Abstract
Ammonia is considered as an alternative fuel resource for a sustainable green future. The production of ammonia involves the electrochemical nitrogen reduction reaction (NRR), which has gained considerable attention due to its eco-friendly resources and nonharmful byproducts. Even with the manifold works on NRR, the technique has not reached the industrial scale because of the impediments of NRR electrocatalysts, and in addition, state-of-the-art electrocatalysts have not yet been discovered. In this review, first, the mechanism of the NRR, key metrics, and operational procedures for NRR electrochemistry are presented. Then, the electrocatalyst designs for efficient NRR are briefly introduced, followed by a discussion on the influence of the electrolytes that enhance NRR performance. The counterion effects of electrolytes on NRR performance and strategies for suppressing the HER by electrolyte additives are also discussed. Later, the NRR mechanisms are upgraded, and a comprehensive review of metal-N2 batteries is provided. This review summarizes the effective methods for performing the NRR and strategies to suppress the HER on various electrocatalysts by tuning electrolytes and their additives. The review concludes by discussing the prospects of metal-N2 batteries.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world’s population is expected to reach 11 billion by the end of this century (2100) from 7.7 billion people in 2019, according to a report launched by the United Nations (UN) [1]. Modern energy demands, due to this profusion, have put forward an essential threat to seeking renewable, sustainable, economical, and eco-friendly strategies for tackling the upcoming energy crisis. The development of electrocatalysts paved an efficient, economical path to obtain products, such as hydrogen (H2), ammonia (NH3), and hydrocarbons, and the production of value-added chemicals from earth-abundant materials, such as water, nitrogen (N2), oxygen (O2) and carbon dioxide (CO2). Over decades, scientists have relentlessly designed electrocatalysts that target the nitrogen reduction reaction (NRR) [2], hydrogen evolution reaction (HER) [3], oxygen reduction reaction (ORR) [4], carbon dioxide reduction reaction (CO2RR) [5], and many other [6] electrochemical reactions that facilitate the formation of fuel products and value-added chemicals.
Among the valuable fuel products, ammonia (NH3) has stood out greatly, exhibiting better storage and transport facilities and more cost-efficient and eco-friendly production pathways than hydrogen, and ammonia is a long-term energy resource that serves as a low-cost hydrogen energy carrier [7]. Additionally, it serves as a source of fertilizers for agriculture and raw materials for explosives and coolants. The efficient production of ammonia, as shown in Eq. (1), has invariably been an important part of research interests for decades [8]. Currently, major ammonia production follows the polluting Haber–Bosch process. The Haber–Bosch technique uses high temperatures of approximately 350 °C and pressures ranging from 250 to 350 bar (1 bar = 100 000 Pa), and the minimum energy consumption is approximately 27.4–31.8 GJ \({\mathrm{t}}_{{\mathrm{NH}}_{3}}^{-1}\) with an efficiency of 65% [9]. This higher energy consumption in the Haber–Bosch process can be lessened by alternative routes, an enzymatic pathway and an electrochemical pathway. Along the enzymatic pathway, nitrogenase enzymes produce more hydrogen than the required ammonia, which shows the unreliability of the enzymatic pathway [10]. Ultimately, the choices are reduced to the electrochemical NRR, which is a promising candidate for green ammonia production.
Electrochemical ammonia production involves NRR that adsorbs N2 molecules on the catalyst surface. The electrochemical NRR uses electricity derived from renewable sources and N2, along with protons from water dissociation.
Figure 1 represents the electrochemical ammonia production from water and renewable electricity and its application in various industrial and transportation sectors. Electrochemical NRR in an electrochemical cell is promoted by using an electrocatalyst that efficiently adsorbs N2 gas onto the electrode surface and reduces it to ammonia. Electrocatalysts with a large surface area, highly porous structures with intrinsic catalytic activity, and excellent bulk conductivity exhibit better nitrogen adsorption energies and stability to catalyse the electrochemical reaction. However, this electrochemical NRR is suppressed by the slower kinetics of nitrogen adsorption onto the surface, and the splitting of the N2 triple bond leads to reduced faradaic efficiency (FE) and lower ammonia production rates [11]. Studies show that the limiting potential of the HER is smaller than that of the NRR, which indicates that the HER competes with the NRR with smaller potentials for H2 evolution in an electrocatalyst [12]. Additionally, from a kinetic point of view, protons and electrons undergo H2 formation rather than NH3 formation, which accounts for the extremely low faradaic efficiency (FE) and the reduction in ammonia yield of the electrocatalysts [13]. Thus, electrocatalysts are classified as noble metal-based, nonnoble metal-based, metal-free, and single-atom catalysts (SACs) that exhibit different orders of activity depending on their unique surface adsorption properties and affinity towards N2 triple bond splitting.
HER suppression is of utmost importance for effective NRR performance. The active sites of the electrocatalyst are heavily utilized by the protons from the electrolyte because of the electronic effects between the electrocatalyst and the H+ ions. This usage has a negative impact on the ammonia yield and the FE of the electrocatalyst, reducing the NRR performance. Hence, selectivity towards N2 must be increased for electrocatalysts over the HER. Strategies such as tuning the d-bands of the electrocatalyst [14] and electrolyte modifications [15,16,17] have been followed and promising results are shown in reducing the HER. In later sections, we clearly discuss the important strategies that inhibit the HER for efficient NRR performance.
Upgrading the electrochemical NRR mechanisms into an energy storage device has proven to be the most needed plan for the current energy-demanding society. The importance of electrochemical energy storage (EES) devices has increased in the modern era of technology for electric vehicles and smart grid power storage [18, 19]. Various EES devices, such as rechargeable batteries, flow batteries, metal gas batteries, supercapacitors, and fuel cells, can store energy from smart grids and supply as needed. Among these devices, metal N2 batteries have started attracting attention due to their readily available resources and the importance of the final product. Metal-N2 batteries have importance due to ammonia production with simultaneous electricity generation. The history of metal-N2 batteries dates back to 2017 when Zhang et al. [20,21,22,23,24,25] decided to fabricate a rechargeable Li-N2 battery inspired by the high energy density of metal gas batteries, Al-CO2, Li-CO2, Li-SO2, Na-CO2, and Li-O2. Later, several reports were published in response to the novelty of the work and prime expectations of metal-N2 battery systems, as shown in Fig. 2 [26]. Soon after the first Zn-N2 battery was reported in 2019 under an aqueous alkaline electrolyte, novel design strategies for the electrocatalyst were proposed and demonstrated as cathodes for the Zn-N2 battery. The aprotic electrolytes in metal-N2 batteries make their construction and maintenance tedious. Fortunately, alkaline metal-N2 batteries have provided a safer, promising, and reliable approach due to the wide availability of resources and low maintenance costs. However, the limited NRR performance of alkaline metal-N2 batteries shows the incubatory stage of these batteries for which various electrocatalysts have been constantly engineered to meet the current demands.

© 2017, 2019, 2020, 2021 Elsevier Ltd. Adapted with permission from Ref. [27]. Copyrights © 2021, American Chemical Society. Adapted with permission from Refs. [31, 32, 47, 50]. Copyrights © 2019, 2020, 2021, Royal Society of Chemistry. Adapted with permission from Ref. [189]. Copyrights (c) 2019, John Wiley & Sons, Inc
Presently, various metal-N2 batteries are available, each having its own advantage owing to its cathode reactivity and anode configuration. An active electrocatalyst as the cathode is the integral unit for the battery because it effectively increases the activity by offering minimum resistance for the charge‒discharge mechanisms. To date, only a few metal-N2 batteries have been demonstrated (Li-N2, Na-N2, Al-N2, Zn-N2), and further upgrades to increase the faradaic efficiency (FE) and ammonia yield of electrocatalysts with a higher power density are foreseen. The real challenge lies in the activation of the strong triple bond of the nitrogen molecule, which prevents the battery system from reaching maturity.
Metal-N2 batteries simultaneously undergo redox reactions at the cathode and metal anode to drive the charge storage mechanism. In the case of metal-N2 batteries, N2 reduction and oxidation reactions occur at the cathode for the discharge‒charge cycles, respectively [20, 27]. Therefore, simultaneous ammonia production and electricity generation can be manifested over metal-N2 batteries, which show importance over conventional metal-air batteries [27, 28]. Among the reported metal-N2 batteries, including Li-N2 batteries [20], Na-N2 batteries [29], and Al-N2 batteries [26], Zn-N2 batteries hold great prospects due to the stability of the Zn anode in an alkaline environment and the fabrication of the battery in an ambient atmosphere [30]. In addition, Zn is widely available in the Earth’s crust, is eco-friendly, has high capacity and stable discharge potential, and is a mature technology compared to other batteries [31, 32]. Therefore, Zn-N2 batteries hold assuring commercial value and can be easily developed to provide industrial-scale applications.
The present review article focuses on the rational design strategies of an electrocatalyst from electrochemical NRR into metal-N2 batteries. The review includes discussions on the novel electrocatalyst design strategies and the mechanisms of different electrocatalyst structures. The important role of electrolytes and the counterions in the electrolytes in driving the electrochemical NRR are reviewed. The advantage of suppressing the HER by electrolyte modifications is also explained. Moreover, incorporating NRR electrocatalysts into the metal-N2 battery system and future aspects of metal-N2 batteries are also discussed. Based on this discussion, the review develops a research orientation to incorporate an electrocatalyst as an active cathode for potential metal-N2 battery applications.
2 Fundamentals of Electrochemical NRR and Metal-N2 Batteries
The reduction of nitrogen to ammonia via the electrochemical NRR route is a feasible green method, and this approach is more energy efficient than the conventional Haber–Bosch process because of the low operating temperature. The electrochemical NRR process uses water, rather than natural gas, as a H2 source [33]. NRR occurring at the electrocatalyst surface must be immune to hydrogen adsorption. The merits of the electrocatalyst include improving NRR selectivity and increasing ammonia yield and FE. NRR at the electrocatalyst surface occurs at more negative potentials, which can be compromised by HER. From a thermodynamic perspective, we can observe the HER being more favourable than the NRR due to the lower free energy of formation required for H2 [34].
2.1 Theory of N2 Adsorption
Nitrogen (N2), a homonuclear nonpolar molecule, is a Lewis base surrounded by a lone pair of electrons. It does not possess a permanent dipole moment because of the even distribution of charges and equal sharing of electrons, as shown in Fig. 3a. Moreover, N2 has a triple bond, with each atom holding 7 electrons with an electronic configuration of 1s22s22p3, which has pairs of electrons in the s orbitals with opposite spin and electrons in the p orbital with the same spin. N2 forms a triple bond that exhibits excellent stability. However, ammonia production solely lies in N2 bond cleavage and initial protonation. Further difficulties can be offered by the thermodynamic constraints from the intermediates during catalytic N2 reduction [35, 36]. The adsorption of N2 onto the electrocatalyst surface occurs at specific electron-deficient active sites. The active sites include transition metal sites with unoccupied d orbitals or heteroatoms with sp2 orbitals that actively adsorb N2.
As shown in Fig. 3b, c, the unoccupied d-orbitals receive an electron from N2 to form a σ-bond and donate another occupied d-orbital electron back into the vacant π⃰ antibonding orbital, creating a π-back-donation effect, called the “donor–acceptor” mechanism. The donor–acceptor mechanism on N2 activates the triple bond and forces it to remain adsorbed in the active site. Similarly, in the case of nonmetals, the vacant sites and the Lewis acid sites with an empty orbital accept an electron from N2 to form a σ-bond and supply an electron from the neighbouring site into the π⃰ orbital of N2, promoting the activation of the triple bond, as shown in Fig. 3d. This donor–acceptor mechanism of activating the N2 triple bond has provided a general idea for designing electrocatalysts with transition metals and nonmetal substrates that exhibit higher affinity towards N2 adsorption [36, 37].
2.2 Mechanism of N2 Reduction
Ammonia can be produced by reducing N2 in an electrochemical cell. Successful electrochemical N2 reduction may follow an indirect pathway or a direct pathway. The indirect pathway is mediated through nonaqueous aprotic electrolytes and involves forming intermediates. These intermediates are usually the corresponding metal nitrides that can be hydrolysed to produce ammonia. Because of the instability, recyclability, and scalability of nonaqueous electrolytes, indirect ammonia synthesis does not compete with the current demands. However, because of the limited proton content, the indirect approach effectively suppresses the HER and side reactions, which produce higher ammonia yields with higher FE [38,39,40,41,42]. The Li-mediated ammonia synthesis (LiMEAS) pathway has been recognized by researchers and shows promising ammonia yields involving nitrides, but the process is energetically expensive and involves a two-step ammonia production method [40, 41, 43]. The direct pathway can be divided into two categories based on the nature of the electrolyte, viz., nonaqueous and aqueous. Ammonia production in nonaqueous electrolytes uses sacrificial proton donors, such as alcohols or cationic complexes, to protonate the adsorbed N2 into ammonia [38, 39, 41, 42]. Ammonia production in aqueous electrolytes utilizes water as the proton source, which may be acidic, alkaline, or neutral in nature, for direct ammonia synthesis. The aqueous pathway is the most feasible approach to produce ammonia from atmospheric N2, protons from water, and electricity from renewable sources.
Direct ammonia synthesis occurs under various pathways, of which dissociative and associative pathways are primarily followed. The dissociative and associative mechanisms are differentiated by either direct dissociation of the N2 triple bond or by protonation of the adsorbed N2 molecule, respectively. Furthermore, the associative mechanism is classified into two pathways: associative alternating and associative distal, as shown in Fig. 4. The dissociative pathway involves a large kinetic barrier that consumes high input energy for N2 triple bond dissociation (∆H = 941 kJ mol−1) [44]. The hydrogenation of the dissociative adsorbed N usually occurs with the H2 produced from natural gas, producing CO2 as the byproduct.
The associative pathway reduces the nitrogen molecule into ammonia in six proton-coupled electron transfer (PCET) steps. In the associative pathway, the N‒N bond remains intact until all or some of the hydrogenation process is under the influence of end-on adsorption of dinitrogen to the catalyst surface. Moreover, this associative mechanism is further classified into alternating and distal pathways based on the sequential addition of hydrogen to the activated dinitrogen. In the associative alternating pathway, hydrogenation occurs simultaneously on both nitrogen atoms, and the ammonia molecule is released in the subsequent steps, as shown in Fig. 4b. In the associative distal pathway, the hydrogenation series occurs only on the first nitrogen atom, and only after the release of the first ammonia molecule does protonation of the other nitrogen occur, as shown in Fig. 4c [35].
Apart from these mechanisms, another pathway preferentially occurs on the surface of transition metal nitrides (TMNs) called the Mars–van Krevelen (MvK) mechanism, as shown in Fig. 4d [45]. In this pathway, first, the nitrogen atoms on the surface of the catalyst are protonated to reduce to ammonia (NH3), leaving behind a vacancy. Later, the vacancy is replenished with externally supplied nitrogen gas and is reduced to ammonia following the distal pathway [46]. In particular cases, the nitrogen atoms from the bulk are transferred to the N-vacant sites and are consecutively reduced to ammonia. This bulk migration of the N atoms to the surface transforms the electrocatalyst, hindering its stability [46].
2.3 Metal-N2 Battery
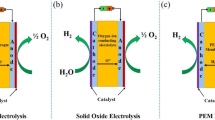
Metal-air batteries have had a large impact on the scope of efficient energy storage devices. Inspired by the dual role of metal-CO2 batteries in gas conversion with simultaneous electricity generation, researchers envision the metal-N2 battery system for greater utilization of the conversion of atmospheric N2. Similar to metal-air batteries, the metal-N2 battery is constructed with a metal anode and NRR electrocatalyst-coated electrode as the cathode [20, 32, 47]. The electrode terms cathode and anode for the electrocatalyst-coated electrode and metal electrode remain the same, irrespective of the charge‒discharge cycles. The mechanism of the metal-N2 battery with the electrode terms is explained below for further clarification. The redox reaction in a metal-N2 battery varies with the nature of the electrolyte, viz., nonaqueous and aqueous electrolytes. In a nonaqueous metal-N2 battery, during discharge, N2 reduction at the cathode by the corresponding metal ions forms nitrides (Fig. 5a), which can later be hydrolysed to ammonia [20, 29, 47]. During charging, under an external bias, the nitrides at the cathode dissociate into metal ions and gaseous nitrogen, as shown in Fig. 5b [20, 29, 47]. The cathode serves as a conductive substrate as well as a nucleation site for anchoring the discharge products in nonaqueous metal-N2 batteries. The common nonaqueous batteries fabricated thus far are the Li-N2 battery, Na-N2 battery, and Al-N2 battery [20, 29, 47].
Because of the economic, operational, and maintenance difficulties of using aprotic electrolytes, research has been targeted to design aqueous metal-N2 batteries [26, 27, 30,31,32, 48, 49]. In aqueous metal-N2 batteries, the cathode proceeds through the nitrogen reduction reaction (NRR) and oxygen evolution reaction (OER) during discharging and charging, respectively [32]. During discharging, the cathodic reaction is the NRR, which draws electrons and protons from the oxidized metal anode and electrolyte, respectively, forming ammonia (Fig. 5c) [26, 31]. Thus, simultaneous ammonia production and electricity generation can be manifested [49, 50].
During charging, the external bias reduces the oxidized metal, and the direction of electron flow is reversed (Fig. 5d) [32]. Accordingly, the anodic reaction over the cathode evolves oxygen as a result of the OER. The oxidation reaction at the electrocatalyst incorporates electrons into the cathode from the electrolytic hydroxide anions. Thus, in an alkaline metal-N2 battery, the discharge process at the cathode occurs with the NRR, resulting in ammonia formation, and the charging process at the cathode occurs with the OER.
The NRR during discharge usually follows the associative pathway or the MvK mechanism, as stated previously, depending on the nature of the electrocatalyst. The mechanism of the OER following a 4-electron pathway during charging is stated below [51, 52].
where * represents the surface adsorption site of the bifunctional electrocatalyst at the cathode. Initially, upon charging, the first hydroxide ion is adsorbed on the electrode active site by one electron transfer (Eq. 2). Then, the second and third hydroxide ions react with the adsorbed hydroxide ion to form oxide and oxyhydroxides, which involve the second and third electron transfer steps (Eqs. 3, 4), respectively. Finally, the fourth OH¯ ion reacts with the oxyhydroxide to release water and oxygen molecules into the electrolyte (Eq. 5).
2.4 Key Metrics in Determining NRR Performance
The key parameters for determining the rate of the NRR are ammonia yield and faradaic efficiency (FE). Graphs are drawn for the applied potential (V) vs. the obtained production rate of ammonia and FE, respectively. These parameters determine the activity of the electrocatalyst towards the NRR and its selectivity over the HER.
2.4.1 Calculation of Ammonia (NH3) Yield
The NH3 yield rate indicates the amount of ammonia produced per catalyst loading mass/area and unit time, which directly relates to the adsorption (N2)/desorption (NH3) properties of the electrocatalyst. Experimentally, the ammonia yield is measured by using chronoamperometry analysis for a period of time and evaluated through Eq. (6).
where [NH4+] is the measured concentration of ammonia from UV‒vis spectrometry and the calibration curves of the standard ammonia solution, V is the volume of the electrolyte, t is the reaction time, and A is the electrode surface area [53].
2.4.2 Calculation of Faradaic Efficiency (FE)
Faradaic efficiency (FE) is given by the ratio of the faradaic current used for the NRR to the total current supplied to the electrochemical system. This parameter determines the selectivity of the electrochemical process of the NRR over the competing HER. The FE of any catalyst surface can be obtained using Eq. (7).
where 3 is the number of electrons for producing one NH3 molecule, F is the Faraday constant (F = 96 485.33), and j is the current density measured for the corresponding ammonia yield \({r}_{{\mathrm{NH}}_{3}}\) [53].
2.4.3 Determination of Cell Voltage
Experimentally, the cell potential is calculated by determining the potential difference between the two electrodes (cathode and anode) and is expressed in volts (V), as shown in Eq. (8) [54].
2.5 Determination and Analysis Techniques
Detecting and quantifying the produced ammonia, postsynthesis, is essential for reviewing the NRR activity of the electrocatalyst. The detection techniques are performed on the sampled electrolyte, and the methods for ammonia detection must be extremely delicate, dependable, precise, and reproducible. Ammonia quantification must be accurate because of various factors that contribute to overestimating ammonia, such as the time of quantification, the pH of the solution, foreign agents that produce ammonia-containing products other than the catalyst, and the nature of the electrolyte. Initially, ammonia detection is performed on the sampled electrolyte by the UV‒vis spectrophotometric method, involving the colouring agents of –(N=N)–. Later, the quantification of the produced ammonia using 15N2 feeding gas was observed to verify the source of nitrogen used in ammonia synthesis [55, 56].
2.5.1 Spectrophotometry Method
The spectrophotometry method, also known as the colourimetric method, is based on the Berthelot reaction, which involves the indophenol blue method to form a dye product with produced ammonia. This product can be observed under UV‒vis spectroscopy to record the absorption spectrum at certain wavelengths corresponding to the abovementioned methods. The indophenol blue method, because of its lower detection limit (0–0.6 mg NH3–N L−1), better reproducibility, and consistent colour development, is preferred. In this method, the sampled electrolyte is treated with a colouring solution of NaOH containing salicylic acid and sodium citrate. To this solution, a catalyst solution of sodium nitroprusside in DI water and oxidizing solution made of sodium hypochlorite (NaOCl) in NaOH is added [7, 57, 58]. The resulting solution is left undisturbed for an hour, and the UV‒vis absorption spectra are recorded. The spectra reveal an absorption band at approximately 680 nm corresponding to the blue region, indicating the presence of ammonia. The higher the intensity of the absorption peak is, the higher the concentration of ammonia in the sample is. To calculate the concentration of the produced ammonia, concentration–calibration graphs are drawn for the standard solution of ammonia, and peak fitting gives the obtained ammonia concentration.
The peak fitting of the concentration–absorbance graph at the absorption peak maximum gives a linear equation y = mx + c. The straight-line equation is the linear relation between absorbance and the concentration of the standard ammonia solution, as shown in Eq. (9).
where A is the absorbance intensity, ε is the molar extinction coefficient (the slope) obtained from the concentration–calibration graph, C is the concentration of the ammonia in the sample, and b is the y-intercept of the concentration–calibration graph. The absorbance values for a particular voltage for an electrocatalyst are applied to the linear equation to calculate the concentration of the produced ammonia in the sample. Irrespective of the method used for ammonia determination, a second procedure for quantifying ammonia is also needed to account for the source of produced ammonia.
2.5.2 Isotope Labelling Method
The isotope labelling procedure is followed to quantify the source of the produced ammonia, which is either N from the substrate or the N2 feeding gas. The isotope labelling method for ammonia quantification uses 15N2 as the feeding gas, whose chemical properties do not greatly differ from those of 14N2. NRR experiments under 15N2 feeding gas can be used to quantify the source of the produced ammonia. The sampled electrolyte after NRR experiments with 15N2 feeding gas must contain only 15NH4+ ions; therefore, any other contaminants from the catalyst or impurities from the electrolyte can be easily neglected to determine the actual ammonia yield rate due to the NRR. For the 15N2 feeding gas experiment, the amount of detected 15NH4+ must be greater than that of 14NH4+, and the total ammonia yields for the 15N2 and 14N2 feeding gases must be similar. The similar ammonia yield results corroborate that no external impurities or degradation of the catalyst contributed to the final ammonia yield [59]. Thus, determining and quantifying the produced ammonia are essential parameters of the NRR, and purified gas must be provided to avoid any contamination and false determination.
3 Electrocatalysts for Ammonia Production
Electrochemical NRR is a possible route for synthesizing ammonia under ambient conditions. The stable nonpolar bonds of N2 make the molecule chemically inert, which inhibits N2 bond cleavage in an ambient atmosphere. This problem outlines the need for an electrocatalyst with high selectivity towards N2 adsorption and sufficient energy to activate the triple bonds. The electrocatalysts must offer a few amenities that promote N2 adsorption and NH3 desorption, thereby limiting the HER, which can be fabricated as an electrode in a metal-N2 battery. The Sabatier principle states that “the binding energy between the catalyst and the reactant must be neither too strong nor too weak.” This rule is the key aspect of designing any electrocatalyst and is of major concern when screening electrocatalytic materials that exhibit sustainability over cost [60].
Activity, selectivity, and stability are the three parameters that determine the performance of an electrocatalyst. For efficient activity, the catalyst must have many available active sites and sufficient pore sizes for the reactants to access the active sites. In selectivity, the material must have surface adsorption potentials that favour the electrochemical NRR by inhibiting the inimical HER. Finally, the catalyst must have a stable structure and tolerate corrosion to sustain long-term operation that eventually reduces the maintenance cost and achieves the efficient use of materials for designing electrocatalysts.
3.1 Screening Metals for NRR Electrocatalysis
An indispensable aspect of electrocatalytic material is selectivity for the valuable NRR over the hindering HER. Theoretically, the best electrocatalysts are transition metals, which were analysed by Skúlason et al. [35] for efficient NRR electrolysis and derived from a volcano plot. The plot sorts out the possibility of using d-metals as catalysts for their preferred activity and selectivity towards the NRR, as shown in Fig. 6a. The top of the diagram indicates that Rh, Ru, and Ir have the highest H adsorption energies, while Pd and Pt are at more negative energies, which indicate prime H adsorption. Moreover, transition metals such as Zr, Sc, Y, and Ti show more negative N adsorption energies, as observed from theoretical studies. Later, researchers used screening procedures to compile and engineer materials suitable for the requirements using simulations and experimental procedures. In addition, Dražević et al. [61] studied the N2 binding energy parameters from experimental data and the limiting potentials from DFT studies. The study includes the results from Trasatti et al. [62], who calculated the possible HER reactivity of metals and derived a plot to demonstrate the binding energies of the M‒H bond over various metals (Fig. 6b).

Copyright © 2011, The Royal Society of Chemistry. b Volcano plots for the exchange current density of HER vs. MH bond strengths for different metals; 1 kcal mol−1 = 4 185.85 J mol−1. c Volcano plots for 31 metals for the estimated limiting potentials of the first and fifth PCET vs. Ecell (MN) values. d Extended inset plot of (c) for the elements on the right leg of the volcano plot. Reprinted with permission from Ref. [61]. Copyright © 2020, Elsevier Ltd
a Volcano plot for the reduction of nitrogen on transition metal surfaces through dissociative and associative mechanisms. Reprinted with permission from Ref. [35].
The result of the study of M‒N binding energy was a volcano plot, as shown in Fig. 6c, d, which revealed Mn, Ga, and In as potential NRR candidates and unidentified elements on top of the plot. Combining the experimental HER exchange current densities and the Pourbaix diagrams, the author confirmed the preferable activity of Mn, Ga, and In towards the NRR in water over the HER. However, contrary to previous studies, Fe, Au, Cu, Bi, and Pd required smaller potentials to initiate the HER than the NRR. Similar studies have been performed to evaluate the selectivity for N2 adsorption for efficient HER suppression and preferential NRR pathways over modified transition metal-incorporated carbon-based electrocatalysts [63, 64]. Thus, an effective screening of various metals helps experimentalists to design an electrocatalyst preferring the NRR over the HER with an appropriate choice of metals.
The electrocatalysts are primarily classified into three categories based on the materials used for their construction, viz., noble metal-based, nonnoble metal-based, and metal-free catalysts. Researchers have developed technologies for screening efficient metal and nonmetal surfaces by theoretical studies that indicate the redox potentials available on the electrocatalyst surface to estimate the catalytic activity for the noxious HER and the expected NRR [46]. To date, a large amount of work has concentrated on designing electrocatalysts to perform in aqueous electrolytes. Nonaqueous electrocatalysts, mostly gas diffusion electrodes, are made of Cu electrodes, stainless steel cloth, Mo foil, bare carbon cloth, and Au-coated carbon fibrous paper (Au/CP) [40, 43, 65,66,67]. They primarily target the adsorption of N2, and the absence of protons in the electrolyte facilitates increased ammonia yield and higher FE. However, because of the unreliable, non-eco-friendly nature of nonaqueous systems, aqueous-based ammonia production techniques have gained increased attention.
3.2 Noble Metal-Based Catalysts
Various electrocatalysts have been reported for the NRR, including noble metal-based electrocatalysts, such as Pt [68], Pd [69], Au [70], Ru [71], and Rh [72]. Noble-metal SAC electrocatalysts exhibit excellent NRR activity with high NH3 yield and considerable faradaic efficiency (FE), but they are susceptible to corrosion, and their lack of availability is also a detriment to efficient catalysis [73, 74]. Researchers have utilized noble metals to their fullest potential via strategies that enhance accessibility to metal active sites. Strategies such as designing a firm substrate for metal loadings, do** noble metals with transition metals, single-atom catalysts (SACs) and phase interface structures are followed to minimize the catalyst loading and extract the highest performance from the limited metal availability. To utilize noble metal atoms effectively, Yu et al. [75] adopted the SAC method. They developed a mesoporous Rh film with B- and S-do** on Ni foam (B, S-mRh/NF) to show the efficient NRR catalytic activity of noble metals and the effective utilization of scarce metals. B- and S-do** greatly enhances the catalytic activity by tuning the electronic density of Rh, which increases the N2 adsorption ability, and S-do** maintains the selective hydrogenation of N2 to NH3. The synergistic effects of the noble metal with the heteroatoms for a selective function of the electrochemical NRR and an effective choice of a substrate directly influence the electrocatalytic activity.
Another strategy for the effective use of noble metal catalysts is through alloy engineering of either two noble metal atoms or a transition metal and a noble metal atom (Fig. 7b) [76]. On the basis of the alloy structures, Zhao et al. designed a bimetallic nanoalloy (NA) made of Ru and Rh, forming an fcc crystal lattice (RhxRu1−x NA) [7]. The coupled effects of the Rh-Ru metal atoms induce electronic interactions between metals and the substrate with an ammonia yield of 57.75 μg h−1 mgcat−1 and an FE of approximately 3.39%.

Copyright © 2021, Elsevier Ltd. b General strategies for alloy engineering of two noble metals. c Comparison of the ammonia yields and FE of Pd/α-MnO2, Pd/β-MnO2 and Pd/γ-MnO2. Reprinted with permission from Ref. [77]. Copyright © 2020, MDPI. d LSV curves for WO3 and single atoms and nanoparticle Pt atoms on WO3 and Pt nanoparticles, respectively, in an Ar-saturated 0.1 M K2SO4 (1 M = 1 mol L−1) electrolyte. e Comparison of the ammonia yield and FE of Pt SA/WO3 and Pt NP/WO3 for various applied voltages in 0.1 M K2SO4. Reprinted with permission from Ref. [68]. Copyright © 2020, John Wiley & Sons, Inc
a Schematic of the electrochemical cell with BS-mRh/NF as the cathode. Reprinted with permission from Ref. [75].
The interfacial activity of noble metals with modified transition metal substrates exhibits considerable NRR performance. Sun et al. [77] reported that the phase interface of Pd/γ-MnO2 was active for nitrogen reduction to ammonia. Additionally, this study suggested an increased activity of the γ-MnO2 substrate compared with the α-MnO2 and β-MnO2 substrates. This increased activity can be attributed to the combined planar and pyramidal oxygen atoms on the γ-MnO2 substrate (Fig. 7c) [78]. The Mn atoms initially adsorb N2 on the surface because of their strong N2 adsorption ability. Pd atoms then protonate the adsorbed N2 by the Grotthuss-like proton hop** mechanism. Thus, utilizing the individual properties of different phases can enhance ammonia production because of the modified interfacial properties. Although Pt is a state-of-the-art electrocatalyst for the HER, single Pt atoms have been effectively implanted on suitable substrates to improve NRR performance and suppress the HER. Hao et al. [68] deposited isolated Pt atoms on a WO3 nanoplate (Pt SAs/WO3) substrate, which suppressed the HER and improved the NRR activity to deliver higher ammonia yields and FE. The observed NRR performances reveal that the single-atom Pt catalysts (Pt SA/WO3) exhibit better NRR activity than the nanoparticle Pt catalysts (Pt NP/WO3), as shown in Fig. 7d, e. Thus, the single-atom catalyst strategy is a worthy candidate for expressing higher yield and FE by noble metal-based NRR electrocatalysts. Thus, the appropriate use of a noble metal and a better substrate choice can enhance the electrocatalytic activity of the metal loadings and the doped heteroatoms, and despite its availability and cost, it delivers outstanding activities.
3.3 Transition Metal-Based Catalysts
Although noble metals are efficient electrocatalysts for the electrochemical NRR, their lack of availability in the Earth’s crust and their high cost are detrimental to the cost-efficient NRR process. Transition metals are available in abundance and facilitate active sites for N2 adsorption with their partially filled d-orbitals [79]. Transition metal derivatives such as oxides [53], nitrides [80], carbides [81], dichalcogenides [82,83,84], bimetallic alloys [85, 86], MXenes, and MBenes [87, 88], as well as metal–organic frameworks (MOFs) [70] and single-atom catalysts [89], provide a platform for incorporating more accessible active sites, improved reaction kinetics, selectivity for the NRR, and stability. Transition metal-based electrocatalysts adopt various design strategies and morphologies that substantially enhance the NRR.
3.3.1 Metal–Organic Framework Electrocatalysts
Researchers have been obsessed with designing an efficient structure capable of providing large pore sizes for mass transport, as well as an effective conducting path for electrons. In this context, Cong and his group synthesized porphyrin-based single-site MOFs with Fe (Fe-TCPP) nodal sites as the catalytic centres for artificial N2 fixation (Fig. 8a) [8f). Although a handful of theoretical works implement a COF into electrocatalysts, experimental insights into the same structures are most expected. These structures can possibly meet the requirements of electrode materials for air-battery applications, as well as flexible, rechargeable battery applications [92, 93]. The prominent nonnoble metal-based electrocatalysts are derivatives of transition metal complexes. They are made to tune the intrinsic electronic properties, and they are planted onto a substrate that enhances the electronic conductivity of the electrocatalysts.
3.3.2 Single-Atom Electrocatalysts
Single-atom catalysts as metallic electrocatalysts offer an efficient design strategy for metal species that are well distinguished by their mononuclear active sites.
The SAC strategy is adopted to effectively utilize the metal species and increase the specific activity of the electrocatalyst. SACs anchored on substrates exhibit higher exposure to electrolytic ions, which enhance adsorption towards N2. Similar to noble metal SACs, transition metals such as Mo [94], Mn [95], and Fe [96] were reported as SACs with mostly M–N–C coordinated sites, which expose more active sites for selective N2 adsorption.
The M–N–C active sites can efficiently perform heterogeneous catalysis with their isolated single atoms dispersed along the substrate. For instance, Han et al. [94] reported single-atom catalysts (SACs) and nanoclusters (NCs) of Mo atoms embedded on nitrogen-doped porous carbon (SA-Mo/NPC) for the NRR (Fig. 9a-c). When Mo atoms form clusters, the activity is obvious, and their active site density is reduced compared to that of a single Mo atom. The improved activity of a SAC can be discerned through comparison with the NRR performance of its nanocluster counterpart. Additionally, the authors synthesized SA-Co/NPC, and their nanocluster assemblies exhibit superior activity for single-atom Co/NPC but not higher than SA-Mo/NPC.

Copyright © 2019, John Wiley & Sons, Inc. d Scheme representing the active sites for N adsorption of the Mn–N–C SAC electrocatalyst. Comparison of the e ammonia yields and f faradaic efficiencies of the Mn–N–C single-atom catalyst and NC-nanosheets. Reprinted with permission from Ref. [95]. Copyright © 2021, Springer Nature Ltd
a Illustration of SA-Mo/NPC and the respective atomic structure model. b EDS map** of SA-Mo/NPC. c Ammonia yield for various Mo loading in SA-Mo/NPC Reprinted with permission from Ref. [94].
Similarly, Wang et al. [95] developed a 2D Mn–N–C SAC via an acid self-assembly strategy that exposes Mn atoms for selective N2 adsorption and higher active sites (Fig. 9d). The well-exposed Mn–N–C coordinated sites on the 2D matrix reveal a high ammonia yield of approximately 21.43 μg h−1 mg−1cat and an FE of nearly 32.02% (Fig. 9 e, f). The Mn–N–C coordinated sites show excellent selectivity towards the NRR over the HER, which contributes to the higher ammonia yield and FE. Therefore, studies have concluded the superior activity of SACs in the NRR. Thus, SACs are bound to exhibit excellent NRR electrocatalytic activity with their large active site density and maximized metal utilization [97].
3.3.3 Transition Metal Oxides
Transition metal oxides have ruled the electrocatalytic field for decades and have proven their superiority in almost all redox reactions owing to their defect modulation ability and electron affinity behaviour, which actively tunes the intrinsic electronic configuration of the electrocatalysts [98, 99]. ** on N doped carbon supported noble metal catalysts. J. Catal. 375, 456–465 (2019). https://doi.org/10.1016/j.jcat.2019.06.039 " href="/article/10.1007/s41918-023-00186-6#ref-CR146" id="ref-link-section-d50593638e4110">146]. Several design strategies, including heteroatom dopant engineering and vacancy engineering methods, and utilizing various metal-free materials, such as graphitic-carbon nitride (g-C3N4), graphene, boron nitride (BN), black phosphorous (BP), and organic frameworks (MOF/COFs), have been successful in achieving efficient NRR electrocatalysts. Dopant engineering involves the do** of heteroatoms (B, N, S, P) into carbonaceous materials, which modulate the electronic structures of the materials to achieve higher NRR performances [147,148,149,150]. Boron-based electrocatalysts have been found to exhibit enhanced NRR performance due to their electron deficiency nature and inactivity towards the HER [151].
As an example of a do** strategy, Yu et al. [148] reported boron-doped graphene (BC3) as an NRR electrocatalyst that exhibits excellent NRR properties at the B site, enhancing the N2 binding capability (Fig. 14a). The HOMO–LUMO plots and the charge density difference diagrams reveal the charge-deficient regions for possible N adsorption sites (Fig. 14b, c). Hence, this work highlights the potential ability of the boron-doped metal-free matrix for efficient NRR. The carbon framework provides structural stability and enhanced electron conductivity, as well as mass transport, which provides the added advantage of choosing a framework for electrocatalytic applications. Similarly, Song et al. [149] engineered N, P-codoped MOF-5 carbon (NP-C-MOF-5), exhibiting an ammonia yield of nearly 1.08 μg h−1 mgcat−1 with considerable evolution of N2H4. Likewise, Tian et al. [150] reported N, S codoped graphene (NSG) as a durable NRR electrocatalyst that exhibited a high ammonia yield of nearly 7.7 μg h−1 mg−1 with a high selectivity towards NRR. Heteroatom do** in carbonaceous materials has been studied to generate charge distributions in the carbon matrix that enhance electron conductivity [146], tune the selectivity towards NRR [152], promote N2 adsorption [153], and create more defective sites for effective NRR performance.

Copyright © 2018, Elsevier Ltd. d Representation of the S dopant position in the graphene sheets for NRR. Reprinted with permission from Ref. [164]. Copyright © 2020, Elsevier Ltd. e Illustration of exfoliated black phosphorous sheets with different charge densities around various positions. Reprinted with permission from Ref. [155]. Copyright © 2019, John Wiley & Sons, Inc. f Representation of boron-doped black phosphorous nanosheets showing the NRR mechanism. Reprinted with permission from Ref. [151]. Copyright © 2019, Royal Society of Chemistry. g Illustration of the synthesis route for Eex-COF in a N2 atmosphere. h Modified charge distribution on the COF substrate after electrochemical excitation. Reprinted with permission from Ref. [145]. Copyright © 2019, Springer Nature Ltd
a Illustration of NRR on boron-doped carbon graphene sheets with N2 adsorption activity. b Diagram representing the HOMO–LUMO of BC3 sheets. c Modified charge density distribution on the BC3 nanosheets. Reprinted with permission from Ref. [148].
As metal-free noncarbonaceous NRR electrocatalysts, boron nitride (BN) has been anticipated to achieve better selectivity towards NRR, and its layered structure provides highly accessible active sites. For instance, Zhao et al. [154] designed mesoporous boron nitride (MBN) electrocatalysts that show a large surface area and highly accessible active sites due to their mesoporous structure. Compared to the bulk BN, a high ammonia yield can be observed in the MBN electrocatalysts. In addition, black phosphorous has also been observed to exhibit excellent NRR activity due to its anisotropic structure and abundant edge sites that enhance ammonia productivity. Zhang et al. [155] reported exfoliated black phosphorous (FL-BP) nanosheets that were observed to produce high ammonia yields and FE on the zigzag edges of FL-BP (Fig. 14e, f).
Similar to MOFs, Liu et al. [145] reported a facile synthesis procedure for 2D boron-rich COF materials that are electrochemically excited (Eex-COF/NC) to exhibit better NRR performances and suppress HER over the B-active sites (Fig. 14g, h). The COF structures linked by boroxine rings (B3O3), boronate esters (C2O2B), amide and imide connecting structures are addressed to be Lewis acid sites that can efficiently adsorb a weak Lewis base, such as the N2 molecule. Importantly, these framework structures exhibit poor electron conductivities despite the boron rings that actuate electrochemical NRR. To overcome these difficulties, the authors embedded these COFs on a nitrogen-doped carbon template to offer external electron conductivity and a stable substrate. Thus, metal-free electrocatalysts play a crucial role in NRR electrocatalysis by inhibiting the HER by electronic modifications in a carbon matrix and incorporating more accessible active sites, increasing the specific activity.
3.5 Bifunctional Electrocatalysts for NRR and OER
In the case of aqueous metal-N2 batteries, the bifunctional NRR and OER performances during discharging and charging reactions become an essential aspect [32]. The electrocatalyst for NRR must exhibit a high ammonia yield rate and suppressed HER for a higher FE. Similarly, for the OER, the electrocatalyst must exhibit a low overpotential and faster reaction kinetics for effective performance [116]. Previous studies show better OER performances with low overpotentials and lower Tafel slope values and reveal improved NRR performances of the electrocatalyst. For instance, Li et al. [126] synthesized CoxFe-MOF as an effective NRR and OER electrocatalyst because of the presence of abundant active sites and the positive synergistic effect of Co-Fe metals. CoxFe-MOF exhibits a very low Tafel slope of 38 mV dec−1 and a lower overpotential of 280 mV @ 10 mA cm−2 for the OER. The electrocatalysts also reveal a high ammonia yield and FE of 25.64% at a very low potential (− 0.2 V vs. RHE), which hold prospects for a metal-N2 battery. Similarly, Sun et al. [156] designed 2D NiFe nanomesh arrays on nickel foam, which imposes the combined effects of Ni–Fe and the hierarchical porosity from arranged nanolayers for higher OER/NRR performance. The electrocatalyst exhibits an even lower OER overpotential of approximately 191 mV and a considerably lower Tafel slope of approximately 43 mV dec−1. The electrocatalyst delivers better NRR performance with a considerable yield and an FE of approximately 9.8%. Therefore, the electrocatalysts must hold efficient electrocatalytic activity for NRR and OER, which reduces the required charging potential and increases the discharge cell voltage, respectively (Table 1).
Thus, electrocatalysts can be designed with various strategies that effectively promote the NRR and suppress the HER. Electrocatalysts for nonaqueous-based systems have not attracted interest because of the high maintenance and instability of electrocatalysts in nonaqueous media. However, research on electrocatalysts has been targeted for aqueous-based systems for which the kinetics has been sluggish but shows promising results. Moreover, the bifunctional performances of the electrocatalysts are highly anticipated to achieve efficient rechargeability of aqueous metal-N2 batteries. Metal-N2 batteries can be fabricated with electrocatalysts having a stable architecture, increased active site density, large surface areas, selective adsorption towards N2, and prominent HER mitigation.
4 Electrolytes for NRR
Electrocatalysts are the most studied entity in the NRR process. However, electrolytes form the environment for efficient NRR electrocatalysis, which plays a major role in determining the better performance of the electrocatalyst and the metal-N2 battery. They are found to actively inhibit the progress of the HER and supply protons to the surface of the electrocatalyst [171].
Despite acidic electrolytes being the prime proton donors for the HER in aqueous solution, they can catalyse ammonia production in a controlled environment. However, the long-term stability of electrocatalysts in an acidic electrolyte poses a serious threat. Additionally, the evolution of hydrogen from the excess protons can lead to a higher HER than the expected NRR. Alkaline electrolytes tackling all the abovementioned issues of proton migration are found to suppress the intrinsic HER.
Alkaline electrolytes are the most preferred medium for most nitrogen reduction experiments. They are inexpensive, easy to handle, do not corrode the electrochemical cell and provide sufficient H+ ions for protonation. Mukherjee et al. [172] studied ZIF-8 frameworks for electrocatalytic NRR for various pyrolysis temperatures, varied concentrations of Fe do** and different electrolytes (0.1 M KOH, 0.1 M NaOH and 0.1 M HCl). For the C-ZIF-1000–1 h catalyst, the electrolytes KOH and NaOH with varied cations are examined, which indicates a higher ammonia yield (Fig. 15b, c). Including the concentration effects, it is observed that the NaOH electrolyte is always one order behind the KOH electrolyte, indicating feasible K+ ion transport in the electrolyte, suggesting favourable NRR kinetics in the KOH electrolyte.
In addition to acidic and alkaline electrolytes, neutral electrolytes play an efficient role in electrochemical N2 reduction. Notably, phosphate buffer saline (PBS) electrolytes have found importance in the NRR by suppressing the HER and exhibiting better catalytic activity than NaOH and H2SO4 electrolytes. For example, Wei et al. [15] extensively studied the flow reactor for ammonia synthesis and electrolytes of varied pH (H2SO4, K2SO4, PBS and KOH) (Fig. 16a). The study finds effective HER suppression in the neutral electrolyte initially, followed by the alkaline electrolyte (PBS > K2SO4 > KOH > H2SO4) (Fig. 16b, c). The buffer effects of H2SO4/PBS, where PBS is a neutral medium, effectively reduce the proton concentration, which increases the pH of the electrolyte. Hence, the electrocatalytic surfaces are limited to adsorbing protons, and relatively high N2 species are adsorbed on the active sites. However, protonation of the adsorbed nitrogen is essential for forming ammonia, where the limited proton availability enhances the ammonia production rate and suppresses the HER. Therefore, increasing the pH of the electrolyte suppresses the HER, and the limited proton availability is facilitated to enhance the NRR. Additionally, the PTFE (polytetrafluoroethylene)-coated gas diffusion layer (GDL) prevents the electrode from becoming wettable, which increases N2 diffusion and inhibits hydrogen production in the flow cell (Fig. 16d, e).
a Scheme representing ammonia production on the GDL embedded with Ru NP under a flow reactor. b Comparison of the cell potential of the flow reactor for NRR under various electrolytes. c Comparison of the ammonia yields and FE under various electrolytes. d Illustration of the synthesis route of Ru/CB NPs and their fabrication onto the GDL. e Scheme representing ammonia production in the GDL embedded with Ru NPs in a flow reactor. Reprinted with permission from Ref. [15].
In addition to the reported electrolytes, a noteworthy strategy is used to suppress the HER by limiting proton migration and improving feasible N2 movement into the electrolyte. This novel scenario involves using an additive in the electrolyte that effectively limits proton migration into the electrolyte.
The molecular crowding effect observed in living cells restricts water activity by hydrogen bonding interactions between water molecules and long-chain macromolecules (Fig. 17a, b) [173,174,175,75], strain engineering [171], heterostructure formation [183], alloy engineering [125], and crystal facet regulation [184], which improve the structural and electronic effects in electrocatalysts, achieving superior performances. Structural and morphological engineering incorporates highly accessible active sites and the stability of the structures. Electronic modifications alter the conduction and valence bands of metals near the Fermi level, resulting in higher activity and selectivity towards NRR [185]. Studies have shown promising improvement in ammonia yield by tuning the electrocatalyst for a higher number of active sites.
Second, competitive side reactions such as the HER must be preferably suppressed to increase the selectivity for N2 adsorption, resulting in higher ammonia yields and FE. HER can be effectively suppressed by electrocatalyst modification by appropriately tuning the electronic structures of the active sites [37], adopting early transition metal-based electrocatalysts [35], and main group metal-based electrocatalysts [127]. Additionally, the HER can be suppressed by a reliable electrolyte engineering strategy. Reducing the proton concentration [15], resisting proton migration [16], and increasing the potential window for electrolysis [17] are proven to be effective strategies for inhibiting the HER in aqueous electrolytes. Adopting nonaqueous electrolytes with various proton sources is also an effective route to generate better NRR performance and inhibit the HER [43].
Third, the intrinsic electrochemical characteristics, such as conductivity and electrochemical active surface area (ECSA), of the electrocatalyst are improved [186, 187]. The electrocatalyst must exhibit lower charge transfer resistances and electrode–electrolyte resistances to achieve faster electronic conductivity and faster reaction kinetics. The electrocatalysts are incorporated on a N-doped carbon matrix, which can be designed by several methods to achieve faster conductivity due to the highly polarized electronically tuned substrates [188]. Moreover, increasing the ECSA of the electrocatalyst incorporates higher exposure of active sites for N2 adsorption and electrolytic ions. Hence, the abovementioned strategies can be followed to achieve higher ammonia yield and FE and to exhibit better electrochemical performances for the electrocatalyst.
Nevertheless, there is a high risk of overestimating or underestimating the ammonia yield due to the low sensitivity of detection methods, the nitrogenous contents of the electrode and the presence of impurities in the electrolyte, membrane/separators and analyte gas contents [59]. Proper purification procedures and careful observation of the contents of electrocatalysts and electrolytes are mandatory while performing NRR studies.