Abstract
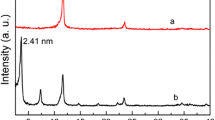
There is an ever-growing global concern of water pollution and water scarcity. Numerous strategies are investigated within a holistic approach that includes water treatment using inorganic and composite adsorbents. In this context, MgAlCO3-layered double hydroxide (LDH) was synthesized by co-precipitation at pH 10 and characterized by XRD, FTIR, TGA, N2-adsorption–desorption and XPS. MgAlCO3-LDH was evaluated as an adsorbent to remove methyl orange (MO), a model organic pollutant, from aqueous solutions. The effects of initial dye concentration, contact time and pH on the adsorption process were investigated. For 150 ppm MO initial concentration, a maximum adsorption capacity of 120 mg g−1 was obtained at 25 °C and pH 6 after 120 min contact time. The equilibrium adsorption data were found to fit the Freundlich rather than the Langmuir adsorption model (R2 = 0.9971). The adsorption process was found to be chemisorption that most likely involves the anion exchange mechanism. Kinetically, the experimental data fitted very well the pseudo-second order and gave a good representation for the pseudo-first-order, with adsorption rate constants K2 = 4 × 10−6 g mg−1 min−1 and K1 = 0.0082 min−1.











Similar content being viewed by others
References
Tiwari A, Joshi M, Salvi N, Gupta D, Gandhi S, Rajpoot K, Tekade RK (2022) Toxicity of pharmaceutical azo dyes. Pharmacokinetics and toxicokinetic considerations. Elsevier, Amsterdam, pp 569–603
Bujak T, Zagórska-Dziok M, Ziemlewska A, Nizioł-Łukaszewska Z, Lal K, Wasilewski T, Hordyjewicz-Baran Z (2022) Flower extracts as multifunctional dyes in the cosmetics industry. Molecules 27:922. https://doi.org/10.3390/molecules27030922
Wijaya A, Siregar PMSBN, Priambodo A, Palapa NR, Taher T, Lesbani A (2021) Innovative modified of Cu-Al/C (C = biochar, graphite) composites for removal of procion red from aqueous solution. Sci Technol Indones. https://doi.org/10.26554/sti.2021.6.4.228-234
Ebrahim S, Mosaad M, Othman H, Hassabo A (2021) A valuable observation of eco-friendly natural dyes for valuable utilisation in the textile industry. J Text Color Polym Sci. https://doi.org/10.21608/jtcps.2021.97342.1090
Palaskar SS, Kale RD, Deshmukh RR (2021) Application of natural yellow (curcumin) dye on silk to impart multifunctional finishing and validation of dyeing process using BBD model. Color Res Appl 46:1301–1312. https://doi.org/10.1002/col.22678
Fried R, Oprea I, Fleck K, Rudroff F (2022) Biogenic colourants in the textile industry—a promising and sustainable alternative to synthetic dyes. Green Chem 24:13–35. https://doi.org/10.1039/D1GC02968A
Lach CE, Pauli CS, Coan AS, Simionatto EL, Koslowski LAD (2022) Investigating the process of electrocoagulation in the removal of azo dye from synthetic textile effluents and the effects of acute toxicity on Daphnia magna test organisms. J Water Process Eng 45:102485. https://doi.org/10.1016/j.jwpe.2021.102485
Li B, Zhang Y, Zhou X, Liu Z, Liu Q, Li X (2016) Different dye removal mechanisms between monodispersed and uniform hexagonal thin plate-like MgAl–CO32–LDH and its calcined product in efficient removal of Congo red from water. J Alloys Compd 673:265–271. https://doi.org/10.1016/j.jallcom.2016.02.248
Abderrazek K, Najoua FS, Srasra E (2016) Synthesis and characterization of [Zn–Al] LDH: study of the effect of calcination on the photocatalytic activity. Appl Clay Sci 119:229–235. https://doi.org/10.1016/j.clay.2015.10.014
Huang J, Huang D, Zeng F, Ma L, Wang Z (2021) Photocatalytic MOF fibrous membranes for cyclic adsorption and degradation of dyes. J Mater Sci 56:3127–3139. https://doi.org/10.1007/s10853-020-05473-x
Eslami H, Zarei Mahmoudabadi T (2022) Modified coagulation processes using polyferric chloride and polytitanium tetrachloride for the removal of anionic dye from aqueous solution. Int J Environ Sci Technol 19:1811–1818. https://doi.org/10.1007/s13762-021-03293-3
Nazim M, Khan AAP, Asiri AM, Kim JH (2021) Exploring rapid photocatalytic degradation of organic pollutants with porous CuO nanosheets: synthesis, dye removal, and kinetic studies at room temperature. ACS Omega 6:2601–2612. https://doi.org/10.1021/acsomega.0c04747
Beydaghdari M, Hooriabad Saboor F, Babapoor A, Karve VV, Asgari M (2022) Recent advances in MOF-based adsorbents for dye removal from the aquatic environment. Energies 15:2023. https://doi.org/10.3390/en15062023
Lin Q, Zeng G, Yan G, Luo J, Cheng X, Zhao Z, Li H (2022) Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: oil/water separation and dyes removal. Chem Eng J 427:131668. https://doi.org/10.1016/j.cej.2021.131668
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YA-G, Elsamahy T, Jiao H, Fu Y, Sun J (2022) A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf 231:113160. https://doi.org/10.1016/j.ecoenv.2021.113160
Zhang P, Qian G, Shi H, Ruan X, Yang J, Frost RL (2012) Mechanism of interaction of hydrocalumites (Ca/Al-LDH) with methyl orange and acidic scarlet GR. J Colloid Interface Sci 365:110–116. https://doi.org/10.1016/j.jcis.2011.08.064
Sun D, Zhang X, Wu Y, Liu X (2010) Adsorption of anionic dyes from aqueous solution on fly ash. J Hazard Mater 181:335–342. https://doi.org/10.1016/j.jhazmat.2010.05.015
Hou J, Liu Y, Weng R, Li L, Liu Y, Sheng J, Song Y (2022) Purification of dye-contaminated water using Si-doped mesoporous Fe3O4 prepared with rice husk SBA-15 as a template: behavior and mechanism. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02379-3
Nargis F, Duong A, Rehl E, Bradshaw C, Kazemian H (2022) Highly efficient and low-cost clay-based adsorbent for glyphosate removal from contaminated water. Chem Eng Technol 45:340–347. https://doi.org/10.1002/ceat.202100437
Tsarpali M, Kuhn JN, Philippidis GP (2022) Hydrothermal carbonization of residual algal biomass for production of hydrochar as a biobased metal adsorbent. Sustainability 14:455. https://doi.org/10.3390/su14010455
Irtiseva K, Mosina M, Tumilovica A, Lapkovskis V, Mironovs V, Ozolins J, Stepanova V, Shishkin A (2022) Application of granular biocomposites based on homogenised peat for absorption of oil products. Materials (Basel) 15:1306. https://doi.org/10.3390/ma15041306
Zulkefli NN, Seladorai R, Masdar MS, Mohd Sofian N, Wan Isahak WNR (2022) Core shell nanostructure: impregnated activated carbon as adsorbent for hydrogen sulfide adsorption. Molecules 27:1145. https://doi.org/10.3390/molecules27031145
Shamsayei M, Yamini Y, Asiabi H (2022) A novel diatomite supported layered double hydroxide as reusable adsorbent for efficient removal of acidic dyes. Int J Environ Anal Chem 102:1849–1865. https://doi.org/10.1080/03067319.2020.1743833
Gidado SM, Akanyeti İ (2020) Comparison of remazol brilliant blue reactive adsorption on pristine and calcined ZnAl, MgAl, ZnMgAl layered double hydroxides. Water Air Soil Pollut 231:146. https://doi.org/10.1007/s11270-020-04522-0
Zhu S, Khan MA, Kameda T, Xu H, Wang F, **a M, Yoshioka T (2022) New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J Hazard Mater 426:128062. https://doi.org/10.1016/j.jhazmat.2021.128062
Ahmed IM, Gasser MS (2012) Adsorption study of anionic reactive dye from aqueous solution to Mg–Fe–CO3 layered double hydroxide (LDH). Appl Surf Sci 259:650–656. https://doi.org/10.1016/j.apsusc.2012.07.092
Mishra G, Dash B, Pandey S (2018) Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci 153:172–186. https://doi.org/10.1016/j.clay.2017.12.021
Elkhattabi EH, Lakraimi M, Berraho M, Legrouri A, Hammal R, El Gaini L (2018) Acid Green 1 removal from wastewater by layered double hydroxides. Appl Water Sci 8:45. https://doi.org/10.1007/s13201-018-0658-1
Ni Z-M, **a S-J, Wang L-G, **ng F-F, Pan G-X (2007) Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J Colloid Interface Sci 316:284–291. https://doi.org/10.1016/j.jcis.2007.07.045
Abdellaoui K, Pavlovic I, Barriga C (2019) Nanohybrid layered double hydroxides used to remove several dyes from water. ChemEngineering 3:41. https://doi.org/10.3390/chemengineering3020041
Wang J, Peng F, Wu X, Wang D, Zheng A, Cao L, Yu C, Liu X, Jiang X (2021) Biocompatibility and bone regeneration of PEO/Mg-Al LDH-coated pure Mg: an in vitro and in vivo study. Sci China Mater 64:460–473. https://doi.org/10.1007/s40843-020-1392-5
**e Y, Yuan X, Wu Z, Zeng G, Jiang L, Peng X, Li H (2019) Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J Colloid Interface Sci 536:440–455. https://doi.org/10.1016/j.jcis.2018.10.066
Fan G, Li F, Evans DG, Duan X (2014) Catalytic applications of layered double hydroxides: recent advances and perspectives. Chem Soc Rev 43:7040–7066. https://doi.org/10.1039/C4CS00160E
Everaert M, da Silva RC, Degryse F, McLaughlin MJ, Smolders E (2018) Limited dissolved phosphorus runoff losses from layered double hydroxide and struvite fertilizers in a rainfall simulation study. J Environ Qual 47:371–377. https://doi.org/10.2134/jeq2017.07.0282
Mittal J (2021) Recent progress in the synthesis of layered double hydroxides and their application for the adsorptive removal of dyes: a review. J Environ Manag 295:113017. https://doi.org/10.1016/j.jenvman.2021.113017
Zaghloul A, Benhiti R, Ait Ichou A, Carja G, Soudani A, Zerbet M, Sinan F, Chiban M (2021) Characterization and application of MgAl layered double hydroxide for methyl orange removal from aqueous solution. Mater Today Proc 37:3793–3797. https://doi.org/10.1016/j.matpr.2020.07.676
Zhu Z, Ouyang S, Li P, Shan L, Ma R, Zhang P (2020) Persistent organic pollutants removal via hierarchical flower-like layered double hydroxide: adsorption behaviors and mechanism investigation. Appl Clay Sci 188:105500. https://doi.org/10.1016/j.clay.2020.105500
Zheng G, Wu C, Wang J, Mo S, Wang Y, Zou Z, Zhou B, Long F (2019) Facile synthesis of few-layer MoS2 in MgAl-LDH layers for enhanced visible-light photocatalytic activity. RSC Adv 9:24280–24290. https://doi.org/10.1039/C9RA03858B
dos Santos RMM, Gonçalves RGL, Constantino VRL, Santilli CV, Borges PD, Tronto J, Pinto FG (2017) Adsorption of acid yellow 42 dye on calcined layered double hydroxide: effect of time, concentration, pH and temperature. Appl Clay Sci 140:132–139. https://doi.org/10.1016/j.clay.2017.02.005
Dietmann KM, Linke T, del Nogal Sánchez M, Pérez Pavón JL, Rives V (2020) Layered double hydroxides with intercalated permanganate and peroxydisulphate anions for oxidative removal of chlorinated organic solvents contaminated water. Minerals 10:462. https://doi.org/10.3390/min10050462
Yang M, McDermott O, Buffet J-C, O’Hare D (2014) Synthesis and characterisation of layered double hydroxide dispersions in organic solvents. RSC Adv 4:51676–51682. https://doi.org/10.1039/C4RA08505A
Vu VN, Pham THT, Chanthavong M, Do TH, Nguyen THL, Nguyen QD, Tran TKN (2022) Enhanced photocatalytic degradation of rhodamine-b under led light using CuZnAl hydrotalcite synthesized by co-precipitation technique. Inorganics 10:89. https://doi.org/10.3390/inorganics10070089
Cursino ACT, da Silva Lisboa F, dos Santos Pyrrho A, de Sousa VP, Wypych F (2013) Layered double hydroxides intercalated with anionic surfactants/benzophenone as potential materials for sunscreens. J Colloid Interface Sci 397:88–95. https://doi.org/10.1016/j.jcis.2013.01.059
Jabłońska M, Chmielarz L, Węgrzyn A, Góra-Marek K, Piwowarska Z, Witkowski S, Bidzińska E, Kuśtrowski P, Wach A, Majda D (2015) Hydrotalcite derived (Cu, Mn)–Mg–Al metal oxide systems doped with palladium as catalysts for low-temperature methanol incineration. Appl Clay Sci 114:273–282. https://doi.org/10.1016/j.clay.2015.05.027
Karami Z, Jouyandeh M, Hamad SM, Ganjali MR, Aghazadeh M, Torre L, Puglia D, Saeb MR (2019) Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog Org Coat 136:105278. https://doi.org/10.1016/j.porgcoat.2019.105278
Zhang W, Liang Y, Wang J, Zhang Y, Gao Z, Yang Y, Yang K (2019) Ultrasound-assisted adsorption of Congo red from aqueous solution using Mg Al CO3 layered double hydroxide. Appl Clay Sci 174:100–109. https://doi.org/10.1016/j.clay.2019.03.025
Wang X, Bai Z, Zhao D, Chai Y, Guo M, Zhang J (2013) New synthetic route to Mg–Al–CO3 layered double hydroxide using magnesite. Mater Res Bull 48:1228–1232. https://doi.org/10.1016/j.materresbull.2012.11.096
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Iyi N, Sasaki T (2008) Deintercalation of carbonate ions and anion exchange of an Al-rich MgAl-LDH (layered double hydroxide). Appl Clay Sci 42:246–251. https://doi.org/10.1016/j.clay.2008.01.016
Mushtaq M, Bhatti HN, Iqbal M, Noreen S (2016) Eriobotrya japonica seed biocomposite efficiency for copper adsorption: isotherms, kinetics, thermodynamic and desorption studies. J Environ Manag 176:21–33. https://doi.org/10.1016/j.jenvman.2016.03.013
Shoukat S, Bhatti HN, Iqbal M, Noreen S (2017) Mango stone biocomposite preparation and application for crystal violet adsorption: a mechanistic study. Microporous Mesoporous Mater 239:180–189. https://doi.org/10.1016/j.micromeso.2016.10.004
Shabbir R, Gu A, Chen J, Khan MM, Wang P, Jiao Y, Zhang Z, Liu Y, Yang Y (2022) Highly efficient removal of congo red and methyl orange by using petal-like Fe–Mg layered double hydroxide. Int J Environ Anal Chem 102:1060–1077. https://doi.org/10.1080/03067319.2020.1730343
Sriram G, Bendre A, Altalhi T, Jung H-Y, Hegde G, Kurkuri M (2022) Surface engineering of silica based materials with Ni–Fe layered double hydroxide for the efficient removal of methyl orange: isotherms, kinetics, mechanism and high selectivity studies. Chemosphere 287:131976. https://doi.org/10.1016/j.chemosphere.2021.131976
Alhaji NMI, Nathiya D, Kaviyarasu K, Meshram M, Ayeshamariam A (2019) A comparative study of structural and photocatalytic mechanism of AgGaO2 nanocomposites for equilibrium and kinetics evaluation of adsorption parameters. Surf Interfaces 17:100375. https://doi.org/10.1016/j.surfin.2019.100375
Riaz Q, Ahmed M, Zafar MN, Zubair M, Nazar MF, Sumrra SH, Ahmad I, Hosseini-Bandegharaei A (2022) NiO nanoparticles for enhanced removal of methyl orange: equilibrium, kinetics, thermodynamic and desorption studies. Int J Environ Anal Chem 102:84–103. https://doi.org/10.1080/03067319.2020.1715383
Dao TTU, Nguyen THT, Phan TTQ, Do TS, Vo DVN, Tien NA (2020) Comparative study on removal of Monodyes by using Ni-Al layered double hydroxides. IOP Conf Ser Mater Sci Eng 736:022068. https://doi.org/10.1088/1757-899X/736/2/022068
Nazir MA, Khan NA, Cheng C, Shah SSA, Najam T, Arshad M, Sharif A, Akhtar S, ur Rehman A (2020) Surface induced growth of ZIF-67 at Co-layered double hydroxide: removal of methylene blue and methyl orange from water. Appl Clay Sci 190:105564. https://doi.org/10.1016/j.clay.2020.105564
Wiyantoko B, Kurniawati P, Purbaningtias TE, Jauhari MH, Yahya A, Tamyiz M, Fatimah I, Doong R (2022) Assessing the effect of calcination on adsorption capability of Mg/Al layer double hydroxides (LDHs). Mater Res Express 9:035505. https://doi.org/10.1088/2053-1591/ac5ef7
Zaghouane-Boudiaf H, Boutahala M, Arab L (2012) Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem Eng J 187:142–149. https://doi.org/10.1016/j.cej.2012.01.112
Buvaneswari N, Kannan C (2011) Plant toxic and non-toxic nature of organic dyes through adsorption mechanism on cellulose surface. J Hazard Mater 189:294–300. https://doi.org/10.1016/j.jhazmat.2011.02.036
Shan R, Yan L, Yang Y, Yang K, Yu S, Yu H, Zhu B, Du B (2015) Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J Ind Eng Chem 21:561–568. https://doi.org/10.1016/j.jiec.2014.03.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest relevant to the content of this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toumi, S., Snoussi, Y. & Abderrabba, M. MgAl-Layered Double Hydroxide: An Efficient Material for Adsorptive Removal of Methyl Orange from Aqueous Solutions. Chemistry Africa 6, 323–333 (2023). https://doi.org/10.1007/s42250-022-00503-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00503-4