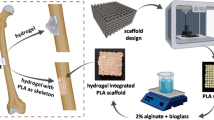
Abstract
Osteoconductive function is remarkably low in bone disease in the absence of bone tissue surrounding the grafting site, or if the bone tissue is in poor condition. Thus, an effective bone graft in terms of both osteoconductivity and osteoinductivity is required for clinical therapy. Recently, the three-dimensional (3D) kagome structure has been shown to be advantageous for bone tissue regeneration due to its mechanical properties. In this study, a polycaprolactone (PCL) kagome-structure scaffold containing a hyaluronic acid (HA)-based hydrogel was fabricated using a 3D printing technique. The retention capacity of the hydrogel in the scaffold was assessed in vivo with a rat calvaria subcutaneous model for 3 weeks, and the results were compared with those obtained with conventional 3D-printed PCL grid-structure scaffolds containing HA-based hydrogel and bulk-type HA-based hydrogel. The retained hydrogel in the kagome-structure scaffold was further evaluated by in vivo imaging system analysis. To further reinforce the osteoinductivity of the kagome-structure scaffold, a PCL kagome-structure scaffold with bone morphogenetic protein-2 (BMP-2) containing HA hydrogel was fabricated and implanted in a calvarial defect model of rabbits for 16 weeks. The bone regeneration characteristics were evaluated with hematoxylin and eosin (H&E), Masson’s trichrome staining, and micro-CT image analysis.
Graphic abstract







Similar content being viewed by others
References
Kwong FNK, Harris MB (2008) Recent developments in the biology of fracture repair. J Am Acad Orthop Surg 16(11):619–625. https://doi.org/10.5435/00124635-200811000-00001
Roberts TT, Rosenbaum AJ (2012) Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis 8(4):114–124. https://doi.org/10.4161/org.23306
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10(Suppl 2):S96–S101. https://doi.org/10.1007/s005860100282
Ben-Nissan B (2003) Natural bioceramics: from coral to bone and beyond. Curr Opin Solid State Mater Sci 7(4–5):283–288. https://doi.org/10.1016/j.cossms.2003.10.001
Fernandez JM, Molinuevo MS, Cortizo MS et al (2011) Development of an osteoconductive PCL–PDIPF–hydroxyapatite composite scaffold for bone tissue engineering. J Tissue Eng Regen Med 5(6):e126–e135. https://doi.org/10.1002/term.394
Nazirkar G, Singh S, Dole V et al (2014) Effortless effort in bone regeneration: a review. J Int Oral Health 6(3):120
Bae JC, Lee JJ, Shim JH et al (2017) Development and assessment of a 3D-printed scaffold with rhBMP-2 for an implant surgical guide stent and bone graft material: a pilot animal study. Materials 10(12):1434. https://doi.org/10.3390/ma10121434
Gautschi OP, Frey SP, Zellweger R (2007) Bone morphogenetic proteins in clinical applications. ANZ J Surg 77(8):626–631. https://doi.org/10.1111/j.1445-2197.2007.04175.x
Wei Y, Zhu G, Zhao Z et al (2021) Individualized plasticity autograft mimic with efficient bioactivity inducing osteogenesis. Int J Oral Sci 13(1):1–8. https://doi.org/10.1038/s41368-021-00120-w
James AW, LaChaud G, Shen J et al (2016) A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev 22(4):284–297. https://doi.org/10.1089/ten.TEB.2015.0357
McKay WF, Peckham SM, Badura JM (2007) A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int Orthop 31(6):729–734. https://doi.org/10.1007/s00264-007-0418-6
van Hout WM, van der Molen ABM, Breugem CC et al (2011) Reconstruction of the alveolar cleft: can growth factor-aided tissue engineering replace autologous bone grafting? A literature review and systematic review of results obtained with bone morphogenetic protein-2. Clin Oral Invest 15(3):297–303. https://doi.org/10.1007/s00784-011-0547-6
Li RH, Wozney JM (2001) Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol 19(7):255–265. https://doi.org/10.1016/s0167-7799(01)01665-1
Mumcuoglu D, Fahmy-Garcia S, Ridwan Y et al (2018) Injectable BMP-2 delivery system based on collagen-derived microspheres and alginate induced bone formation in a time- and dose-dependent manner. Eur Cells Mater 35:242–254. https://doi.org/10.22203/eCM.v035a17
Kempen DH, Lu L, Hefferan TE et al (2008) Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 29(22):3245–3252. https://doi.org/10.1016/j.biomaterials.2008.04.031
Lee JW, Kang KS, Lee SH et al (2011) Bone regeneration using a microstereolithography-produced customized poly (propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials 32(3):744–752. https://doi.org/10.1016/j.biomaterials.2010.09.035
Lee SJ, Lee D, Yoon TR et al (2016) Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater 40:182–191. https://doi.org/10.1016/j.actbio.2016.02.006
Park JY, Gao G, Jang J et al (2016) 3D printed structures for delivery of biomolecules and cells: tissue repair and regeneration. J Mater Chem B 4(47):7521–7539. https://doi.org/10.1039/c6tb01662f
Jang CH, Kim MS, Cho YB et al (2013) Mastoid obliteration using 3D PCL scaffold in combination with alginate and rhBMP-2. Int J Biol Macromol 62:614–622. https://doi.org/10.1016/j.ijbiomac.2013.10.011
Kang SW, Kim JS, Park KS et al (2011) Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone 48(2):298–306. https://doi.org/10.1016/j.bone.2010.09.029
Park JY, Shim JH, Choi SA et al (2015) 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mater Chem B 3(27):5415–5425. https://doi.org/10.1039/c5tb00637f
Deng CJ, Lin RC, Zhang M et al (2019) Micro/nanometer-structured scaffolds for regeneration of both cartilage and subchondral bone. Adv Funct Mater 29(4):1806068. https://doi.org/10.1002/adfm.201806068
Zhang B, Gui X, Song P et al (2022) Three-dimensional printing of large-scale, high-resolution bioceramics with micronano inner porosity and customized surface characterization design for bone regeneration. ACS Appl Mater Interf 14(7):8804–8815. https://doi.org/10.1021/acsami.1c22868
Zhang B, Wang W, Gui X et al (2022) 3D printing of customized key biomaterials genomics for bone regeneration. Appl Mater Today 26:101346. https://doi.org/10.1016/j.apmt.2021.101346
Pei X, Wu L, Lei H et al (2021) Fabrication of customized Ti6AI4V heterogeneous scaffolds with selective laser melting: optimization of the architecture for orthopedic implant applications. Acta Biomater 126:485–495. https://doi.org/10.1016/j.actbio.2021.03.040
Lee SH, Cho YS, Hong MW et al (2017) Mechanical properties and cell-culture characteristics of a polycaprolactone kagome-structure scaffold fabricated by a precision extruding deposition system. Biomed Mater 12(5):055003. https://doi.org/10.1088/1748-605X/aa8357
Lee SH, Lee KG, Hwang JH et al (2019) Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater Sci Eng C 98:949–959. https://doi.org/10.1016/j.msec.2019.01.050
Cho YS, Quan M, Kang NU et al (2020) Strategy for enhancing mechanical properties and bone regeneration of 3D polycaprolactone kagome scaffold: nano hydroxyapatite composite and its exposure. Eur Polymer J 134:109814. https://doi.org/10.1016/j.eurpolymj.2020.109814
Wang AJ, McDowell D (2004) In-plane stiffness and yield strength of periodic metal honeycombs. J Eng Mater Technol 126(2):137–156. https://doi.org/10.1115/1.1646165
Yun BG, Lee SH, Jeon JH et al (2019) Accelerated bone regeneration via three-dimensional cell-printed constructs containing human nasal turbinate-derived stem cells as a clinically applicable therapy. ACS Biomater Sci Eng 5(11):6171–6185. https://doi.org/10.1021/acsbiomaterials.9b01356
Kim BS, Jang J, Chae S et al (2016) Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication 8(3):035013. https://doi.org/10.1088/1758-5090/8/3/035013
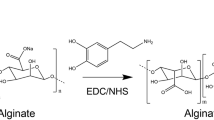
Kim J, Park Y, Tae G et al (2008) Synthesis and characterization of matrix metalloprotease sensitive-low molecular weight hyaluronic acid based hydrogels. J Mater Sci Mater Med 19(11):3311–3318. https://doi.org/10.1007/s10856-008-3469-3
Ku JK, Lee KG, Ghim MS et al (2021) Onlay-graft of 3D printed kagome-structure PCL scaffold incorporated with rhBMP-2 based on hyaluronic acid hydrogel. Biomed Mater 16(5):055004. https://doi.org/10.1088/1748-605X/ac0f47
Kim J, Kim IS, Cho TH et al (2007) Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 28(10):1830–1837. https://doi.org/10.1016/j.biomaterials.2006.11.050
Reyes RL, Ghim MS, Kang NU et al (2022) Development and assessment of modified-honeycomb-structure scaffold for bone tissue engineering. Addit Manuf 54:102740. https://doi.org/10.1016/j.addma.2022.102740
Patterson J, Siew R, Herring SW et al (2010) Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 31(26):6772–6781. https://doi.org/10.1016/j.biomaterials.2010.05.047
Kim J, Park Y, Tae G et al (2009) Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. J Biomed Mater Res A 88(4):967–975. https://doi.org/10.1002/jbm.a.31947
Lutolf MP, Lauer-Fields JL, Schmoekel HG et al (2003) Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci 100(9):5413–5418. https://doi.org/10.1073/pnas.0737381100
Schultz KM, Kyburz KA, Anseth KS (2015) Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc Natl Acad Sci 112(29):E3757–E3764. https://doi.org/10.1073/pnas.1511304112
Kim J, Kim IS, Cho TH et al (2010) In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res Part A 95(3):673–681. https://doi.org/10.1002/jbm.a.32884
Mumcuoglu D, Siverino C, Tabisz B et al (2017) How to use BMP-2 for clinical applications? A review on pros and cons of existing delivery strategies. J Trans Sci 3(5):1–11. https://doi.org/10.15761/JTS.1000195
Li J, Mooney DJ (2016) Designing hydrogels for controlled drug delivery. Nat Rev Mater 1(12):1–17. https://doi.org/10.1038/natrevmats.2016.71
Shim JH, Kim JY, Park M et al (2011) Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 3(3):034102. https://doi.org/10.1088/1758-5082/3/3/034102
Shim JH, Lee JS, Kim JY et al (2012) Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng 22(8):085014. https://doi.org/10.1088/0960-1317/22/8/085014
Lee F, Chung JE, Kurisawa M (2009) An injectable hyaluronic acid–tyramine hydrogel system for protein delivery. J Contr Release 134(3):186–193. https://doi.org/10.1016/j.jconrel.2008.11.028
Bhakta G, Rai B, Lim ZX et al (2012) Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 33(26):6113–6122. https://doi.org/10.1016/j.biomaterials.2012.05.030
Ishidou Y, Kitajima I, Obama H et al (1995) Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J Bone Mine Res 10(11):1651–1659. https://doi.org/10.1002/jbmr.5650101107
Spector JA, Luchs JS, Mehrara BJ et al (2001) Expression of bone morphogenetic proteins during membranous bone healing. Plastic Reconstruct Surg 107(1):124–134. https://doi.org/10.1097/00006534-200101000-00018
La WG, Kang SW, Yang HS et al (2010) The efficacy of bone morphogenetic protein-2 depends on its mode of delivery. Artif Organs 34(12):1150–1153. https://doi.org/10.1111/j.1525-1594.2009.00988.x
Yang HS, La WG, Cho YM et al (2012) Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp Mol Med 44(5):350–355. https://doi.org/10.3858/emm.2012.44.5.039
Uludag H, Golden J, Palmer R et al (1999) Biotinated bone morphogenetic protein-2: in vivo and in vitro activity. Biotechnol Bioeng 65(6):668–672. https://doi.org/10.1002/(sici)1097-0290(19991220)65:6%3c668::aid-bit7%3e3.0.co;2-8
Haidar ZS, Hamdy RC, Tabrizian M et al (2009) Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett 31(12):1817–1824. https://doi.org/10.1007/s10529-009-0099-x
Acknowledgements
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI14C2143), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (NRF-2021R1A2C2009665). We thank the Laboratory of Animal Research core facility at the ConveRgence mEDIcine research cenTer (CREDIT), Asan Medical Center for support and instrumentation.
Author information
Authors and Affiliations
Contributions
SHL, KGL, JL, YP, YSC, and BKL contributed to conceptualization; SHL, KGL, and JL contributed to writing-original draft preparation and visualization; JL and KGL contributed to synthesis of HA-hydrogel system; SHL, YSC, and MSG contributed to 3D printing; KGL and SK contributed to animal experiment; SHL, KGL, and JL contributed to analysis of experimental results; SHL, KGL, SJH, YP, YSC, and BKL contributed to review and editing; and YP, YSC, and BKL contributed to supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
IACUC approve numbers: 2017-12-002 and 2017-12-068. All institutional and national guidelines for the care and use of laboratory animals were followed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, SH., Lee, KG., Lee, J. et al. Three-dimensional kagome structures in a PCL/HA-based hydrogel scaffold to lead slow BMP-2 release for effective bone regeneration. Bio-des. Manuf. 6, 12–25 (2023). https://doi.org/10.1007/s42242-022-00219-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00219-x