Abstract
Acid mine drainage (AMD) which produced in the process of mining seriously pollutes the water resources and endangers the ecological environment due to its physicochemical characteristics, such as low pH, high salinity and high heavy metal concentrations. In recent decades, the treatment of AMD has become a key issue in the field of environmental protection. One important method of AMD treatment is adsorption method, and the selection of adsorbent is the key of this technique. Bentonite and steel slag are usually sintered at high temperatures to prepare bentonite–steel slag composite. AMD treatment with bentonite–steel slag composite, as a new adsorbent, is emerging as a promising treatment method by physical adsorption, ion exchange and chemical neutralization. The bentonite–steel slag composites mainly include bicomponent composite with bentonite–steel slag and multicomponent composite with bentonite–steel slag modifier. The author found that this important research question was rarely paid attention to, therefore, and the author combined with previous research and theories to promote the explanation of this problem. In this review, the technology of treatment of AMD with bentonite–steel slag composite is comprehensively discussed. Also, the role of its mechanism is also discussed in-depth. This paper provides a scientific reference on the remediation of contaminated environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mineral resources are the lifeblood of human society and national economic development. At present, more than 200 kinds of mineral resources have been discovered in the world, mainly in Russia, the USA, Saudi Arabia, Canada, China and other countries, among which coal, oil and natural gas are equivalent to 120 trillion tons of standard coal [6]. Acid mine drainage (AMD) is produced in the process of mining and seriously endangers the health of plants, animals and human. One important method of AMD treatment is adsorption method. AMD treatment with bentonite–steel slag composite, as a new adsorbent, is emerging as a promising treatment method by physical adsorption, ion exchange and chemical neutralization [21].
Steel slag is a by-product of industrial steelmaking and mainly composed of Ca3SiO5, Ca2SiO4, Ca2Fe2O5, CaFeO4, MgFe2O4 and FeO [29]. It can be used as a water treatment agent to adsorb heavy metal ions. Its adsorption mechanism is mainly physical adsorption and chemical adsorption. The physical adsorption mainly depends on the van der Waals force between steel slag and pollutants. The physical adsorption of steel slag is determined by its specific surface area and porosity. The larger the specific surface area and the more voids, the stronger the physical adsorption capacity (Yan et al. 2018). When there are electron transfer, chemical bond formation and fracture between adsorbate and adsorbent, it is called chemical adsorption. Chemical adsorption mainly includes chemical precipitation, reduction, cation exchange and surface coordination. According to different steelmaking methods, steel slag can be divided into alkaline oxygen furnace slag, electric arc furnace slag, blast furnace slag and modified steel slag. Steel slag of alkaline oxygen furnace is the product of further refining iron in alkaline oxygen furnace. Electric arc furnace steel slag is the melting product of waste materials recovered in electric arc furnace. Blast furnace slag is a by-product in the process of blast furnace ironmaking. The modified steel slag can improve the removal efficiency of pollutants in water. The modified steel slag mainly includes fast cooling alkaline oxygen furnace steel slag and thermally modified steel slag. The constructed wetland with alkaline oxygen furnace steel slag, electric arc furnace steel slag and blast furnace steel slag as the matrix has a higher removal rate of total phosphorus in sewage, and the removal rate of total phosphorus in simulated sewage is greater than that in actual sewage. Steel slag, like clay minerals, is porous, has large surface area, contains more alkali metal elements and has strong adsorption on ammonia nitrogen. Therefore, the application of steel slag in the constructed wetland can enhance the removal of nitrogen in sewage. The content of calcium and magnesium in steel slag is large, and the metal oxides and hydroxyl oxides on the surface of steel slag can adsorb a small amount of phosphorus [12]. Ca (OH)2 in steel slag is dissolved in water, and the released Ca2+ precipitates with phosphate to form calcium phosphate, so as to promote the removal of phosphorus in sewage by constructed wetland. By mixing steel slag with other substrates, the particle size of the substrate can be changed and the removal efficiency of organic matter in sewage can be improved. Because of the component characteristics of steel slag, steel slag has the ability of adsorption and neutralizing the acidity in AMD [39].
Therefore, it can effectively remove the pollutants in AMD. For example, the steel slag filter was constructed to treat AMD at the abandoned Mc Carty mine site in West Virginia, and the wastewater was discharged into the tributaries after treatment [8]. The steel slag filter was also used to treat AMD in southeastern Ohio [5]. In some mining areas, the reverse osmosis system made of steel slag, lime and soda ash was used to treat AMD [18]. Although there are some applications of steel slag in the treatment of AMD, the adsorption capacity of steel slag needs to be improved. Therefore, steel slag can be combined with other adsorbents such as lignite and Bentonite [30]. Bentonite and steel slag have similar mineral composition and different constituent content which changes with the producing area. For example, the chemical composition of bentonite and steel slag of Liuzhou steel mill in Guangxi, China, is shown in Table 1 [28]
In recent years, bentonite and steel slag are often combined by sintering to be used in mine environment restoration [26]. In 2019, Wang Guifang et al. combined Ca-bentonite with steel slag to treat AMD containing Cu2+, and the wastewater after treatment reached China's comprehensive sewage discharge standard (GB 8978-1996) [28]. Thus, it is a new direction for AMD treatment with bentonite and steel slag composite (B-SS). And, the characteristic of this material is high reserves, wide sources and low price [4]. Some researchers in China have been working on the research of B-SS composites to treat AMD. Although it has not been widely applied in the field, these composites will be of great research value. The author sorted out the method of treating AMD with bentonite and steel slag on most journal database. The B-SS composites for treating AMD include bicomponent composites with bentonite–steel slag and multicomponent composites with bentonite–steel slag modifier.
2 Bicomponent composites with bentonite–steel slag
Bentonite and steel slag have large specific surface area and high adsorption capacity. Also, K+, Mg2+ and Na+ in bentonite can exchange with the wastewater. Furthermore, the basic oxide in the steel slag can neutralize the acid in the solution. Therefore, bentonite and steel slag composites are suitable for the treatment of AMD produced in mining activities. Some researchers sinter the bentonite and steel slag into particles to treat AMD and achieve good results and neutralization of acid in wastewater. The following research mainly analyzes the removal of Fe2+, Mn2+, Cu2+, Zn2+ ions and the treatment of waste acid.
2.1 Treatment of AMD containing Cu2+
Kakaei S et al. modified the bentonite with imidazole compounds to treat Cu2+ in the wastewater. After 30 min, the saturated adsorption capacity of cu2+ reached 88.60 mg/g [9]. In this experiment, organic compounds were incorporated into the bentonite structure, which improved the specific surface area of bentonite. Although the modified bentonite can effectively adsorb Cu2+, the acid in the wastewater is not treated, and the other agents are needed to neutralize the acidity.
At present, some scholars studied the composites that can fully absorb Cu2+ in the wastewater and neutralize the acidity of the solution. Luan Xuefei sintered Na-bentonite and steel slag with mass ratios of 5:5 and 8:2, respectively, to make powdery composites [31, 32]. The data of bentonite and steel slag are shown in Table 2.
In this experiment, the concentration of Cu2+ was about 50 mg/L, pH value was 3.5 ~ 4, and constant temperature was 25℃. The two powdery composites were injected into the AMD at 2 g/L, the removal rate of Cu2+ reached 95.8%, 93.94%, and the pH value of the wastewater was 8.67 and 7.21, respectively. The surface of B-SS composite materials was rough by SEM analysis, which significantly increased the specific surface area and the number of adsorption sites [10]. This process was relatively consistent with the pseudo-second-order kinetic model, indicating that the adsorption process was dominated by chemical adsorption, supplemented by micro-diffusion of particles and liquid film [2].
Wang guifang et al. selected Ca-bentonite and steel slag to make composite particles to treat AMD containing Cu2. The data of bentonite and steel slag are shown in Table 3. The pH values of bentonite and steel slag were 8.27 and 11.59, respectively, which were alkaline.
In this experiment, the concentration of Cu2+ was about 200 mg/L, the pH value was 5 in the AMD, and the mass ratio of bentonite to steel slag was 0:10, 3:7, 5:5, 7:3 and 10:0, respectively [28]. After the treatment, the removal rate of Cu2+ by 3:7 and 5:5 ratio composites was 99.96%, higher than that of single material, and the pH value of the wastewater by 3:7 ratio composite material was 7.31, which met China's comprehensive sewage discharge standard (GB 8978-1996).
The above AMD treatment with B-SS composites increased the pH of the wastewater and inhibited the adsorption competition between H+ and metal ions, and precipitated some contaminants. Different compositions of bentonite and steel slag lead to different adsorption effects. And this method solved the problem that Kakaei S cannot neutralize the acidity of the solution. Furthermore, if the acidity of the wastewater is very high, the mass ratio of the steel slag material should be further increased. When the acid concentration of the wastewater is high, it is uneconomical to completely use steel slag to neutralize the acidity, and the alkali solution needs to be added to neutralize the wastewater. The appropriate pH value needs to be further discussed.
2.2 Treatment of AMD containing Mn2+
In recent years, some researchers studied the adsorption of Mn2+ with new mineral adsorbents from a wide range of sources [19]. In 2010, Silva AM et al. used the limestone to adsorb Mn2+ in AMD [20]. The final concentration of Mn2+ in the treated wastewater was less than 1 mg / L, and the pH value was about 5.5. Zuo Weiyuan used bentonite and activated carbon to adsorb Mn2+ in AMD. The initial concentration of Mn2+ in the AMD was 50 mg / L, and the pH was 6. After treatment, the saturated adsorption capacity of the adsorbent reached 27.78 mg/g [41]. The above two adsorbents cannot neutralize the acidity of the solution, so the adsorbents can be combined with the steel slag to treat AMD.
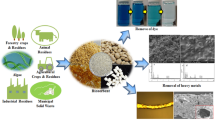
** et al. mixed Na-bentonite with steel slag powder at the mass ratio of 5:5, then sintered them with 5% Na2CO3 at high temperature. The data of bentonite and steel slag are shown in Table 4. The composites were put into a cylindrical container to treat AMD. The dynamic device diagram of three kinds of adsorbent material is shown in Fig. 1.
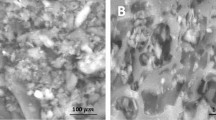
When the wastewater flew through the container at the rate of 1 mL/min, after treatment, the concentration of Mn2+ was about 100 mg/L and the pH value was 3 ~ 3.5, the color of the material gradually deepened, and the adsorption capacity of Mn2+ reached 28.871 mg/g, and the pH value of the wastewater was about 6 after 12 days [37]. The alkaline oxides in the steel slag of the composites, such as MgO and CaO, hydrolyzed to release OH− and Mn2+ which was oxidized by O2 in the air to brown precipitate Mn(OH)2. Also, OH− gradually neutralized H+ to reduce the acidity of the wastewater. The bentonite in the composite material has a special layered structure (Fig. 2) [11], which enables it to adsorb through Van der Waals force and chemical bonding force.
The above B-SS composites can significantly remove Cu2+ and neutralize the acidity of the AMD. However, as time goes on, the ability of the composites to neutralize the acidity gradually decreases, and the adsorption capacity reaches the maximum. Manganese is one of the pollutants widely distributed and seriously harmful in the leakage groundwater of uranium mine tailings pond. It is suggested that the coagulant is added to B-SS particles to make it react with manganese ions in AMD, so as to form flocs and improve the removal rate of manganese as much as possible. The common coagulants include aluminum salt and iron salt, which are inorganic coagulants. These coagulants are generally used in large quantities and at low prices.
2.3 Treatment of AMD containing Zn2+
Mishraa P C et al. treated AMD containing Zn2+ with bentonite, steel slag and fly ash [17]. In this experiment, the maximum adsorption of Zn2+ with bentonite and fly ash was higher than the steel slag. The adsorption capacity of the adsorbents was obviously affected by the pH value of the solution. The optimal experimental conditions were a pH of 6. When the pH of AMD was relatively high, the H+ inevitably inhibited the adsorption effect of each adsorbent.
** sintered bentonite and steel slag with the mass ratio of 5:5 and 8:2 to make two composites as adsorption materials. In this experiment, the concentration of Zn2+ was about 50 mg/L and the pH value was 3 ~ 4 in the AMD. As the dosage of the composites was 0.5 g, the removal rate of Zn2+ by 8:2 ratio was 85.86%, higher than the mass ratio of 5:5. As the dosage was 1.0 g, the removal rate of Zn2+ was up to more than 99%, and with the increase in time, the removal rate increased slowly. The composites had a larger specific surface area, which made Zn2+ combine with Zn–Si–O as minerals and polymerize and precipitate with macromolecular exfoliates [31, 32]. And the powder material significantly improved the pH of solution, which neutralized H+ and prevented the competitive adsorption of H+ and Zn2+ in the wastewater. When the mass ratio of bentonite to steel slag was 8:2, the composite had higher removal rate of Zn2+ and lower neutralization ability of water acidity. After treatment, the removal rate of Zn2+ and pH met China's comprehensive sewage discharge standard (GB 8978-1996). The specific surface area of the composites has further influence on the adsorption effect, but the experiment still lacks the comparison of shape influencing factors, so it is suggested to further study its microscopic mechanism. By sintering the adsorbed particles, this type of adsorbent can be well fixed in the sewage area, which is convenient for field application. However, if applied in the field, whether the adsorbent formed by sintering is convenient for regeneration is a restrictive problem.
2.4 Treatment of AMD containing Fe2+
In 2019, Francisca F M studied the application of basic slag in the environmental protection. The permeation barrier made of the basic slag could absorb contaminants Fe2+ from the wastewater and increase the pH of the solution [3]. In order to improve the adsorption, bentonite can be added into the barrier.
** et al. mixed bentonite and steel slag with the mass ratio of 5:5 and added the binder to make composite particles to treat AMD with shock adsorption method [33,34,35,36]. When a variety of metal ions coexisted, the adsorption interference and competition occurred. The electronegativity of Cu2+ was slightly higher than that of other ions, so the adsorption capacity of Cu2+ was slightly higher, and the hydration radius of Cu2+ was small, so the removal rate of Cu2+ was better than that of other ions.
For the treatment of AMD containing a variety of ions, the effect of pH change, material proportion, material shape and other factors on the treatment results was not thoroughly studied. In order to promote the wide application of this technology in AMD processing, it is necessary to conduct further research on the above problems (Weng and Hsu 2017).
2.6 Comparison of the results of treating AMD with bentonite–steel slag composites
Based on the above research results, the comprehensive effect of B-SS composites treating AMD containing Fe2+, Mn2+, Cu2+ and Zn2+ is significantly better than that of single modified bentonite, steel slag and limestone etc. The results of treating AMD with B-SS composites and some single modified adsorbents are shown in Table 6.
As can be seen from Table 6, during the treatment of AMD with B-SS composite, the removal rate of metal ions gradually increase with the increase in composite dosage. The shape of the composites affects the specific surface area of the material, and the larger specific surface area is beneficial to the adsorption of metal ions. When the bentonite and steel slag compound at the mass ratio of 5:5 and 8:2, composite materials can fully absorb metal ions. And, if the pH of AMD is too high, it is necessary to readjust the composite proportion of materials. It is most important to note that for the materials to be called adsorbent, it must not change the pH of the solution and it must be stable and never under goes mass change that can help to determine the adsorption rate.
The hydrolysis of basic oxides in steel slag can change the pH of wastewater, inhibit the competitive adsorption of H+ and facilitate ion exchange. The steel slag shows good properties in all aspects in this removal process, but if the dosage of steel slag is too high, it will increase the pH of the wastewater. The bentonite and steel slag need to be pretreated in the process of treating AMD and choose the optimal mass ratio. Although steel slag has many advantages, it is difficult to produce adsorption products with uniform particle size and performance due to different particle sizes of steel slag products [40]. Therefore, the effect of granular materials with different particle size in the treatment process should be considered in the current research and compared.
3 Multicomponent composites with bentonite–steel slag modifier
Although bicomponent composite with bentonite–steel slag has a good effect in treating AMD, it should be further optimized and modified by researchers in order to solve the complex environmental pollution problems in mines, improve the utilization efficiency of materials, and reducing the costs. The modification of bentonite and steel slag materials has been studied in a large amount in the world. The application of modifier combined with B-SS composite material to AMD treatment will be an important research direction in the environmental protection of mine.
3.1 Multicomponent composites with bentonite–steel slag polymer
Some scholars had prepared multicomponent composites with bentonite–steel slag polymer to treat AMD. In 2016, Sato K modified Na-bentonite with polymer, which had a positive effect on the swelling of bentonite and improved its adsorption performance (Sato et al. 2017). Merino D et al. prepared amine modified polyacrylamide / bentonite composite (Am-PAA-B) by intercalation in situ polymerization to adsorb Cu2+ in the wastewater, the removal rate reached 99.0% at pH of 5 [14]. In this experiment, the composites significantly improved the adsorption capacity.
Li Ying et al. prepared composite particles with Na-bentonite and steel slag powder according to the mass ratio of 5:5, and treated AMD containing Mn2+ with a concentration of about 50 mg/L and a pH value of 3 ~ 3.5 at 25℃ [33,34,35,36]. In this experiment, the saturated removal rates of Mn2+, Cu2+ and Zn2+ were 94.22%, 83.2% and 76.30%, respectively, after 650 h. The removal rates of Mn2+, Cu2+ and Zn2+ after regeneration were 74.14%, 16.28% and 40.28% higher than single bentonite particles. After regeneration, the adsorption capacity of Fe2+ decreased a lot. The SEM of saturated and regenerated B-SS composites adsorbed Fe2+ and Mn2+ was tested [7]. After regeneration of adsorbent absorbed Fe2+, the framework of montmorillonite structure collapsed, making it unable to recover the adsorption capacity. After regeneration of adsorbent absorbed Mn2+, the structure of montmorillonite remains unchanged. The metal ions form sulfide precipitation and fixed, and the adsorbent of composite particles is simultaneously regenerated and fixed. In order to improve the reuse efficiency of bentonite–steel slag composites, more regeneration agents should be studied.
4 Conclusion
The development and production of mineral resources inevitably cause serious environmental pollution. The method to treat AMD with bentonite–steel slag composites is very economic, environmentally friendly and efficient. This paper reviews the technology to treat AMD and puts forward the following conclusions and suggestions.
-
1.
The adsorption effect of bentonite–steel slag composites on Fe2+, Mn2+, Cu2+ and Zn2+ in AMD is better than that of single modified bentonite and steel slag material. In the process of treating AMD, many problems have not been thoroughly studied. It is suggested that the further research should focus on composite proportion of bentonite and steel slag, the influence of material shape on adsorption performance, the influence of operating conditions on adsorption performance and the synthesis mechanism. The technology will be a new direction of the treatment for AMD.
-
2.
The removal rate and removal time of metal ions in AMD by multicomponent composites with bentonite–steel slag polymer have accuracy improvement than that by bicomponent composites with bentonite–steel slag material. It is suggested to carry out the research on the compound of various polymer compounds with bentonite and steel slag, to further study the compound mechanism, improve the surface area of the compound, maintain the stability of the composite material, and make full use of the characteristics of the polymer material to treat AMD.
-
3.
The bentonite–steel slag composite is suitable for reducing bacteria to reproduce under specific environmental conditions. Reducing bacteria combine with metal ions to form precipitation, make adsorbent regeneration, and fix metal ions. This method has a large treating capacity for AMD. It is suggested to carry out a variety of reducing bacteria and bentonite–steel slag composites to treat AMD, further study the reproduction of reducing bacteria in the composites, the mechanism of different reducing bacteria composites.
-
4.
In the process of treating AMD by multi-component composites with bentonite–steel slag-metal salt, metal salts enable the adsorbent to be fixed and regenerated. Na2S can significantly improve the regeneration adsorption effect of the composites on Mn2+, Cu2+ and Zn2+, except Fe2+. To improve the reuse of bentonite–steel slag composites, it is suggested to carry out the experiment of regeneration effect of various metal salts on bentonite–steel slag composites, further study the theory of regeneration, to find effective, cheap metal salt which is used for regenerating the adsorbents.
Change history
30 April 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s42452-022-05041-9
References
Bengtsson A, Pedersen K (2017) Microbial sulphide-producing activity in water saturated Wyoming MX-80, Asha and Calcigel bentonites at wet densities from 1500 to 2000kgm−3. Appl Clay Sci 137:203–212
Bhattacharyya KG, Gupta SS (2007) Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: Influence of acid activation. J Colloid Interface Sci 310(2):411–424
Francisca FM, Glatstein DA (2019) Environmental application of basic oxygen furnace slag for the removal of heavy metals from leachates. J Hazard Mater. https://doi.org/10.1016/j.jcis.2005.01.073
Fisher LV, Barron AR (2019) The recycling and reuse of steelmaking slags-a review. Resour Conserv Recycl 146:244–255
Goetz ER, Riefler RG (2014) Performance of steel slag leach beds in acid mine drainage treatment. Chem Eng J 240:579–588
Hyeong K, Ju SJ, Billett D, Rowden A (2018) Preface for the special volume on exploration and environmental considerations of deep seabed mineral resources. Ocean Sci J 53(2):285–286
Huling SG, Hwang S (2010) Iron amendment and Fenton oxidation of MTBE-spent granular activated carbon. Water Res 44(8):2663–2671
Jennifer S, Paul Z, D. Courtney B, (2002) Use of steel slag leach beds for the treatment of acid mine drainage. Mine Water Environ 21(2):91–99. https://doi.org/10.1007/s102300200024
Kakaei S, Khameneh ES, Rezazadeh F, Hosseini MH (2019) Heavy metal removing by modified bentonite and study of catalytic activity. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.126989
Luan XF (2016) The mechanism of acid mine drainage containing heavy metal ions by bentonite-steel slag composite particle, MSc thesis, Liaoning Technical University
Li Y (2017) The dynamic experimenta study on the treatment of Fe2+, Mn2+ in acid mine drainage by bentonite and steel slag composite partical, MSc thesis, Liaoning Technical Univ
Liu J, Li C (2019) Experimental study on flocculant treatment of mineral processing wastewater containing heavy metal lead and zinc. Appl Chem Eng 48(5):1114–1118
Mohapatra D, Mishra D, Chaudhury GR, Das RP (2007) Arsenic adsorption mechanism on clay minerals and its dependence on temperature. Korean J Chem Eng 24(3):426–430
Merino D, Mansilla AY, Casalongue CA, Alvarez VA (2019) Effect of nanoclay addition on the biodegradability and performance of starch-based nanocomposites as mulch films. J Polym Environ 27(9):1959–1970
Meng FQ (2017) Polymer modified bentonite and its absorption of orange II, MSc thesis, **an University
Motamedi M, Karland O, Pedersen K (1996) Survival of sulfate reducing bacteria at different water activities in compacted bentonite. FEMS Microbiol Lett 141(1):83–87
Mishra PC, Patel RK (2009) Removal of lead and zinc ions from water by low cost adsorbents. J Hazard Mater 168(1):319–325
Masindi V, Osman MS, Abu-Mahfouz AM (2017) Integrated treatment of acid mine drainage using bof slag, lime/soda ash and reverse osmosis (ro): implication for the production of drinking water. Desalination 424:45–52
Pawar R, Gupta P (2018) Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int J Biol Macromol 114:1315–1324
Silva AM, Cruz FLS, Lima RMF, Teixeira MC (2010) Manganese and limestone interactions during mine water treatment. J Hazard Mater 181(1/2/3):514–520. https://doi.org/10.1016/j.jhazmat.2010.05.044
Savun B, Gineste C (2019) From protection to persecution: threat environment and refugee scapegoating. J Peace Res 56(1):88–102
Singh CK, Sahu JN, Mahalik KK, Mohanty CR, Mohan BR, Meikap BC (2008) Studies on the removal of Pb(II) from wastewater by activated carbon developed from Tamarind wood activated with sulphuric acid. J Hazard Mater 153(1–2):221–228
Setiadi EA, Sebayang P, Ginting M, Sari AY, Simamora P (2016) The synthesization of Fe3O4 magnetic nanoparticles based on natural iron sand by co-precipitation method for the used of the adsorption of Cu and Pb ions. J Phys Conf Ser 776(1):012020
Sato K, Barast G, Razakamanantsoa AR, Irini DM, Katsumi T, Levacher D (2016) Comparison of prehydration and polymer adding effects on Na activated Ca-bentonite by free swell index test. Appl Clay Sci 142(2017):69–80
Tohdee K, Kaewsichan L (2018) Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. J Environ Chem Eng 6(2):2821–2828
Toor M, ** B, Dai S, Vimonses V (2015) Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J Ind Eng Chem 21:653–661
Weng CH, Hsu MC (2008) Regeneration of granular activated carbon by an electrochemical process. Sep Purif Technol 64(2):227–236
Wang GF, Chen C, Meng GD, Li HJ, Wei L, Tang CH (2018) Study on treatment of mine wastewater by new bentonite composite. Non-Metallic Mines 41(06):7–9
Wu QS, Gu XH, Yang T, Zhang CS, Min ZA, Wu Y (2019) Analysis of mechanical performance and microstructure of steel slag processed with accelerated carbonation. Mater Sci Forum 944:1240–1251
Westholm LJ, Repo E, Sillanp M (2014) Filter materials for metal removal from mine drainage-a review. Environ Sci Pollut Res 21(15):9109–9128
**ao LP, Luan XF, Bai JC, Guo Y (2015) The adsorption-precipitation effect on the treatment of acid mine drainage containing cu2+ by environment mineral material. Non-Metallic Mines 5:71–73
**ao LP, Liu Y, Qiu QH, Bai JC (2015) Bentonite-slag Composite for Treatment of Acid Mine Wastewater Containing Zn2+. Non-Metallic Mines 38(03):80–82
**ao LP, Liu Z, Wei B (2016) Study on Fe2+ adsorption mechanism by bentonite-steel slag composite particles. Non-Metallic Mines 39(04):43–45
**ao LP, Geng XH, Pei G (2016) Immobilization and regeneration of heavy metal ions by composite saturated bentonite adsorbent. Chin J Environ Eng 10(4):1645–1650
**ao LP, Li Y, Liu Z (2016) Adsorption –coagulation of acid mine wastewater containing Mn2+ by environment mineral material and PAM. Non-Metallic Mines 39(2):28–30
**ao LP, Geng XH, Pei G (2016) Treatment of acid mine drainage by bentonite composite particles cooperate with SRB. Chin J Environ Eng 10(11):6457–6463. https://doi.org/10.12030/j.cjee.201506182
**ao LP, Song JG, Wang RJ, Li Y (2018) Treatment of Mn2+ acid mine wastewater by fixed bed adsorption. Chin J Environ Eng 12(02):475–481
Yan YS, Li YF, Zhao LB (2019) Research status of heavy metal ions adsorption by modified steel slag. Multipurp Util Mineral Resour 01:8–13
Yang HF, Fu PF, Zhou F (2008) Adsorption and reduction of Cr(VI) in water by steel slag particles. J Process Eng 3:499–503
Zhao J, Yan P, Wang D (2017) Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J Clean Prod 156:50–61
Zuo WY, Tian HJ, Shi BF (2016) Adsorption of Mn(II) by bentonite-activate carbon compound adsorbent. Inorg Chem Ind 48(7):58–62
Funding
This study is financially supported by Department of Education of Liaoning Province (L2020026) and Science and Technology Department of Liaoning Province (202102).
Author information
Authors and Affiliations
Contributions
Data collection and analysis were performed by Le Tong and Qiushi Zhang. The first draft of the manuscript was written by Yi Pan, Shuangchun Yang and Ronggui Fan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest/The authors declare that they have no competing interests.
Consent for publication
Written informed consent for publication was obtained from all participants.
Availability of data and material (data transparency)
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s42452-022-05041-9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tong, L., Fan, R., Yang, S. et al. RETRACTED ARTICLE: A technology review on treatment of acid mine drainage with bentonite–steel slag composite. SN Appl. Sci. 4, 10 (2022). https://doi.org/10.1007/s42452-021-04888-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04888-8