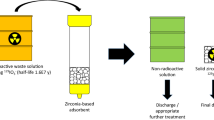
Abstract
Abstract
Iodine-129 poses a significant challenge in the drive towards lowering radionuclide emissions from used nuclear fuel recycling operations. Various techniques are employed for capture of gaseous iodine species, but it is also present, mainly as iodide anions, in problematic residual aqueous wastestreams, which have stimulated research interest in technologies for adsorption and retention of the radioiodine. This removal effort requires specialised adsorbents, which use soft metals to create selectivity in the challenging chemical conditions. A review of the literature, at laboratory scale, reveals a number of organic, inorganic and hybrid adsorbent matrices have been investigated for this purpose. They are functionalised principally by Ag metal, but also Bi, Cu and Pb, using numerous synthetic strategies. The iodide capacity of the adsorbents varies from 13 to 430 mg g−1, with ion-exchange resins and titanates displaying the highest maximum uptakes. Kinetics of adsorption are often slow, requiring several days to reach equilibrium, although some ligated metal ion and metal nanoparticle systems can equilibrate in < 1 h. Ag-loaded materials generally exhibit superior selectivity for iodide verses other common anions, but more consideration is required of how these materials would function successfully in industrial operation; specifically their performance in dynamic column experiments and stability of the bound radioiodine in the conversion to final wasteform and subsequent geological storage.
Article highlights
-
Metallated adsorbents for the capture and retention of radioiodine in the nuclear industry are assessed.
-
The strengths and weaknesses of organic, inorganic and hybrid support matrices and loading mechanisms are discussed.
-
Pathways for progression of this technology are proposed.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Radioiodine in recycling used nuclear fuel
The role of nuclear technology, in reducing carbon emissions, is growing ever more important, with global power output of the industry projected to increase by 55% before 2040 [1]. There is an international drive to increase recycling of used nuclear fuel (UNF), to access the energy potential of the remaining uranium-235 and plutonium-239 [2]. At the same time, the industrial release of chemical species into the environment, particularly radionuclides, is coming under unprecedented scrutiny. A 2018 Nuclear Energy Agency (NEA) report stated a target for “Near Zero” future radionuclide discharges from advanced recycle processes to meet future waste management and environmental impact requirements [3].
There are a number of challenging volatile (tritium, carbon-14, krypton-85) and semi-volatile (technicium-99, ruthenium-106, cesium-137) species among the fission products of U-235. Pre-eminent though, are four isotopes of iodine, of which iodine-129 and iodine-131 are the species of greatest concern, due to their potential for environmental damage and toxicity to humans and other organisms [4, 5]. I-131 radiation is highly ionising, but this isotope has a short half-life of 8.02 days and thus, the main associated threat is environmental release in the event of nuclear accident. By the time UNF enters recycling (typically > 5 years after leaving a reactor), I-131 is no longer a significant hazard. In contrast, the I-129 half-life is 15.7 million years and it is essential that strategies are implemented for effective management of iodine-bearing waste-streams. Considering the aerial radionuclide discharges from the UK’s most recent recycle plant, Thorp [6], I-129 represented ~ 75% of the total calculated absorbed dose from aerial release.
Within the overall challenge of the abatement of radioiodine during used nuclear fuel (UNF) recycling, aqueous removal is particularly noteworthy. Dissolution of UNF in nitric acid (3 M, Eh ≡ 1 V/NHE) typically causes 94–99% of the total iodine species present to volatilise and enter the dissolver off-gas stream [7]. This fraction is captured either by wet caustic scrubbing or adsorption on to a solid phase [8]. Gaseous iodine absorption has been extensively researched for decades and is the subject of a number of excellent review articles [7, 9, 10]. Although there remains potential for progression and development in terms of optimisation of adsorbent materials, there are a number of systems in full-scale operational use (at least 11 known) [7].
In contrast, there is currently no effective method for the adsorption of iodine from the aqueous phases of nuclear wastestreams. The iodine speciation immediately downstream of the dissolver is very complicated and includes I−, I2, HIO, IO3− and colloidal species formed by complexation with fission products [11]. This, along with the concentrated HNO3 conditions makes direct iodine removal by adsorption unlikely. Nonetheless, in a typical UNF recycle plant (Fig. 1), the dissolver raffinate travels through a number of separation processes and tanks, with further sequential release of iodine into the off-gas systems [12], meaning there is potential for an aqueous iodine-removal step at numerous stages of unit operations for treatment of the various off-gas condensates. These aqueous streams encompass a wide range of conditions, but generally feature very high dissolved salt content and variable acidity, from 1 M HNO3 [13] to spent caustic scrubbing liquor (pH ~ 14) [14] and even more basic low-activity wastewater (2.43 M OH−) [15]. However, the crux of the issue is that there is no existing process to convert the aqueous iodine scrubbed from off-gas streams into an inert final wasteform. The combination of the labile oxidation state of iodine and reducing conditions encountered in geological disposal tend to enable reductive dissolution and anion-exchange mechanisms, promoting leaching [16, 17]. Because of these challenges, current practice follows a “dilute and disperse” approach, releasing this fraction (after neutralisation) into the ocean [18]. This is done within allowed limits for environmental release and is currently the best available technology (BAT) for “Near Zero” discharge. A “concentrate and contain” strategy may represent a pathway towards lower emissions, but requires both an efficient adsorbent and a feasible wasteform conversion process to be pursued.
1.2 The challenges of aqueous iodine adsorption in the nuclear industry
Although the target iodine species in aqueous waste-streams are generally anionic, the aim of selective remediation via conventional adsorption means is difficult. Whilst an anion-exchange mechanism appears to be the most plausible means of removal, some aqueous liquors within a UNF recycling plant can contain up to 400 g L−1 dissolved solids, in the form of nitrate, nitrite and carbonate [14]. Another major source of competition would be the chelating oxo-anion molybdate, which is the dominant Mo species at pH > 7 [19]. Other common anions present at concentrations exceeding those of iodine species are fluoride, chloride and sulfate [14, 20]. Regardless of where an iodine removal step is placed with respect to unit operations, there will always be significant co-anions present, which can interfere with the extraction. It must also be considered that many of the aqueous streams, from which iodine removal could be attempted, feature extreme pHs [21]. Even mildly acidic conditions change iodine speciation from predominantly iodide to more hydrophobic species, while a high pH entails high concentrations of hydroxide and possibly carbonate anions [22], which can severely limit the exchange of anions on to the solid phase, due to competition for binding sites [23,24,25].
The “go to” technology for a dynamic industrial adsorption process is an ion-exchange (IX) resin column. IX is used widely for water treatment within the nuclear industry [26, 27]. However, the harsh operational conditions already detailed preclude the use of conventional IX technology [23]. The competition from the many coexisting anionic species would cause low loading efficiency, which directly impacts the size of column required and operating lifespan. Furthermore, within this remit, the binding of iodine would be preferably close to irreversible, to ensure its retention in the final wasteform taken by the spent adsorbent. This would for example, likely involve being resistant to significant changes in pH and reducing conditions after the adsorption is completed [16]. Classical IX processes, by their very nature, are reversible and ions intended to be desorbed on exposure to concentrated salt solutions and/or pH changes to allow resins to be regenerated for many cycles of use. There is furthermore a fundamental disparity between the organic matrix of the adsorbent and the target adsorbate. This is because it has historically been shown that the most effective chemistry for radioiodine immobilisation and retention is conversion to a stable iodide salt [7, 15, 28]. Yet, the hydrophobic nature of the resin matrix promotes interactions with more hydrophobic iodine species [23, 29]. This complicates control of speciation in the adsorbent and the final wasteform stability. A further critical problem is the potential for radiolytic breakdown of the organic material and remobilisation of the iodine from the final wasteform. Although the styrene/divinylbenzene resin beads are radiolytically robust, this is still a concern for containment of a long-lived radionuclide such as I-129; particularly since anion-exchange resins are less resistant than cation-exchange [30].
The compatibility of IX resins with both the capture and wasteform ends of the intended process are discussed further in due course.
1.3 “Hard-soft-acid–base” (HSAB) concept
One way of achieving selectivity and strong binding for anion uptake is the use of metal-bearing adsorbents, which function according to hard-soft-acid–base (HSAB) theory. This states that smaller, strongly-polarising “hard” Lewis acids (metal ions) preferentially interact with smaller, less-polarisable “hard” Lewis bases (and visa-versa for “soft” metals and bases). Most metal ions are multivalent and capable of interacting and binding with multiple different species simultaneously, to form complexes via coordinate bonds. Essentially, this is due to proximal energy levels and thus favourable overlap of electron orbital shells. However, the theory can also apply to purely ionic interactions (formation of insoluble salts). The strength of interaction can be approximately measured by the magnitude of stability constants for the resulting complexes and sometimes, but not always, solubility product (Ksp) values for the relevant salts. Similarly to common ion-exchange mechanisms, a Lewis base with high affinity for a metal ion will displace an existing lower affinity species in the form of either a ligand-exchange reaction or salt formation. A brief summary of common hard, soft and borderline metals and ligands is shown in Table 1. Note the classification of iodide and iodate as soft bases.
The potential superiority of the HSAB strategy for selective iodide removal from solution, over conventional IX is shown in Fig. 2.
Key considerations are not only the strength of interaction between metal and anion, but also that between metal and bulk solid-phase. If the second parameter is not of sufficient magnitude (especially in harsh chemical conditions), the metal will desorb from the resin and either an aqueous complex will form, containing the anion of interest, or the insoluble salt will immediately precipitate, either nucleating on the resin surface or simply aggregating in solution [31]. This is not always a problem, depending on the design of the adsorption, but the presence of free fine salt particles in a resin bed would cause pressure drop variations in a dynamic system. For this reason, the adsorbent matrix, as well as the loading metal require careful design.
1.4 Metals for iodine capture
As may be imagined, metallated adsorbents for selective radioiodine capture are restricted to soft metals, to promote favourable binding. Ideally, the affinity for iodine species, principally iodide, needs to be several orders of magnitude above that for the many competing anions that will be present in the wastestream. For this reason, Ag is a popular choice in adsorbent design, due to the remarkably low Ksp of AgI. It is followed, somewhat distantly, by Cu, Bi and Pb. Of the other economically feasible soft metals (Table 1), Hg is a possibility for iodine capture processes, as demonstrated in the Mercurex acidic scrubbing process [7]. However, the labile oxidation states, lipophilicity and general instability of their organometallic complexes [32] make both Hg and Sn less attractive choices. For the four most favoured metals, Table 2 shows the solubility parameters for their iodide salts and other relevant salts that might represent competing reactions in adsorption scenarios.
It should be noted that the data presented in Table 2 should not be considered the sole determining factor in terms of the desired favourable interactions with aqueous iodine. For example, Fe oxyhydroxides have recently been identified as having potential in this remit and cerium has been used historically for iodate immobilisation [35].
In addition, all the other well-known parameters, which characterise a successful adsorbent material, must be considered in any assessment. These include high exchange capacity, fast uptake kinetics, provable performance in dynamic systems and high chemical durability.
Research into the uptake of the fluoride ion, with metallated ion-exchange resins and other metal-bearing adsorbents, which will not be discussed here, is quite extensive [31, 36,37,38]. Surprisingly, relatively few equivalent studies have been devoted to iodide, both in general terms and with a nuclear waste focus. This is possibly because the iodide anion is rather high in the known series of selectivity for conventional anion-exchange resins and in some conditions, established IX technology is sufficient for its effective removal [29, 39]. However, with the remarkable chemical conditions that must be overcome within this remit, the application of HSAB is close to essential [23, 24, 40]. To the best of our knowledge, this is the first comprehensive review of metallated adsorbents for aqueous iodide immobilisation.
1.5 Structure and scope of review
Figure 3 shows a summary of the various popular classes of adsorbent matrices, along with the metallation strategies available to the researcher for each. The review will be divided accordingly. It will not be further sub-divided by choice of loading or compositional metal ions, since the number of metals studied in any detail for this purpose is small and the metals used are similar across all adsorbent classes. Table 3 shows the performance range of each material class across a number of parameters. A more detailed comparison of individual materials is seen in Table S1. The reader is referred to these two tables throughout the review, for corroboration.
It should be noted that, for reasons of brevity, this review will mainly focus on development of the field within the last ~ 20 years. Within this timeframe, the concept of designing novel materials and incorporating soft metals strategically for enhanced iodine selectivity has become firmly established. It should be noted however, that the idea of immobilisation of radioiodine by adsorption certainly predates this timeframe. An excellent summary of earlier developments is seen in the review of Mattigod et al. and references therein [28], which includes some discussion on early novel metal-functionalised materials.
2 Carbon-based materials
2.1 Activated carbon
Activated carbon (AC) offers an attractive range of characteristics as an iodide adsorbent. It is resistant to radiolysis, having extensive delocalised π-electrons. It is highly porous, affording a potentially large number of surface adsorption sites. AC columns are also already deployed in the industry to capture gaseous iodine species [51]. More generally, AC can be manufactured from renewable resources and effectively repurpose ‘waste’ carbon sources, such as biowaste. AC may be impregnated or doped (the two terms being used interchangeably) with Ag(0), rendering the adsorbent capable of reducing iodide concentrations in solution to < 2 g L−1 [52]. Such materials are available commercially [15], but the metallation procedure generally appears to be contact of the AC with an aqueous suspension of Ag(0) nanoparticles, performed in the dark [53, 54]. Ag-doped AC was investigated, without further modification, for removal of radioiodine, but was found to have inferior kinetics (~ 1 day to reach equilibrium) and much lower Ag content than the equivalent zeolite [15]. Ag-impregnated AC (commercially available) has exhibited a high iodide capacity of ~ 88 mg g−1 in optimal uptake conditions. However, this figure was no higher than that achieved for unmodified AC at pH 5 in the same study [55].
Another functionalisation strategy involves the metal being present as AgCl, rather than Ag(0). This type of material was first reported in 1981 by Ho and Kraus [51], with loading achieved by treatment of the AC with aqueous AgNO3, followed by HCl. The material was shown to be viable for small-scale column experiments and it was noted that conversion to AgI occurred throughout the bulk of the precipitated AgCl particles, rather than merely surface layers. However, the kinetics of conversion were slower than expected [51]. More recent work used a similar methodology, with Ag(0)-impregnated AC being converted to an AgCl-loaded material by treatment with HCl, with the objective of increasing stability and reducing Ag leaching. However, this material had low capacity (13 mg g−1) and again slow kinetics (3 days required to reach equilibrium). In column experiments, ~ 10% of the loaded Ag bled from the column, at a pH of 4.5 [41].
AC matrices have also been Cu-loaded, using a hydrothermal method, incorporating a polyethylenimine (PEI) ligand [42]. The Cu was believed to be present as both Cu(0) and Cu2O in the material, which had a moderate capacity (41 mg g−1), but much improved kinetics to the Ag-loaded carbons, with 90% uptake achieved in 20 min. This may be due to the Cu ions being chelated to the remaining ligand, as the material was not heated above the decomposition temperature of PEI [56] and nitrogen was detected in XPS analysis. However, the adsorbent suffered 6% dissolution at moderate pH and the presence of chloride and sulfate ions decreased performance.
The major barriers to the use of ACs appear to be first, the low maximum possible metal loadings, despite high porosity (Table S1). Because of this, studies involving AC tend to attempt the iodine extraction from low concentration solutions < 30 mg L−1, which is an order of magnitude below what would be expected in, for example, a spent caustic scrubbing solution wastestream. The second barrier is the relative weakness of the metal binding by the AC matrix, essentially due to variable and inconsistent functional group distribution on the surface [57]. Indeed, the reversibility of platinum group metal adsorption on to activated carbon is the phenomenon which underpins gold hydrometallurgy [58].
2.2 Ion-exchange resins
Commercial polymeric resins have the advantage of being optimised, over many years of development, for dynamic systems, which can be seen in their iodide uptake performance in simulated column experiments [23]. As mentioned in 1.2., IX resin columns are widely deployed throughout the nuclear industry for water treatment. Therefore there is an existing industry knowledge base concerning their use and implementation. Commercial resins may also be easily modified by treatment with aqueous solutions of metal ions to create iodine affinity. The metals chelate, or otherwise bind to the chosen resin functionality and remain as pseudo organometallic complexes and iodine species may either be bound as ligands, or metal iodides may precipitate in-situ. An example is seen in the work of Decamp & Happel [13], who created an Ag-loaded resin. No information could be found on what functionality was used to immobilise the metal, but the resin was an extraction chromatography medium, so was likely solvent-impregnated. Dynamic experiments were performed, with a 1 M HNO3 feed solution from which the calculated amount of iodine adsorbed was 25 mg g−1, which may again reflect the low Ag loading of 0.75 mass% However, the experimental flow rates were extremely high (5,400–11,200 mL hr−1). Molybdate was necessarily removed from the system using an alumina pre-column.
Another method of creating a metallated IX resin is via adsorption by a chelating functional group [31, 59]. A bispicolylamine resin has been loaded with Cu2+ ions, which was stable at pH 2–10 and adsorbed > 160 mg I− g−1 with a column feed of 50 mg L−1 iodide and 10 molar equivalents of nitrate and molybdate ions [24]. However, because of a REDOX reaction between Cu2+ and I−, the more hydrophobic I3− anion was formed in-situ. This species is known to interact weakly with organic adsorbents by partitioning [24, 60]. This is problematic for the stability of final wasteforms (Fig. 4), as any iodine species not directly bonded to a metal ion would be at risk of volatilising at raised temperature, rather than being converted to a stable metal iodide salt.
Further recent research has demonstrated a different loading technique, in which the commercial resins were treated with an acidic solution of metal ions, followed by NaOH. This caused precipitation of metal oxides upon the adsorbent surface, creating a true hybrid material [35]. Some less common metals were trialled, including Bi, Ce and Fe, with a CeO2-loaded resin proving the most efficient material. The remit of this work was in clean-up on contaminated groundwater, with trace levels of I-129 present mainly as iodate and so direct comparisons to the other two studies here mentioned are not helpful. Nonetheless, it is clear that other species, apart from the four key metals focussed on in this review deserve consideration when identifying an adsorbent for a specific remediation task.
3 Inorganic materials
Metal-bearing inorganics have been extensively researched for the capture of iodine species within both nuclear waste treatment and other remits. Unlike organic materials, they generally are not prone to radiolytic degradation and therefore there are no concerns around breakdown of the final wasteform and release of radionuclides into the environment (even long-lived species, such as I-129). Some inorganic adsorbents are naturally occurring and therefore have lower energy production costs. There are also an attractive range of amenable wasteform options, further discussed in Sect. 4. Most adsorbents used can collectively be termed as “metal-exchanged ceramics” and include aluminas, silicates, zeolites and mordenites. The common method of loading is essentially reaction of aqueous metal ions with vacant -OH groups on the surface of the adsorbent particles [46, 47, 50]. However, the metal ions may also be immobilised by an organic component on the surface [20] and in certain adsorbents, the metals with the iodine affinity constitute the main mineral phase of the adsorbent. The latter category is discussed first.
3.1 Minerals of natural iodine affinity
The most obvious attraction of these materials is that the metal of high iodine affinity actually forms the bulk mineral phase and therefore, the ‘loading’, when compared to a metal-functionalised adsorbent, is very high. Additionally, the number of processing stages required to produce the adsorbent is reduced. For example, argentite (Ag2S) can be synthesised for iodine remediation [15]. Its most obvious attraction is the very high Ag content (~ 90 mass%). However, in competitive studies verses Ag-loaded silicas and zeolites, uptake kinetics were slower, taking 15 days to reach equilibrium. The fundamental flaw is that the Ksp for Ag2S is even lower than for AgI (8.0 × 10–51). Furthermore, it is questionable whether, in a world of dwindling natural resources (especially precious metals), argentite is a sensible choice for permanent radioactive waste sequestration, since most of its Ag content is not gainfully used to capture the iodine.
Minerals composed of other metals, such as Cu, are less disadvantaged in this regard; although Cu is itself a valuable metal with high environmental impacts. Malachite Cu2CO3(OH)2, azurite Cu3(CO3)2(OH)2 and cuprite (Cu2O) have been investigated for iodine affinity [49, 62]. The highest uptake yet reported is 23 mg g−1, with equilibrium taking ~ 4 h [49]. However, significant interferences from chloride, carbonate and hydroxide anions were observed. Cu2O has furthermore been sparingly functionalised with Ag to enhance performance [48]. This increased capacity slightly to ~ 26 mg g−1 and improved both kinetics and selectivity, although carbonate still lessened performance. An interesting photocatalytic oxidation of iodide anions was observed under visible light, which led to I2 being adsorbed on the particle surfaces and again, potential volatilisation issues (Fig. 4). A mineral adsorbent of the composition (BiPbO2(NO3)) has been reported to remove iodide from a simulant spent caustic scrubbing solution (pH 14), although uptake values were not determined [14]. Carbonate was again a problematic interference and equilibrium required ~ 10 h. Nonetheless, the iodide affinity is notable, considering the affinity of both metals in the mineral phase for hydroxide and carbonate ions (Table 2).
An adsorbent type which has generated some interest in the field is bismuth oxides. By using a solvothermal technique, these materials can take on interesting nanostructures and be rendered porous, increasing the available area for iodide adsorption [25, 63], whereas the previously mentioned minerals with poorer capacities are non-porous. Uptake capacities of up to 285 mg g−1 iodide are reported, proceeding via formation of Bi4I2O5 although, similar to the adsorbents already discussed, iodide (and iodate) percentage uptake was reduced by > 50% by the presence of chloride and carbonate in the system [25]. The adsorbent would possibly be difficult to implement for column studies, given the undefined particle size distribution.
Mineral adsorbents for iodine are notable for the vast range of adsorbent-to-solution ratios and iodine concentration ranges examined in the literature. The first parameter ranges from 0.2–200 g adsorbent per L of water treated, while the second has been studied from 0.5–12,700 mg L−1. This is because the objective behind some work is to demonstrate that iodine concentration can be reduced to below detectable or acceptable levels, with a sufficient adsorbent mass [35], whereas other researchers place more value in the maximal adsorption capacity for the material [23], while both approaches yield valuable data, it does make direct comparison of adsorbents problematic.
3.2 Metal-exchanged zeolites
Similarly to AC, the most popular metal to immobilise in a zeolite matrix has been Ag. In research by Tauanov and Inglezakis, a synthetic, mixed-phase zeolite, derived from fly ash was first activated with NaOH, then treated with AgNO3 solution, to produce an adsorbent loaded with Ag(0) nanoparticles [45]. Although the iodide uptake capacity was low (20 mg g−1, from initial concentrations of 75–450 mg L−1), the material demonstrated good resistance to a number of common co-anions and was stable under alkaline leaching. Control over the oxidation state of the loaded Ag can present processing challenges, as this material had to be stored in the dark. The Ag nanoparticles were reported to be in the size range of 4–45 nm. This may explain the slow kinetics (> 10 days to reach equilibrium), as only a fraction of the silver atoms present would initially be exposed to iodine species and work as active adsorption sites [12].
Other researchers, using titanates as the adsorbent matrix, have retained the loaded metal as Ag+, rather than Ag(0). These materials have shown improved capacity, relative to the previously mentioned zeolite, with 430 mg g−1 and 213 mg g−1 iodide captured, relative to 41% and 6% mass metal-loading respectively [47, 50]. A further similar approach was taken by Chen et al., using a brucite (Mg(OH)2) matrix. This achieved 370 mg g−1 iodide uptake with 35% Ag loading [46]. These three adsorbents may be directly compared, as assessment was conducted with an equivalent adsorbent/solution ratio and similar iodide concentrations. The high uptakes may be related to porosity, which was > 50 m2 g−1 for all materials, allowing for increased metallation efficiency. In the latter case however, because of a photocatalytic mechanism, the adsorbed species was a polyiodide and sacrificed stability in favour of capacity, with iodine volatilisation observed at 350 °C [50]. Ag+-loaded adsorbents have faster uptake kinetics than Ag(0)-loaded, which require an in-situ oxidation process for ultimate conversion into AgI. Compared to other adsorption processes, the kinetics for zeolitic adsorbents are however quite slow, with equilibrium still requiring > 1 h [46], whereas ligand-functionalised silicas and IX resins can reach equilibrium within a few minutes [64, 65].
3.3 Silica and organosilica materials
Ag-loaded silica-based materials have been investigated. Silica “aerogels” are a preferred support matrix, which are commercially available ultra-light porous silicates. An advantage of silica matrices is that organic functionalities can easily be incorporated into the structure of the material by copolymerisation of silanes or grafting on to activated silica particles [66,67,68]. Aerogels can for example be functionalised with mercaptopropylsilanes, using supercritical CO2 as the reaction solvent, then treatment with aqueous AgNO3, before finally a reduction process using H2 in Ar. This allows formation of Ag(0) nanoparticles on the aerogel surface [69]. Such adsorbents were originally designed with gas-phase capture in mind [69, 70]. More recently they were essayed for uptake of aqueous iodide and iodate, in what is thus far the only study of its kind [20]. The iodine uptake capacity of 88 mg g−1 did not match the performance of the material in previous gaseous iodine uptake experiments (> 300 mg g−1) [69], which was believed to be due to the hydrophobic silica matrix. Uptake was complete within 1 h from analytical iodide solutions, but far slower (days) from simulated off-gas condensate. Also, iodate affinity was greatly reduced in these more realistic conditions by a factor of ~ 10 [20]. Notably, adsorption and resistance to leaching was not tested at high pH, presumably because of the known hydrolysis of the silica matrices under these conditions [71].
4 Trends and future challenges
4.1 Appropriate choice of functionalising metal and adsorbent matrix
Despite the high economic cost of Ag-loaded media, other soft-metals have long been considered by the industry to be inferior to silver for iodine removal, on the basis of established gas-phase capture work [10, 12], although more recent gas-phase studies have investigated Bi- and even Sn- loading [72, 73]. Indeed, the range of adsorbents covered in this review clearly indicates that successful gas-phase adsorbents have been influential to the materials choices made by aqueous-phase researchers. A full discussion of the different adsorption challenges posed by the two phases is beyond the scope of this work, but it should be noted that both the iodine speciation and the potentially competing adsorbates are not alike, as plant off-gas streams contain mainly I2, with high pressures of water vapour and NOx [10]. Thus, it can be predicted that adsorbent performance may not always be transferable to aqueous streams.
This said, the literature does broadly show that Ag-bearing adsorbents are superior in selectivity to Cu, Pb and other equivalents, verses competing ions such as chloride and carbonate [42, 48], with reported distribution coefficients at least two orders of magnitude higher [15]. The same is true for adsorption in alkaline conditions, relevant to spent caustic scrub solution [15, 20, 24] and can be rationalised by considering the Ksp values of the relevant metal hydroxides and carbonates (Table 2). Very little work has been carried out at strongly acidic pH, partially because of the invariable loss of metal from the adsorbent surface [13, 24], which could cause downstream processing issues, but also because iodine can be driven into the gaseous phase from acidic solutions by sparging [11].
The assessment of selectivity of silver-loaded adsorbents for iodide anions in the literature is problematic however. Only common environmental anions such as chloride, nitrate, sulfate and carbonate tend to be trialled for competition effects [20, 41, 45, 46] and being harder bases than iodide and iodate, the lesser effect on performance is predictable. The literature did not reveal any reports of iodine uptake in solutions of high molybdate concentration for example (although it is not present at high concentrations in dissolver off-gas streams). Ag(0)-loaded materials have suggested superior selectivity in some chemical conditions [20, 45], but there are very few direct comparisons between Ag+ and Ag(0) functionalisation and the oxidation state is sometimes labile within the adsorbent [45, 48]. Hence there is minimal strong evidence for one oxidation state being advantageous over the other. The interaction between iodide and Ag(0) particles is thought to proceed by initial Ag oxidation and formation of an Ag2O film on the particle surface [74], followed by Eq. 1:
It may thus be proposed that iodide capture would be more favourable for Ag(0)-based adsorbents in acidic conditions, whereas Ag+ materials would be better in media such as spent caustic scrub. Further research into the favourable Ag oxidation state, relative to aqueous conditions, is required.
Although, as previously noted, AgI is extremely insoluble, the great majority of silver salts share this characteristic to slightly lesser degrees (Table 2). Therefore, conversion, particularly from AgCl to AgI is often very slow [20, 45, 47]. Furthermore, some Ag-loaded materials are prone to reduced performance in solutions of competing ions for similar reasons, as formation of these species, with the anions at high concentrations, is kinetically favourable and the salts must then be slowly attacked by the aqueous iodide anions for the formation of AgI. This demonstrates the previously noted issue in direct transfer of technologies for gas-phase iodine capture to aqueous conditions. Some Ag2O-loaded materials do exhibit reasonable kinetics, having high surface areas, which facilitate distribution of the active species as nanocrystals, which fully react quickly with the iodine species [46, 50]. However, this comes at the expense of workable particle size (which appeared to be < 10 m in both mentioned cases), for fast-flowing dynamic systems.
Indeed, a common trait of papers detailing the performance of inorganic materials is that no dynamic experimentation is performed [20, 45, 48, 50]. This is probably reflective of the fact that the inorganic adsorbents studied have small and/or ill-defined particle size, making them less suitable for column systems. Nevertheless, such experiments are feasible [41, 46], although these data were not modelled to extract a dynamic uptake capacity.
In terms of capacity of the materials, a reasonably high BET surface area (> 50 m2 g−1) seems to be important, to allow access of the aqueous iodine species to both surface and interior active sites on the adsorbent particles (Table S1). However, the relationship between this parameter and ultimate capacity is ill-defined (Figure S1). This is partially because of a lack of data, particularly at lower surface area values, as the adsorbents are frequently not measured, but also because activated carbon materials tend to produce outlier values, having high surface area, but relatively low metal-loading potential, again related to functional group chemistry [57]. More information is required on the pore characteristics of successful materials, such as the pore-size distribution profile. This is important to further clarifying the generally slow kinetics of uptake.
Fast kinetics are generally achievable only by immobilisation of individual metal ions by an organic functional group, followed by iodide binding as a complex ligand, rather than forming salt particles. For example, in contrast to this body of literature, the binding of fluoride to various ligated metal complexes typically reaches equilibrium in < 30 min [31, 36]. However, as has been discussed, this kind of system can itself cause problems with organic partitioning in iodine-capture processes. The favourability of iodine adsorption in these systems is also rather less predictable, as the species formed is a novel metal complex, incorporating both chelating and iodide ligands and at best, only sensible predictions can be made from such sources as the Critical Stability Constants Database [32]. These key challenges for metallated adsorbents are illustrated in Fig. 5. It can be surmised that a “universal” adsorbent for all aqueous iodine remediation challenges is unlikely to be feasible and the correct adsorbent choice for a given wastestream will probably be a compromise, based on economics, required flow rate/water-treatment rate and wasteform destination.
4.2 Suitability of adsorbents for dynamic and static systems
Given the general trends in static kinetic data already mentioned, the differences in adsorbent performance in dynamic verses static systems is a key current knowledge gap. This is because, depending on the precise remit for the adsorbent, one approach may be favourable over the other. For treatment of dissolver off-gas condensate, which has a production rate in the region of 1000 L day−1, a column system has advantages of enhanced decontamination factor, easier management of the abatement material and tracking of the iodine loading [75]. In this case, adsorbents with a workable particle size and faster kinetics would be desirable. However, for management of a finite amount of low-level legacy aqueous waste in temporary containment, where water-treatment rate is not critical (as found at the US Department of Energy Hanford site), a batch system could offer almost complete removal of iodine, with high efficiency [35].
In the latter case, a small, or irregular particle size would be a lesser concern, as the contact solution can simply percolate through the adsorbent bed [75]. The adsorbent and contact solution could be agitated as a slurry [76], as is practiced at the UK Enhanced Actinide Removal Plant, although this increases mechanical complexity. This scenario being the case, an important factor in an experimental setup is the ratio of adsorbent mass to solution volume, to provide high efficiency but also a sufficiently low resulting iodine concentration. This parameter needs to be investigated further in the literature, as in the majority of reviewed studies, it was simply set at an arbitrary value, usually 1 (Table S1).
4.3 Iodine speciation challenges
The majority of work discussed in this review has examined iodine removal from systems of moderate pH, with controlled concentrations of potentially competing anions. In these conditions, the iodide anion is the only significant iodine species. This is certainly a laudable approach, in that it allows competition effects to be quantified. However, in real nuclear industry aqueous waste, the iodine speciation is complex, as it is strongly dependant on pH and REDOX potential [11]. Acidic conditions produce the more hydrophobic species I3− and I2, which weakly interact with organic adsorbents via partitioning, as discussed in 2.2. The implications for wasteform stability have been only sparingly investigated [24] and require further research. Only one study out of those reviewed examined the performance of an iodine-capture material using an industrially-realistic acidic feed [13].
In contrast, basic conditions result in iodide and iodate as the two dominant species. A small number of studies, in recognition of this, have examined iodate removal or compared the uptake of both species [20, 25, 35]. Whilst it is clear that the metallated materials show ability to capture and retain both anions, the small amount of data make it very difficult to draw conclusions as to which adsorbent types might be more appropriate to remediate one species over the other. In studies of Bi2O2.33 nanosheets, it was found that the adsorbent had a rather higher molar adsorption capacity for iodide than iodate. This appeared to be related to differing iodine-containing crystalline phases being formed at differing iodine solution concentrations [25]. In future, co-uptake experiments for iodide and iodate simultaneously are suggested, to examine their relative affinities for a given adsorbent more closely. This would complement the good work that has already been carried out with respect to the final speciation of the iodine on the adsorbent surface [35]. This is important because, in a dynamic column system with both anions present in the feed, it is possible that breakthrough of one species could occur well before the other, which of course impacts the potential choice of downstream treatment (guard column) [75]. This consideration would also influence whether a static adsorption system is chosen over a dynamic one.
4.4 Wasteform considerations
One additional factor to be considered is the amenability of the spent (iodine-loaded) materials to conversion to a stable wasteform. Although much of the work discussed so far assesses the stability of the iodine adsorption by leaching tests [41, 42, 50], this should not be considered equivalent to assessing the stability of the final wasteform material. A full discussion of radioiodine wasteform compatibility is beyond the scope of this article, but the relative merits of the adsorbent classes mentioned must be considered in this regard.
IX resins, despite exhibiting high iodine capacity, are generally poorly compatible with some wasteform routes. For example, any oxidative decomposition treatment risks volatilisation of the iodine, as the temperatures required are considerably above the iodine sublimation point of 183 °C [27]. Perhaps for this reason, there is no literature describing thermal treatment of iodine-loaded adsorption media or wasteforms. However, there has been research into the pyrolysis of IX resins loaded with other radionuclides (Co and Co). This study found that the radionuclides of interest were partially volatilised beyond a temperature of 400 °C [77]. The polymeric beads are more suitable for cementation, with several operating facilities globally processing IX resins in this way. However, the immobilisation requires careful design, with optimisation of parameters such as pH buffering, water to cement ratio, additive requirements and resin mass% loading [78, 79]. The latter parameter is especially important in the case of I-129, because if the maximum possible adsorbent mass% in the wasteform is low, then the “concentrate and contain strategy is clearly not achieved. Non-traditional encapsulating matrices have shown recent promise in this regard recently. For example, novel ‘geopolymers’ have been reported, which can contain ~ 45 mass% of spent radionuclide-containing IX resin [80]. Again however, there appear to be no examples specifically of iodine-loaded materials encapsulated in this way and this represents a critical knowledge gap.
Ag-exchanged zeolites are suitable for consolidation by hot isostatic pressing (HIP), being chemically converted to a silver iodide sodalite (Ag4Al3Si3O12I). However, the wasteform is unstable under reducing groundwater conditions and iodine-loading is performed in idealised conditions, rather than an industrially-realistic capture process [81, 82]. Ag-functionalised silica aerogels are suitable for sintering by HIP, as well as hot uniaxial pressing (HUP) and spark plasma sintering (SPS), after removal of organic moieties by heating [83]. It has furthermore been shown that aerogels can be incorporated into typical cement matrices without the structure of the aerogel breaking down under alkaline hydrolysis. However, the iodine leachability from such materials has not been reported and is yet uncertain. Future work requires more synergy between adsorption and wasteform creation processes, with the adsorbent (i) showing capability of iodine-capture from an accurately simulated aqueous stream, (ii) being converted to its final wasteform, with minimal iodine loss and (iii) resisting further iodine leaching in environmentally-realistic conditions. Indeed, it could be argued that the latter two aspects are of more importance than high adsorption performance, in the drive towards increasing the sustainability of future processes. The ideal material for safe sequestration of I-129 may be one that has merely sufficient capacity and kinetics for a dynamic column system to be feasible, whilst completely fulfilling its wasteform criteria.
5 Conclusions
I-129 removal from wastewater and safe retention in a solid phase is an industrial challenge, that if solved, could significantly reduce environmental impact from the nuclear industry. There is a small, yet diverse body of work on metallated adsorbents for this purpose, which harnesses the favourable chemical interactions between soft metals and anionic iodine. The most commonly investigated metal is silver and such materials demonstrate the best selectivity. However, the performance of two candidate metals, bound to the same adsorbent matrix, is rarely compared.
Generally, inorganic matrices are preferred to organic. This is possibly related to the incompatibility of IX resins with the more hydrophobic iodine species and challenges around wasteform compatibility. Only a small number of inorganic adsorbents provide similar capacity to IX resins and a BET surface area of > 50 m2 g−1 appears to be desirable to utilise the full metal loading of the material. Kinetics are often problematic, because of the relatively slow conversion of the active metal species to metal iodides.
Many materials appear to be resistant to varoius leaching conditions, but it is far more important that iodine-loaded wasteforms are subject to these tests, using conditions representative of those that would be encountered in geological disposal. Dynamic data is generally lacking, although dynamic water-treatment systems are widely deployed in the nuclear industry. This raises question marks as to the suitability of many of the inorganic adsorbents, because of knowledge and control over their particle size and distribution.
There is wide variance in experimental parameters investigated (concentration range, pH, competing ions and static vs dynamic experiments). Some researchers have simulated a targeted aqueous waste stream, specific to a certain plant. More commonly, the adsorbent performance is tested with analytical iodide solutions and carefully-chosen concentrations of other anions, allowing the interference effect to be quantified.
Future work should focus on a more joined-up approach to iodine capture, considering challenges around speciation in the system, dynamic verses static treatment methods and especially how the adsorption step integrates into the life cycle of the adsorbent, with a pathway in mind towards a final, chemically-stable wasteform.
References
International Eneregy Agency (2020) World energy outlook 2020. OECD, Paris
Kovalev NV, Zilberman BY, Goletsky ND, Sinyukhin AB (2020) A new approach to the recycling of spent nuclear fuel in thermal reactors within the REMIX concept. Nucl Energy Tech 6:93–98
Nuclear Energy Agency (2018) State-of-the-art report on the progress of nuclear fuel cycle chemistry. Paris
Grossman CM, Nussbaum RH, Nussbaum FD (2003) Cancers among residents downwind of the Hanford, Washington, plutonium production site. Arch Environ Health 58(5):267–274
Thomas GD, Smith SM, Turcotte JA (2008) Using public relations strategies to prompt populations at risk to seek health information: the Hanford Community Health Project. Health Promot Pract 10:92–101
Paulillo A, Dodds JM, Milliken A, Palethorpe SJ, Lettieri P (2020) The environmental impacts of reprocessing used nuclear fuels: a UK case study. Sustain Mater Technol 25:e00186
Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden JL (2016) Materials and processes for the effective capture and immobilization of radioiodine: a review. J Nucl Mater 470:307–326
International Atomic Energy Agency, "Treatment of Radioactive Gaseous Waste," Vienna, 2014
Huve J, Ryzhikov A, Nouali H, Lalia V, Auge G, Daou TJ (2018) Porous sorbents for the capture of radioactive iodine compounds: a review. RSC Adv 8(51):29248–29273
Haefner DR, Tranter TJ (2007) Methods of gas phase capture of iodine from fuel reprocessing off-gas: a literature survey, Idaho National Laboratory, Idaho Falls
Sakurai T, Takahashi A, Ishikawa N, Komaki Y (1989) The behavior of iodine in a simulated spent-fuel solution. Nucl Technol 85(2):206–212
Jubin RT (1988) Airborne waste management technology applicable for use in reprocessing plants for control of iodine and other off-gas constituents. Tennessee: Oak Ridge National Laboratory
Decamp C, Happel S (2013) Utilization of a mixed-bed column for the removal of iodine from radioactive process waste solutions. J Radioanal Nucl Chem 298(2):763–767
Kodama H (1999) Removal of iodide ion from simulated radioactive liquid waste. Czech J Phys 49:971–977
Asmussen RM, Neeway JJ, Lawter AR, Wilson A, Qafoku NP (2016) Silver-based getters for I-129 removal from low-activity waste. Radiochim Acta 104(12):905–913
Taylor P (1990) A review of methods for immobilizing iodine-129 arising from a nuclear fuel recycle plant, with emphasis on waste-form chemistry. Manitoba
Kaplan DI et al (2019) Iodine speciation in a silver-amended cementitious system. Environ Int 126:576–584
Raisbeck G, Yiou F (1999) 129I in the oceans: origins and applications. Sci Total Environ 237:31–41
Zhirnov YP, Zhikharev MI, Efremov EP, Malankina RS (1997) Behavior of molybdenum during evaporation of nitric-acid raffinates. At Energ 83(5):848–850
Asmussen RM, Matyas J, Qafoku NP, Kruger AA (2019) Silver-functionalized silica aerogels and their application in the removal of iodine from aqueous environments. J Hazard Mater 379:119364
Hudson PI, Buckley CP (1995) Aerial and liquid effluent treatment in BNFL's Thermal Oxide Reprocessing Plant (THORP). In: Proceedings of the 1995 symposium on nuclear data, Tokai, T. Iguchi and T. Fukahori, Eds., 1995: Japan Atomic Energy Institute, pp. 154–176
Jubin RT (2017) Design and test plan for an integrated iodine scrubber and polishing bed system. Oak Ridge National Laboratory, Oak Ridge
Barton DNT et al (2019) Remediation of radioiodine using polyamine anion exchange resins. J Ind Eng Chem 78:210–221
Robshaw TJ et al (2020) Insights into the interaction of iodide and iodine with Cu(II)-loaded bispicolylamine chelating resin and applications for nuclear waste treatment. Chem Eng J 390:124647–124659
Liu SW, Kang SH, Wang HM, Wang GZ, Zhao HJ, Cai WP (2016) Nanosheets-built flowerlike micro/nanostructured Bi2O2.33 and its highly efficient iodine removal performances. Chem Eng J 289:219–230
Luca V, Bianchi HL, Manzini AC (2012) Cation immobilization in pyrolyzed simulated spent ion exchange resins. J Nucl Mater 424(1–3):1–11
Wang JL, Wan Z (2015) Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog Nucl Energy 78:47–55. https://doi.org/10.1016/j.pnucene.2014.08.003
Mattigod SV, Serne RJ, Fryxell GE (2003) Selection and testing of “Getters” for adsorption of iodine-129 and technetium-99: a review. Richland, Washington
Lambert JL, Fina GT, Fina LR (2002) Preparation and properties of triiodide-quaternary, pentaiodide-quaternary and heptaiodide-quaternary ammonium strong base anion-exchange resin disinfectants. Ind Eng Chem Prod Res Dev 19(2):256–258
Van Loon L, Hummel W (1995) The radiolytic and chemical degradation of organic ion exchange resins under alkaline conditions: effect on radionuclide speciation. Wurenlingen and Villigen, Nagra
Robshaw TJ, Tukra S, Hammond DB, Leggett GJ, Ogden MD (2019) Highly efficient fluoride extraction from simulant leachate of spent potlining via La-loaded chelating resin. an equilibrium study. J Hazard Mater 361:200–209
Martell AE, Smith RM, Motekaitis RJ (1997) Critical stability constants database. ed. College Station, TX, USA: A & M University
Speight JG (2016) Lange’s handbook of chemistry, 17th edn. McGraw-Hill, New York
Grobler SR, Suri SK (1980) Solubilities of the molybdates and tungstates of silver and copper(II) in water by ion-selective electrodes. J Inorg Nucl Chem 42(1):51–53
Cordova EA et al (2020) Hybrid sorbents for I-129 capture from contaminated groundwater. ACS Appl Mater Interfaces 12(23):26113–26126
Viswanathan N, Meenakshi S (2008) Effect of metal ion loaded in a resin towards fluoride retention. J Fluorine Chem 129(7):645–653
Millar GJ, Couperthwaite SJ, Wellner DB, Macfarlane DC, Dalzell SA (2017) Removal of fluoride ions from solution by chelating resin with imino-diacetate functionality. J Water Process Eng 20:113–122
Oke K, Neumann S, Adams B (2011) Selective fluoride removal. Water Today. pp. 76–80
Ye ZX, Chen LF, Liu CC, Ning SY, Wang XP, Wei YZ (2019) The rapid removal of iodide from aqueous solutions using a silica-based ion-exchange resin. React Funct Polym 135:52–57
Parker KE, Golovich EC, Wellman DM (2014) Iodine adsorption on ion-exchange resins and activated carbons– batch testing. Oak Ridge
Karanfil T, Moro EC, Serkiz SM (2005) Development and testing of a silver chloride-impregnated activated carbon for aqueous removal and sequestration of iodide. Environ Technol 26(11):1255–1262
Zhang XY, Gu P, Li XY, Zhang GH (2017) Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem Eng J 322:129–139
Singare PU (2013) Ion-Isotopic Exchange Reaction Kinetics in Characterization of Anion Exchange Resins Dowex 550A LC and Indion-820. Diffus Fundam. Vol. 19
Warchol J, Misaelides P, Petrus R, Zamboulis D (2006) Preparation and application of organo-modified zeolitic material in the removal of chromates and iodides. J Hazard Mater 137(3):1410–1416
Tauanov Z, Inglezakis VJ (2019) Removal of iodide from water using silver nanoparticles-impregnated synthetic zeolites. Sci Total Environ 682:259–270
Bo A, Sarina S, Zheng ZF, Yang DJ, Liu HW, Zhu HY (2013) Removal of radioactive iodine from water using Ag2O grafted titanate nanolamina as efficient adsorbent. J Hazard Mater 246:199–205
Liu SS, Wang N, Zhang YC, Li YR, Han Z, Na P (2015) Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O-Ag/TiO2 composites under visible light irradiation. J Hazard Mater 284:171–181
Mao P, Liu Y, Jiao Y, Chen SW, Yang Y (2016) Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere 164:396–403
Mao P et al (2017) Synthesis of Cu/Cu2O hydrides for enhanced removal of iodide from water. J Hazard Mater 328:21–28
Chen YY, Yu SH, Yao QZ, Fu SQ, Zhou GT (2018) One-step synthesis of Ag2O@Mg(OH)(2) nanocomposite as an efficient scavenger for iodine and uranium. J Colloid Interface Sci 510:280–291
Ho PC, Kraus KA (1981) Adsorption on inorganic materials-VIII. Adsorption of iodide on AgCl-filled carbon. J Inorg Nucl Chem 43:583–587
Watson K, Farre MJ, Knight N (2016) Comparing a silver-impregnated activated carbon with an unmodified activated carbon for disinfection by-product minimisation and precursor removal. Sci Total Environ 542:672–684
Van HT, Nguyen TMP, Thao VT, Vu XH, Nguyen TV, Nguyen LH (2018) Applying activated carbon derived from coconut shell loaded by silver nanoparticles to remove methylene blue in aqueous solution. Water Air Soil Pollut 229(12):1–4
AbdEl-Salam AH, Ewais HA, Basaleh AS (2017) Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J Mol Liq 248:833–841
Hoskins JS, Karanfil T (2002) Removal and sequestration of iodide using silver-impregnated activated carbon. Environ Sci Technol 36(4):784–789
Ogi T, Iskandar F, Nandiyanto ABD, Wang WN, Okuyama K (2012) Influence of polymer decomposition temperature on the formation of rare-earth free boron carbon oxynitride phosphors. J Chem Eng Jpn 45(12):995–1000
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpaa M (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18(2):393–415
Adams CR et al (2020) An alternative to cyanide leaching of waste activated carbon ash for gold and silver recovery via synergistic dual-lixiviant treatment. J Ind Eng Chem 92:120–130
Kanesato M, Yokoyama T, Suzuki TM (1988) Selective adsorption of fluoride-ion by La(III)-loaded chelating resin having phosphonomethylamino groups. Chem Lett 17(2):207–210
Sanemasa I, Yoshida M, Abe A (2008) Uptake of iodine and bromine by ion-exchange resins in aqueous solution. Anal Sci 24(7):921–924
Refat MS, Al Didamony H, Abou El-Nour KM, El-Zayat L (2010) Synthesis and spectroscopic characterization on the tri-iodide charge transfer complex resulted from the interaction between morpholine as donor and iodine sigma-acceptor. J Saudi Chem Soc 14(3):323–330
Lefevre G, Alnot M, Ehrhardt JJ, Bessiere J (1999) Uptake of iodide by a mixture of metallic copper and cupric compounds. Environ Sci Technol 33(10):1732–1737
Liu L et al (2014) Selective capture of iodide from solutions by Microrosette-like delta-Bi2O3. ACS Appl Mater Interfaces 6(18):16082–16090
Pepper SE, Whittle KR, Harwood LM, Cowell J, Lee TS, Ogden MD (2018) Cobalt and nickel uptake by silica-based extractants. Sep Sci Technol 53(10):1552–1562
Robshaw TJ, Dawson R, Bonser K, Ogden MD (2019) Towards the implementation of an ion-exchange system for recovery of fluoride commodity chemicals. Kinetic and dynamic studies. Chem Eng J 367:149–159
Holland BT, Blanford CF, Do T, Stein A (1999) Synthesis of highly ordered, three-dimensional, macroporous structures of amorphous or crystalline inorganic oxides, phosphates, and hybrid composites. Chem Mater 11(3):795–805
Vidal L, Parshintsev J, Hartonen K, Canals A, Riekkola ML (2012) Ionic liquid-functionalized silica for selective solid-phase extraction of organic acids, amines and aldehydes. J Chromatogr A 1226:2–10
Johnston APR, Battersby BJ, Lawrie GA, Trau M (2005) Porous functionalised silica particles: a potential platform for biomolecular screening. Chem Commun 7:848–850
Matyas J, Fryxell GE, Busche BJ, Wallace K, Fifield LS (2011) Functionalized silica aerogels: advanced materials to capture and immobilize radioactive iodine. In: 35th International Conference and Exposition on Advanced Ceramics and Composites, Daytona Beach, FL, Jan 23–28 2011, vol. 32, in Ceramic Engineering and Science Proceedings, 2011, pp. 23–32
Matyas J, Canfield N, Sulaiman S, Zumhoff M (2016) Silica-based waste form for immobilization of iodine from reprocessing plant off-gas streams. J Nucl Mater 476:255–261
Dron R, Brivot F (1992) Thermodynamic and kinetic approach to the alkali-silica reaction.1. concepts. Cem Concr Res 22(5):941–948
Al-Mamoori A, Alsalbokh M, Lawson S, Rownaghi AA, Rezaei F (2020) Development of bismuth-mordenite adsorbents for iodine capture from off-gas streams. Chem Eng J 391:123583
Riley BJ et al (2015) Consolidation of tin sulfide chalcogels and Xerogels with and without adsorbed iodine. Ind Eng Chem Res 54(45):11259–11267
Zhang X, Stewart S, Shoesmith DW, Wren JC (2007) Interaction of aqueous iodine species with Ag2O/Ag surfaces. J Electrochem Soc 154(4):F70–F76
Harland CE (1994) Ion exchange: theory and practice, 2nd edn. Royal Society of Chemistry, Cambridge
Prajitno MY, Taufiqurrakhman M, Harbottle D, Hunter TN (2021) Kinetic studies of Cs+ and Sr2+ ion exchange using clinoptilolite in static columns and an agitated tubular reactor (ATR). Chem Eng 5:9–24
Antonetti P, Claire Y, Massit H, Lessart P, Pham Van Cang C, Perichaud A (2000) Pyrolysis of cobalt and caesium doped cationic ion-exchange resin. J Anal Appl Pyrol 55:81–92
Abdel Rahman RO, Zaki AA (2011) Comparative study of leaching conceptual models: Cs leaching from different ILW cement based matrices. Chem Eng J 173:722–736
Abdel Rahman RO, Zaki AA (2020) Comparative analysis of nuclear waste solidification performance models: spent ion exchanger-cement based wasteforms. Process Safety Environ Prot 136:115–125
Lee W-H, Cheng T-W, Ding Y-C, Lin K-L, Tsao S-W, Huang C-P (2019) Geopolymer technology for the solidification of simulated ion exchange resins with radionuclides. J Environ Manage 235:19–27
Maddrell ER et al (2019) Silver iodide sodalite - Wasteform / Hip canister interactions and aqueous durability. J Nucl Mater 517:71–79
Chong S et al (2020) Iodosodalite synthesis with hot isostatic pressing of precursors produced from aqueous and hydrothermal processes. J Nucl Mater 538:152222
Matyáš J, Engler RK (2013) Assessment of methods to consolidate iodine-loaded silver-functionalized silica Aerogel. Pacific Northwest National Laboratory, Richland
Funding
This research was funded under the £46 m Advanced Fuel Cycle Programme as part of the Department for Business, Energy and Industrial Strategy’s (BEIS) £505 m Energy Innovation Programme.
Author information
Authors and Affiliations
Contributions
TJR wrote the review. JT reviewed the manuscript and provided technical guidance on industry relevance. SK and BW reviewed the manuscript and provided technical insights into wasteform conversion. CAS and MDO reviewed the manuscript and provided technical guidance on separation processes within the nuclear industry.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robshaw, T.J., Turner, J., Kearney, S. et al. Capture of aqueous radioiodine species by metallated adsorbents from wastestreams of the nuclear power industry: a review. SN Appl. Sci. 3, 843 (2021). https://doi.org/10.1007/s42452-021-04818-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04818-8