates, 20 × 15 mm (Legacy, Generation I), as well as the materials used, were manufactured by Ossila, United Kingdom. A 100 nm thick aluminium cathode was deposited under vacuum in a sputtering system. Directly after the encapsulation, the OLEDs were compared.
2.4 Setup of the UV/O3chamber
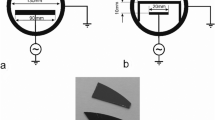
The cost-effective Equipment for surface treatment is based on two 15 W sterilisation lamps from the company BRIGHTINWD, China. The device comprises two cuboid chambers (Hammond Electronics 1402 F(V)), each with a size of 181 mm × 80 mm × 261 mm, which are stacked on top of each other. The upper part contains the following components: the main plug, the current distribution device and a programmable low-cost timer module (Emko EZM-3735). The lower part comprises the two sterilisation lamps, which are mounted onto E14 lamp sockets (Fig. 1). A bent sheet made from anodized aluminium, with a typical reflectivity of 85%, acts as a UV reflector and confines ozone in the area around the substrate. The sample holder was tailored to our specific requirements, including a 4-inch Wafer. The carrier is mounted onto two runners and the front plate consists of a grip in a way that this assembly acts as a drawer. Additionally, the lower chamber contains ventilation slots to allow gas exchange with the environment. This assembly has to be operated within a fume hood to allow a safe outlet of the nascent ozone gas which is generated inside the chamber by photosensitized oxidation process.
Left side: photograph of the equipment in a fume hood with substrate carrier tray open. The upper part houses the electronics, while the lower part acts as the reaction chamber; right side: cross-sectional view of the reaction chamber (lower part): a substrate carrier, b sterilisation lamps, c reflector made of anodized aluminium
2.5 Emission spectra measurement
The used fluorescent lamp is described as a germicidal UV lamp by the distributor without further specification. Therefore, a measurement of the ion spectrum was necessary. The surface modification is based on a photosensitized oxidation process, where the wavelengths of 184.9 and 253.7 nm are crucial. The 184.9 nm radiation can dissociate molecular oxygen continuously. The resulting oxygen radicals can generate ozone by reacting with the remaining atmospheric oxygen. Radiation of 253.7 nm is important for the excitation of the organic residues on the surface. The basic building block of organic materials are hydrocarbons, which absorb this wavelength just like ozone. The photoexcited organics can form radicals, or excited molecules, which reacts strongly with the reactive ozone and atomic oxygen, respectively. The resulting products are simpler volatile molecules that can be desorbed from the surface [29, 37, 42]. Thus, the fluorescent lamp had to be checked for the two required wavelengths. The wavelengths above 200 nm were detected by a spectrometer (FLAME-S-UV-VIS-ES from Ocean Insight, Germany), while the production of ozone was detected semi-quantitatively by an ozone test strip (“ozone test stripes” from Macherey-Nagel, Germany).
2.6 Contact angle measurement
To demonstrate the influence of surface treatment the contact angle measurement method is used. This is a commonly used method in industry for assessing the cleanliness of surfaces. In addition to the cleanliness of surfaces, this method can also measure the direct influence of the pre-treatment on the interface between the substrate and the liquid droplets applied, and thus, for example, show an excitation of the substrate surface [51].
The measurement of the contact angle was performed with the Drop shape analyser 25 (DSA 25), with two pressure dosing systems and the shape of the droplet was recorded by the digital camera CF04. All these devices are from KRUSS GmbH, Germany. The dosing unit was used to deposit a 1 µl water droplet (distilled water (ROTISOLV® HPLC Gradient Grade, Specific electric resistance ≥ 18 MΩ*cm), purchased by Sigma Aldrich, Germany) or a 1 µl droplet of diiodomethane (purchased by MERCK, Germany). The analysing software “ADVANCE”, provided by KRUSS GmbH, measured both contact angles. By using a highly polar liquid (Water) and an almost nonpolar liquid (Diiodomethane) the total SFE, as well as the polar and unipolar SFE values, was calculated using the most common OWRK model. For the determination of the SFE by the OWRK model at least 2 liquids with known disperse and a polar portion of the surface tension are required. Thus, water (polar fraction σpolar = 53.6 and disperse fraction σdisperse = 18.7) and diiodomethane (polar fraction σpolar = 1.3 and disperse fraction σdisperse = 49.5) are ideal test liquids. Thus, in addition to the change in the SFE, a change in polarity can also be detected. This can be used, for example, for coating processes and optimisation for various coatings. The approximation of the droplet shape is acquired by the software through the Ellipse (Tangent-1)-fitting method and the contact angle were automatically calculated. The total SFE is then the sum of the polar and disperse portions.
All measuring points are recorded on two different samples at three different positions. The volume of the deposited distilled water and diiodomethane drops was pre-determined at 1 µl.
3 Results and discussion
3.1 UV/O3-treatment
The UV/O3 treatment of the substrates was carried out in our cost-effective cleaner. The emission spectrum in the range from 200 to 600 nm of the two fluorescent lamps are shown in Fig. 2a. The spectral line distribution is similar to that of a mercury vapour lamp (compare to [52]). In particular, the peak at 253.7 nm was important for the possibility of removing biogradable contamination on the surfaces and was detected with the spectrometer. The ozone test strip consists of a plastic strip with a test paper, which reacts with a colour change from white to brown depending on the ozone level. After an exposure time of 10 min, without direct UV radiation, the test field was compared with the manufacturer’s colour scale. The test strips were placed at different places. All test strips in the immediate vicinity and within the chamber turned dark brown. The discolouration went beyond the measuring range of the scale, which indicated an ozone concentration of well over 210 µg/m³. As an example, two test clearances are shown in Fig. 2b, where the lighter test strip was on top and the darker test strip was in the chamber. Thus, the emission of the wavelength of 184.9 nm could be detected. Due to the strong ozone development, the UV/O3-cleaner should only be operated with a fume cupboard.
3.2 Preliminary results
The first test series were performed with ITO-glass slides (Ossila, surface resistivity 14–16 Ω/sq) and PDMS-coated aluminium substrates. The contact angles of the as-received ITO glasses 90.66° (± 1.80 °) (SFEpolar = 1.44 mN/m) for water and 49.64° (± 1.83 °) (SFEdisperse = 34.48 mN/m) for diiodomethane leading in a total surface free energy of SFEtotal = 35.91 mN/m. Which are typical values for clean but untreated ITO surfaces [53]. The typically used pre-cleaning routine for the ITO films consisted of treating them for at least 5 min in alkaline solution and hot isopropyl alcohol in an ultrasonic bath at 80 °C. This treatment led to a surface with an average contact angle of 10.09 ° (± 2.90 °) (SFEpolar = 33.56 mN/m) for water and an average contact angle of 31.22° (± 1.31 °) (SFEdisperse = 43.71 mN/m) for diiodomethane without UV/O3 treatment. A subsequent UV/O3-cleaning for 10 min led to an average contact angle of 6.17 ° (± 0.41 °) (SFEpolar = 33.44 mN/m) for water and an average contact angle of 28.3° (± 2.47 °) (SFEdisperse = 44.91 mN/m) for diiodomethane. The pictures are shown on the right side in Fig. 3a–d.
The time-dependent measurement of the contact angles between a 1 µl water droplet and an uncleaned ITO substrate and the SFE values, as well as the pictures are shown in Fig. 3. An increase in wettability is visible. The contact angle of water decreases almost continuously with the start of the UV/O3-treatment and drops from above 90° to a contact angle below 10°. After an exposure time of 10 min, the contact angle of 90° in untreated ITO has fallen to 3.3°. This corresponds to a superhydrophilic surface and the change in surface energy is also present. With a surface energy in the untreated stage of 36 mN/m, it doubles the maximum of 78 mN/m after about 5 min. In particular, the increase in the polar proportion from 1.44 to 34.38 mN/m is responsible for the increase in wettability. The disperse fraction increases from 34.48 to 43.63 mN/m, respectively. The measurement uncertainty for all values, outside the range of the strong drop, is below ± 1°, about ± 3° between 1 and 2 min.
Left side: time-dependent change of the surface wettability for water and diiodomethane and the corresponding surface free energies, whereby the total SFE is the sum of the polar and dispersive fractions, for ITO without pre-cleaning; right hand side: picture of the contact angles. a contact angle of water with wet-chemical pre-cleaning and without UV/O3-cleaning, b contact angle of water with wet-chemical pre-cleaning and 10 min UV/O3-cleaning, c contact angle of diiodomethane with wet-chemical pre-cleaning and without UV/O3-cleaning d contact angle of diiodomethane with wet-chemical pre-cleaning and 10 min UV/O3-cleaning, e contact angle of water without wet-chemical pre-cleaning and without UV/O3-cleaning, f contact angle of water without wet-chemical pre-cleaning and 10 min UV/O3-cleaning, g contact angle of diiodomethane without wet-chemical pre-cleaning and without UV/O3-cleaning h contact angle of diiodomethane without wet-chemical pre-cleaning and 10 min UV/O3-cleaning.
Compared to the wet chemical pre-treated samples, hardly any difference in surface energies and contact angles were seen after the 10 min of UV/O3-treatment, which indicated a good effectiveness of the UV/O3-process. For qualitative illustration of the influence of UV/O3 on the substrate, an OLED was prepared as an application example. Despite the good wettability after the preliminary cleaning described above, the UV/O3 -treatment can significantly improve the produced result. This is demonstrated in the top row of the right side of Fig. 4 where OLEDs are depicted without UV/O3-treatment (Fig. 4a) and with UV/O3-treatment of the ITO layer (Fig. 4b). Significant differences were already visible. The sample without UV/O3-treatment provided an inhomogeneous luminous appearance, which was due to uneven layer adhesion. The sample shown in Fig. 4b) illustrates a homogeneous and uniform layer instead. Consequently, the UV/O3-treatment is also recommended in the production process of OLEDs. In notable addition to improved layer formation, the electrical properties of the treated layers can be modified, which leads to an improvement in the radiation behaviour. This can equally be observed in the investigated OLEDs. The OLEDs were operated in a circuit limited to 5 mA. To achieve the observable luminous behaviour at 5 mA, a voltage of 21 V was necessary for the untreated OLED. The current limitation of the OLED treated with UV/O3 was already reached at 9 V. The significant reduction of the operating voltage indicates an improved load carrier transport from the ITO to the HIL layer. This and other possible changes in electrical properties in the ITO layer, resulting from UV/O3 treatment, are discussed in the literature [1, 40, 43, 54].
The PDMS coated aluminium substrates didn’t undergo a pre-cleaning process like the ITO-foils, shown in Fig. 4a, b. Nevertheless, the wettability of the aluminum substrate could be significantly increased by UV/O3-treatment, although a significantly longer exposure time of 30 min was necessary to make the surface hydrophilic (bottom row Fig. 4). In order to rule out a mutual influence of aluminum and PDMS and to reduce the modification effect exclusively to the PDMS, pure PDMS as a substrate was further worked on in the following.
Upper row: photograph of an OLED (ITO 100 nm/PEDOT:PSS 50 nm/F8:F8BT(19:1) 80 nm/Aluminium 100 nm), a spin-coating of PEDOT:PSS without UV/O3-treatment of ITO, b spin-coated PEDOT:PSS after 10 min of UV/O3-treatment of ITO; bottom row: photograph of a water droplet on a PDMS coated aluminium surface with a size of 1 × 1 cm; c before UV/O3-treatment, d after 30 min of UV/O3
3.3 UV/O3cleaning effect on TiO2and PDMS stamps
The 30 nm TiO2 layer and the PDMS substrates, with the size of 2 × 2 cm each, were analysed before and after UV/O3-cleaning varying the exposure time with the contact angle measurement system DSA25. The averaged contact angle of distilled water and diiodomethane with a volume of 1 µl is shown on the top left side of Fig. 5 as a function of UV/O3 exposure time.
The measurements showed the continuously increasing wettability of the samples with increasing exposure time. The initially rather hydrophobic surfaces had an increasingly hydrophilic character, it can be assumed that an accumulation of hydrophilic species on the surface occurred. A wavelength of 253 nm alone led to a change in the surface. According to the studies of Effimenko et al. and Egitto et al. [46], the presence of ozone and atomic oxygen reduces the exposure time, and the increasing wettability of PDMS with and without ozone under UV light was compared there as well. Without ozone, the contact angle decreased significantly slower than with ozone. To summarise, ozone is an important reaction partner, which accelerates the modification of the surface.
The contact angle function is shown for PDMS on the top right side of Fig. 5. The UV/O3-treatment led to photochemical oxidation of the surface [47, 48]. Graubner et al. [48] demonstrate the chain of reactions with X-ray photoelectron spectroscopy (XPS) and infrared spectroscopy. The reactions start with breaking the main chain (Si–O) and the side group of PDMS (Si–C or C–H) through the illumination with 253.7 nm. Subsequently, oxygen or ozone reacts with the silicon or peroxy radicals and rearranges to silanol groups. Further treatment leads to a break of the Si–C bonds, which form Si–O–Si bridges with oxygen, which increases the polar fraction of the SFE [46, 48]. Concerning the comprehensive chemical investigations of Fu et al. [47] this multi-stage conversion process can be used as an explanation for the strong scattering in the first 500–1000 s. Because of the existing penetration depth of the UV radiation, carbon is also separated from the deeper bulk material which diffuses to the surface. This new carbon on the surface competed with the continuous photochemical oxidation on the surface and influenced therefore the modification of wettability [46, 55]. This caused a delayed decrease of the contact angle since this effect must first be compensated. The presence of ozone fulfils a meaningful role in exciting the methyl groups on the surface. As soon as the diffusion of the low molar mass species is compensated to the surface (from about 22.5 min), polar species can accumulate on the surface and the contact angle decreases significantly.
The continuously increased wettability of TiO2 as shown in Fig. 5 could be explained by the illumination with UV-Light. TiO2 has a bandgap in the range of 3.1–3.4 eV, so it can be excited with wavelengths lower than 380 nm. Through this photoexciting process, an electron-hole pair is built. The electrons reduce the Ti4+-Cation to Ti3+ and the holes react with the hydrogen bridges [56]. Water on the surface can react with the oxygen vacancies by forming hydroxyl groups to form a hydrophilic surface. Besides photoexcitation, the removal of organic residues on the surface can enhance the wettability of TiO2 surfaces. A detailed discussion of the dominant effects of forming a hydrophilic surface can be found in Thompson et al. [57].
Upper row: time-dependent change of the surface wettability for PDMS (left) and TiO2 (right) with a sample size of 2 × 2 cm. The contact angle is decreasing with an increased exposure time and therefore the SFE value increases. Lower row: typical pictures of the contact angles of water and diiodomethane on PDMS and TiO2 substrates before UV/O3-cleaning and after
4 Conclusion
As part of the study, the functionality of a simple, self-built and cost-effective UV/O3 cleaner was investigated. To be used as an alternative to commercially available devices, the system must have the following influences on substrate surfaces. This includes the removal of organic residues and the excitation of the surface, whereby, for example, the wettability of the substrate is changed. To achieve this effect, the emission of two wavelengths for cleaning and ozone fabrication is necessary. Photons with a wavelength of 253.7 nm are absorbed by hydrocarbons (the main component of organic materials). These excited molecules can then react with ozone or atmospheric oxygen and thus be dissolved from the surface. The second important wavelength is 184.9 nm, which is responsible for the production of ozone. This reacts with the organic residues on the substrate or with molecules close to the surface, whereby the chemical structure of the surface and thus, for example, the wettability can be influenced.
The emission spectra and the production of ozone of the germicidal lamps used were measured. The wavelength of 253.7 nm could be detected by a spectrometer, the production of ozone by an ozone test strip. Subsequently, various substrate surfaces were modified to demonstrate practical applicability.
A qualitative proof of the functionality was carried out using the application example of an OLED. This example also illustrates the importance and necessity of this type of pre-treatment. The use of UV/O3-pretreatment improved not only the homogeneous emission area but also the electrical properties. This is achieved in particular by increased wettability of the ITO surface, which led to a more uniform distribution of the PEDOT:PSS layer. The improvement of the electrical properties can be shown by the reduced layer resistance. Thus, the UV/O3 treatment significantly reduced the necessary operating voltage, which is necessary to reach the 5 mA from 21 to 5 V. This effect indicates an improved layer adhesion of the hole injections layer. The effect of a modified or adapted strip structure on the surface of the exposed ITO which was discussed in [1, 40, 54] can also be considered as a further component for the improved electrical properties.
Quantitative investigations were performed by measuring the contact angle on PDMS and TiO2 substrates. Both substrate surfaces increased wettability with increasing exposure time until they showed a completely hydrophilic character. In addition to known applications in the semiconductor industry, such as the production of OLED or OPV, such a pretreatment can be used for structuring PDMS [49], wherein certain channels are hydrophilic and others are hydrophobic.
By proving the two critical wavelengths, as well as the application for different material surfaces, it could be shown that the device represents a full-fledged alternative to comparable, commercially available devices. The importance of pretreatment of surfaces was demonstrated using the example of OLED. By using cost-effective components, this device can also be reproduced in all facilities and can be used in the production of homogeneous layers.
In general, the cleaning method must always be adapted to the target and the material. The application of the presented UV/O3 cleaner is specializes in the removal of organic materials at the molecular level. Thus, ultra-pure surfaces can be created. It must be ensured that thick layers, hydrocarbon-free impurities or large particles cannot be eliminated with this method. In addition, the wettability of the surface can be modified to subsequently ensure an ideal adhesion of a liquid. This can be achieved by adjusting the ratio between polar and disperse surface components. In the field of electrical components, the application in the production of OLEDs, in particular, could be shown. The UV/O3 cleaner is therefore a fast, environmentally friendly and easy-to-use device that can produce ultra-pure substrate surfaces on molecular level as long as they don’t have coarse impurities.
The presented device is a prototype, wherein various extensions of the device such as an adjustable irradiation dose or a control of pressure and temperature are possible. Further investigations should be considered to analyze the stability of the induced surface modification. Since coarser impurities on the substrate cannot be removed with the UVO3 cleaning, the influence of pre-cleaning method should be examined.
References
Kato Y, Jung M-C, Lee MV, Qi Y (2014) Electrical and optical properties of transparent flexible electrodes: effects of UV ozone and oxygen plasma treatments. Org Electron 15:721. https://doi.org/10.1016/j.orgel.2014.01.002
Kerner RA, Rand BP (2019) Electrochemical and thermal etching of indium tin oxide by solid-state hybrid organic–inorganic perovskites. ACS Appl Energy Mater 2:6097. https://doi.org/10.1021/acsaem.9b01356
Neutens P, Rutowska M, Van Roy W, Jansen R, Buja F, Van Dorpe P (eds) (2018) Influence of UV light on PECVD silicon nitride waveguide propagation loss. In: 15th international conference on group IV photonics: 29–31 August 2018, Cancun, Mexico. IEEE
Kart D, Gurel DB, Kayaardi S (2018) Cold plasma and ultrasound applications in cleaning of food contact surfaces. Int J Sci Technol Res 4:17
Chu C-L, Lu T-Y, Fuh Y-K (2020) The suitability of ultrasonic and megasonic cleaning of nanoscale patterns in ammonia hydroxide solutions for particle removal and feature damage. Semicond Sci Technol 35:45001. https://doi.org/10.1088/1361-6641/ab675d
Nilsson M, de Maeyer H, Allen M (2022) Evaluation of different cleaning strategies for removal of contaminating DNA molecules. Genes 13. https://doi.org/10.3390/genes13010162
Razab MKAA, Mohamed Noor A, Suhaimi Jaafar M, Abdullah NH, Suhaimi FM, Mohamed M, Adam N, Yusuf NAAN (2018) A review of incorporating Nd:YAG laser cleaning principal in automotive industry. J Radiat Res Appl Sci 11:393. https://doi.org/10.1016/j.jrras.2018.08.002
Marshall JP, Wilcox H (2015) How green and does it clean. In: Developments in surface contamination and cleaning, vol 8, pp 1–69. https://doi.org/10.1016/B978-0-323-29961-9.00001-6
Camacho-Espinosa E, Oliva-Avilés AI, Oliva AI (2017) Effect of the substrate cleaning process on pinhole formation in sputtered CdTe films. J Mater Eng Perform 26:4020. https://doi.org/10.1007/s11665-017-2842-0
Lubna N, Auner G, Patwa R, Herfurth H, Newaz G (2011) Role of cleaning methods on bond quality of Ti coated glass/imidex system. Appl Surf Sci 257:4749. https://doi.org/10.1016/j.apsusc.2010.12.153
Matsuura K, Ogawa S, Kasaki S, Koyama K, Kodama M, Yanase S (2015) Cleaning polymer ink from a glass substrate using microbubbles generated by a hydrogen bubble method. Sep Purif Technol 142:242. https://doi.org/10.1016/j.seppur.2015.01.009
Hattori K, Matsushima D, Demura K, Kamiya M (2020) Particle and pattern discriminant freeze-cleaning method. J Micro/Nanolith MEMS MOEMS. https://doi.org/10.1117/1.JMM.19.4.044401
Nikam AV, Prasad BLV, Kulkarni AA (2018) Wet Chem synthesis metal oxide nanoparticles. Rev CrystEngComm 20:5091. https://doi.org/10.1039/C8CE00487K
Al-Yaseri A, Abbasi GR, Yekeen N, Al-Shajalee F, Giwelli A, **e Q (2022) Effects of cleaning process using toluene and acetone on water-wet-quartz. J Petrol Eng Sci 208
Chen J, Mu H, Hu Q, Jiang Z (2018) Investigation on the process of dry ice cleaning insulators. Mater Sci Eng 394:42112. https://doi.org/10.1088/1757-899x/394/4/042112
Marimuthu S, Sezer HK, Kamara AM (2019) Applications of laser cleaning process in high value manufacturing industries. In: Kohli R, Mittal KL (eds) Developments in surface contamination and cleaning: applications of cleaning techniques, pp 251–288. https://doi.org/10.1016/B978-0-12-815577-6.00007-4
Fan C-L, Lin Y-Z, Huang C-H (2011) Combined scheme of UV/ozone and HMDS treatment on a gate insulator for performance improvement of a low-temperature-processed bottom-contact OTFT Semicond. Sci Technol 26:45006. https://doi.org/10.1088/0268-1242/26/4/045006
Tian Z, Lei Z, Chen X, Chen Y, Zhang L-C, Bi J, Liang J (2020) Nanosecond pulsed fiber laser cleaning of natural marine micro-biofoulings from the surface of aluminum alloy. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118724. ,244:118724
Lu Y, Ding Y, Wang M, Yang L, Wang Y (2021) A characterization of laser cleaning painting layer from steel surface based on thermodynamic model. Int J Adv Manuf Technol. https://doi.org/10.1007/s00170-021-07566-6. ,116:1989
Valtierra N, Courtenay LA, López-Polín L (2020) Microscopic analyses of the effects of mechanical cleaning interventions on cut marks. Archaeol Anthropol Sci 12:1. https://doi.org/10.1007/s12520-020-01153-8
Jia Y, Gong X, Peng P, Wang Z, Tian Z, Ren L, Fu Y, Zhang H (2016) Toward high carrier mobility and low contact resistance: laser cleaning of PMMA residues on graphene surfaces. Nano-micro Lett 8:336. https://doi.org/10.1007/s40820-016-0093-5
Ahmad D, van den Boogaert I, Miller J, Presswell R, Jouhara H (2018) Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A 40:2686 https://doi.org/10.1080/15567036.2018.1511642
Dey T, Naughton D (2016) Cleaning and anti-reflective (AR) hydrophobic coating of glass surface: a review from materials science perspective. J Sol-Gel Sci Technol 77:1. https://doi.org/10.1007/s10971-015-3879-x
Jothi Prakash CG, Prasanth R (2021) Approaches to design a surface with tunable wettability: a review on surface properties. J Mater Sci 56:108. https://doi.org/10.1007/s10853-020-05116-1
Raja RSS, Selvakumar P, Babu PD, Rubasingh BJ, Suresh K (2021) Influence of laser parameters on superhydrophobicity—a review. Eng Res Express 3:22001. https://doi.org/10.1088/2631-8695/abf35f
Sugioka K (2020) Handbook of laser micro- and nano-engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-69537-2
Šimončicová J, Kryštofová S, Medvecká V, Ďurišová K, Kaliňáková B (2019) Technical applications of plasma treatments: current state and perspectives. Appl Microbiol Biotechnol 103:5117. https://doi.org/10.1007/s00253-019-09877-x
Thanu DP, Srinadhu ES, Zhao M, Dole NV,Keswani M (2019) Fundamentals and applications of plasma cleaning. In: Kohli R, Mittal KL (eds) Developments in surface contamination and cleaning: applications of cleaning techniques, pp 289–353. https://doi.org/10.1016/B978-0-12-815577-6.00008-6
Sham ML, Li J, Ma PC, Kim J-K (2009) Cleaning and functionalization of polymer surfaces and nanoscale carbon fillers by UV/ozone treatment: a review. J Compos Mater 43:1537. https://doi.org/10.1177/0021998308337740
Kohli R (2015) UV-ozone cleaning for removal of surface contaminants. In: Developments in surface contamination and cleaning, pp 71–104. https://doi.org/10.1016/B978-0-323-29961-9.00002-8
Liu J, He L, Wang L, Man Y, Huang L, Xu Z, Ge D, Li J, Liu C, Wang L (2016) Significant enhancement of the adhesion between metal films and polymer substrates by UV-ozone surface modification in nanoscale. ACS Appl Mater Interfaces 8:30576. https://doi.org/10.1021/acsami.6b09930
Kruusing A (ed) (2008) Handbook of liquids-assisted laser processing. Elsevier, New York. https://doi.org/10.1016/B978-0-08-044498-7.X5001-8
Weng T-S, Tsai C-H (2014) Laser-induced backside wet cleaning technique for glass substrates. Appl Phys A 116:597.https://doi.org/10.1007/s00339-013-8182-5.
Bausch F, Owusu DD, Graf J, Rosenau T, Potthast A (2022) Shine a light on papyrus: monitoring the aging process. Herit Sci. https://doi.org/10.1186/s40494-022-00687-5
Kusinski J, Kac S, Kopia A, Radziszewska A, Rozmus-Górnikowska M, Major B, Major L, Marczak J, Lisiecki A (2012) Laser modification of the materials surface layer—a review paper. Bull Pol Acad Sci 60:711. https://doi.org/10.2478/v10175-012-0083-9
Žemaitis A, Mimidis A, Papadopoulos A, Gečys P, Račiukaitis G, Stratakis E, Gedvilas M (2020) Controlling the wettability of stainless steel from highly-hydrophilic to super-hydrophobic by femtosecond laser-induced ripples and nanospikes. RSC Adv 10:37956. https://doi.org/10.1039/d0ra05665k
Pizzi A, Mittal KL (eds) (2018) Handbook of adhesive technology. CRC Press, Boca Raton. https://doi.org/10.1201/9781315120942
Kohli R, Mittal KL (eds) (2019) Developments in surface contamination and cleaning: applications of cleaning techniques, vol 12. Elsevier. New York. https://doi.org/10.1016/C2017-0-03847-8
Benor A, Takizawa S, Pérez-Bolívar C, Anzenbacher P (2010) Efficiency improvement of fluorescent OLEDs by tuning the working function of PEDOT:PSS using UV–ozone exposure. Org Electron 11:938. https://doi.org/10.1016/j.orgel.2010.02.014
Hu T, Zhang F, Xu Z, Zhao S, Yue X, Yuan G (2009) Effect of UV–ozone treatment on ITO and post-annealing on the performance of organic solar cells. Synthetic Metals 159:754. https://doi.org/10.1016/j.synthmet.2008.11.024
Zin N, Bakhshi S, Gao M, Ali H, Kashkoush I, Schoenfeld WV (2019) Effective use of UV-ozone oxide in silicon solar cell applications. Phys Status Solidi RRL 13:1800488. https://doi.org/10.1002/pssr.201800488
Choi K, Lee C (2003) Removal efficiency of organic contaminants using ECR H2 plasma and ECR O2 plasma. J Korean Phys Soc42:702. https://doi.org/10.1016/S0169-4332(02)01215-1.
Kim SY, Lee J-L, Kim K-B, Tak Y-H (2004) Effect of ultraviolet–ozone treatment of indium–tin–oxide on electrical properties of organic light emitting diodes. J Appl Phys. 95:2560 https://doi.org/10.1063/1.1635995.
Kuan-**ang Z, wen W, Jian-Hua Z, Ji-Lai H, Yan Z, Ying-Cui F (2012) Hydrophilicity difference of TiO2 thin films induced by different plasmas. Phys Procedia 32:356. https://doi.org/10.1016/j.phpro.2012.03.569
Zhang X, Yao J, Ali M, Wei J, Wang H, Yeo LY, Friend JR, MacFarlane DR (2014) UV/ozone-assisted low temperature preparation of mesoporous TiO2 with tunable phase composition and enhanced solar light photocatalytic activity J. Mater. Chem. A 2:18791. https://doi.org/10.1039/C4TA04020A
Egitto FD, Matienzo LJ (2006) Transformation of poly(dimethylsiloxane) into thin surface films of SiOx by UV/Ozone treatment. Part I: factors affecting modification. J Mater Sci 41:6362. https://doi.org/10.1007/s10853-006-0712-5
Fu Y-J, Qui H, Liao K-S, Lue SJ, Hu C-C, Lee K-R, Lai J-Y (2010) Effect of UV-ozone treatment on poly(dimethylsiloxane) membranes: surface characterization and gas separation performance. Langmuir 26:4392. https://doi.org/10.1021/la903445x
Graubner V-M, Jordan R, Nuyken O, Schnyder B, Lippert T, Kötz R, Wokaun A (2004) Photochemical modification of cross-linked poly(dimethylsiloxane) by irradiation at 172 nm. Macromolecules 37:5936. https://doi.org/10.1021/ma049747q
Ma K, Rivera J, Hirasaki GJ, Biswal SL (2011) Wettability control and patterning of PDMS using UV-ozone and water immersion. J Colloid Interface Sci 363:371. https://doi.org/10.1016/j.jcis.2011.07.036
Strobel A, Schnabel H-D, Reinhold U, Rauer S, Neidhardt A (2016) Room temperature plasma enhanced atomic layer deposition for TiO2 and WO3 films. J Vacuum Sci Technol A 34:01A118. https://doi.org/10.1116/1.4935356
Lamour G, Hamraoui A, Buvailo A, **ng Y, Keuleyan S, Prakash V, Eftekhari-Bafrooei A, Borguet E (2010) Contact angle measurements using a simplified experimental setup. J Chem Educ 87:1403. https://doi.org/10.1021/ed100468u
Ertis IF, Boz I (2017) Synthesis and characterization of metal-doped (Ni, Co, Ce, Sb) CdS catalysts and their use in methylene blue degradation under visible light irradiation. MRC 06:1. https://doi.org/10.4236/mrc.2017.61001
Kim H-J, Piao M-H, Choi S-H, Shin C-H, Lee Y-T (2008) Development of amperometric hydrogen peroxide sensor based on horseradish peroxidase-immobilized poly(thiophene-co-epoxythiophene). Sensors (Basel, Switzerland) 8:4110. https://doi.org/10.3390/s8074110
Mason MG, Hung LS, Tang CW, Lee ST, Wong KW, Wang M (1999) Characterization of treated indium–tin–oxide surfaces used in electroluminescent devices. J Appl Phys 86:1688. https://doi.org/10.1063/1.370948
Schneemilch M, Quirke N (2007) Effect of oxidation on the wettability of poly(dimethylsiloxane) surfaces. J Chem Phys 127:114701. https://doi.org/10.1063/1.2770723.
Thompson TL, Yates JT (2005) TiO2-based photocatalysis: surface defects, oxygen and charge transfer. Top Catal 35:197. https://doi.org/10.1007/s11244-005-3825-1
Thompson TL, Yates JT (2006) Surface science studies of the photoactivation of TiO2–new photochemical processes. Chem Rev 106:4428. https://doi.org/10.1021/cr050172k
Acknowledgements
We want to thank Prof. Dr. Stefan Zigan for the constructive exchange of ideas and discussions as well as for the many helpful hints on form and spelling.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was provided by Freistaat Sachsen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weber, D., Heimburger, R., Schondelmaier, G. et al. Cost-effective equipment for surface pre-treatment for cleaning and excitation of substrates in semiconductor technology. SN Appl. Sci. 5, 21 (2023). https://doi.org/10.1007/s42452-022-05219-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05219-1