Abstract
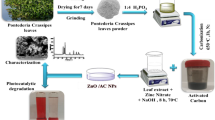
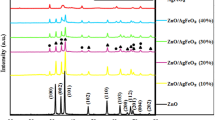
Nanocomposites of spinel aluminate (MAl2O4) based materials decorated with activated carbon (AC) were synthesized using chemical precipitation method. The X-ray diffraction spectra showed the cubic crystalline structure for the nanocomposites. The existence of M–O bond was confirmed by FTIR data. The SEM micrographs revealed the porous behavior of the nanocomposites. The existence of appropriate elements was proved by EDS. HR-TEM results were agreeable with the XRD data. The UV–Visible spectra of the MAl2O4@AC nanocomposites showed a good absorbance behavior in the samples. The nanocomposites were very effective in eliminating the malachite green (MG) dye from water by the photo-degradation reaction. The MG dye before and after degradation was characterized by HPLC-Mass analysis. The photocatalytic degradation of MG follows pseudo-first-order reaction mechanism, i.e., Langmuire and Hinshelwood kinetic model. Among the photocatalysts studied, Co0.85Ni0.15Al2O4-δ@AC nanocomposite exhibited excellent degradation efficiency in removing toxic MG dye completely within 90 min under visible light irradiation.











Similar content being viewed by others
Availability of data and materials
Yes, available.
References
Esmaili H, Kotobi A, Sheibani S, Rashchi F (2018) Photocatalytic degradation of methylene blue by nanostructured Fe/FeS powder under visible light. Int J Miner Metall Mater 25:244–252. https://doi.org/10.1007/s12613-018-1567-x
Gallego-Urrea JA, Hammes J, Cornelis G, Hassellöv M (2016) Coagulation and sedimentation of gold nanoparticles and illite in model natural waters: Influence of initial particle concentration. NanoImpact 3–4:67–74. https://doi.org/10.1016/j.impact.2016.10.004
Al-Anber ZA, Al-Anber MA, Matouq M, Al-Ayed O, Omari NM (2011) Defatted Jojoba for the removal of methylene blue from aqueous solution: thermodynamic and kinetic studies. Desalination 276(1–3):169–174. https://doi.org/10.1016/j.desal.2011.03.043
Dickhout JM, Moreno J, Biesheuvel PM, Boels L, Lammertink RGH, de Vos VM (2017) Produced water treatment by membranes: a review from a colloidal perspective. J Colloid Interface Sci 487:523–526. https://doi.org/10.1016/j.jcis.2016.10.013
Gnanam S, Rajendran V (2018) Facile sol-gel preparation of Cd-doped cerium oxide (CeO2) nanoparticles and their photocatalytic activities. J Alloys Compd 735:1854–1862. https://doi.org/10.1016/j.jallcom.2017.11.330
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Vacca A, Mais L, Mascia M, Usai EM, Palmas S (2020) Design of experiment for the optimization of pesticide removal from wastewater by photo-electrochemical oxidation with TiO2 nanotubes. Catalysts 10(5):512. https://doi.org/10.3390/catal10050512
Deraz NM (2013) Synthesis and characterization of nano-sized nickel aluminate spinel crystals. Int J Electrochem Sci 8:5203–5212
Mathur S, Veith M, Haas M, Shen H, Lecerf N, Huch V, Hufner S, Haberkorn R, Beck HP, Jilavi M (2001) Single-source sol-gel synthesis of nanocrystalline ZnAl2O4: Structural and optical properties. J Am Ceram Soc 84(9):1921–1928. https://doi.org/10.1111/j.1151-2916.2001.tb00938.x
Sampath SK, Cordaro JF (2005) Optical properties of zinc aluminate, zinc gallate, and zinc aluminogallate spinels. J Am Ceram Soc 81(3):649–654. https://doi.org/10.1111/j.1151-2916.1998.tb02385.x
Coromelci-Pastravanu C, Ignat M, Popovici E, Harabagiu V (2014) TiO2-coated mesoporous carbon: conventional vs. microwave-annealing process. J Hazard Mater 278:382–390. https://doi.org/10.1016/j.jhazmat.2014.06.036
Djellabi R, Yang B, Wang Y, Cui X, Zhao X (2019) Carbonaceous biomass-titania composites with Ti–O–C bonding bridge for efficient photocatalytic reduction of Cr(VI) under narrow visible light. Chem Eng J 366:172–180. https://doi.org/10.1016/j.cej.2019.02.035
Mehdi T (2017) Selective adsorption and photocatalytic degradation of dyes using polyoxometalate hybrid supported on magnetic activated carbon nanoparticles under sunlight, visible, and uv irradiation. Int J Photoenergy 2017:8575096. https://doi.org/10.1155/2017/8575096
Ye A, Fan W, Zhang Q, Deng W, Wang Y (2012) CdS–graphene and CdS–CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal Sci Technol 2:969–978. https://doi.org/10.1039/C2CY20027A
Yu H, Zhao Y, Zhou C, Shang L, Peng Y, Cao Y, Wu LZ, Tung CH, Zhang T (2014) Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J Mater Chem A 2:3344–3351. https://doi.org/10.1039/C3TA14108J
Yu W, Xu D, Peng T (2015) Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J Mater Chem A 3:19936–19947. https://doi.org/10.1039/C5TA05503B
Zhang Y, Liu Y, Gao W, Chen P, Cui H, Fan Y (2019) MoS2 nanosheets assembled on three-way nitrogen-doped carbon tubes for photocatalytic water splitting. Front Chem 7:325. https://doi.org/10.3389/fchem.2019.00325
Li Y, Feng X, Lu Z, Yin H, Liu F, **ang Q (2018) Enhanced photocatalytic H2-production activity of C-dots modified g-C3N4/TiO2 nanosheets composites. J Colloid Interface Sci 513:866–876. https://doi.org/10.1016/j.jcis.2017.12.002
Song L, Guo C, Li T, Zhang S (2017) C60/graphene/g-C3N4 composite photocatalyst and mutually-reinforcing synergy to improve hydrogen production in splitting water under visible light radiation. Ceram In 43:7901–7907. https://doi.org/10.1016/j.ceramint.2017.03.115
Velo-Inmaculada G, López-Jesús J, Sanchez-Polo M, Rivera-Utrilla J (2013) Activated carbon as photocatalyst of reactions in aqueous phase. Appl Catal B 142–143:694–704. https://doi.org/10.1016/j.apcatb.2013.06.003
Zhang S, Gu P, Ma R, Luo C, Wen T, Zhao G, Cheng W, Wang X (2019) Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review. Catal Today 335:65–77. https://doi.org/10.1016/j.cattod.2018.09.013
Puma GL, Bono A, Krishnaiah D, Collin JG (2008) Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: a review paper. J Hazard Mater 157(2–3):209–219. https://doi.org/10.1016/j.jhazmat.2008.01.040
Velasco LF, Parra JB, Ania CO (2010) Role of activated carbon features on the photocatalytic degradation of phenol. Appl Surf Sci 256(17):5254–5258. https://doi.org/10.1016/j.apsusc.2009.12.113
Velasco LF, Fonseca IM, Parra JB, Lima JC, Ania CO (2012) Photochemical behaviour of activated carbons under UV irradiation. Carbon 50(1):249–258. https://doi.org/10.1016/j.carbon.2011.08.042
Mestre AS, Carvalho AP (2019) Photocatalytic degradation of pharmaceuticals carbamazepine, diclofenac, and sulfamethoxazole by semiconductor and carbon materials: a review. Molecules 24(20):3702. https://doi.org/10.3390/molecules24203702
Velasco LF, Carmona RJ, Matos J, Ania CO (2014) Performance of activated carbons in consecutive phenol photooxidation cycles. Carbon 73:206–215. https://doi.org/10.1016/j.carbon.2014.02.056
Briche S, Derqaoui M, Belaiche M, Mouchtari EME, Wong-Wah-Chung P, Rafqah S (2020) Nanocomposite material from TiO2 and activated carbon for the removal of pharmaceutical product sulfamethazine by combined adsorption/photocatalysis in aqueous media. Environ Sci Pollut Res Int 27:25523–25534. https://doi.org/10.1016/10.1007/s11356-020-08939-2
Huang H, Wang Y, Jiao W, Cai F, Shen M, Zhou S, Cao H, Lü J, Cao R (2018) Lotus-leaf-derived activated-carbon-supported nano-CdS as energy-efficient photocatalysts under visible irradiation. ACS Sustain Chem Eng 6(6):7871–7879. https://doi.org/10.1021/acssuschemeng.8b01021
Abdelhamid HN, Mathew AP (2022) Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted applications: a review. Coord Chem Rev 451:214263. https://doi.org/10.1016/j.ccr.2021.214263
Nethaji S, Tamilarasan G, Neehar P, Sivasamy A (2018) Visible light photocatalytic activities of BiOBr-activated carbon (derived from waste polyurethane) composites by hydrothermal process. J Environ Chem Eng 6:3735–3744. https://doi.org/10.1016/j.jece.2017.02.037
Soliman AIA, Abdelwahab AMA, Abdelhamid HN (2022) Hierarchical porous zeolitic imidazolate frameworks (ZIF-8) and ZnO@N-doped carbon for selective adsorption and photocatalytic degradation of organic pollutants. RSC Adv 12:7075–7084. https://doi.org/10.1039/D2RA00503D
Abdelhamid HN, Mathew AP (2021) Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: adsorption and catalytic degradation. J Chem Eng 426:131733. https://doi.org/10.1016/j.cej.2021.131733
Arunkumar M, Samson Nesaraj A (2020) Photocatalytic degradation of malachite green dye using NiAl2O4 and Co doped NiAl2O4 nanophotocatalysts prepared by simple one pot wet chemical synthetic route. Iran J Catal 10(3):235–245
Arunkumar M, Samson Nesaraj A (2021) Facile chemical fabrication of Ni doped CoAl2O4 nano-spinel photocatalysts: Physico-chemical properties and photodegradation of toxic malachite green dye under visible light. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1867722
Mohamed RM, Baeissa ES, Mkhalid IA, Al-Rayyani MA (2013) Optimization of preparation conditions of ZnO–SiO2 xerogel by sol–gel technique for photodegradation of methylene blue dye. Appl Nanosci 3:57–63. https://doi.org/10.1007/s13204-012-0074-z
Wang S (1997) Lu GQ (1997) Effects of oxide promoters on metal dispersion and metal−support interactions in Ni catalysts supported on activated carbon. Ind Eng Chem Res 36(12):5103–5109. https://doi.org/10.1021/ie9703604
Sulistianti I, Krisnandi YK, Moenandar I (2017) Study of CO2 adsorption capacity of mesoporous carbon and activated carbon modified by triethylenetetramine (TETA). IOP Conf Ser Mater Sci Eng 188:012041. https://doi.org/10.1088/1757-899X/188/1/012041
Anchieta CG, Tochetto L, Madalosso HB, Sulkovski RD, Serpa C, Mazutti MA, de Almeida ARF, Gündel A, Foletto EL (2015) Effect of thermal treatment on the synthesis of NiAl2O4 spinel oxide using chitosan as precursor. Cerâmica 61(360):477–481. https://doi.org/10.1590/0366-69132015613601925
He C, Ji H, Huang Z, Zhang X, Liu H, Liu S, Liu Y, Fang M, Wu X, Min X (2018) Preparation and photoluminescence properties of red-emitting phosphor ZnAl2O4:Eu3+ with an intense 5D0 → 7F2 transition. Mater Res Express 5(2):025501. https://doi.org/10.1088/2053-1591/aaa7c9
Kumari P, Dwivedi Y, Bahadur A (2018) Analysis of bright red-orange emitting Mn2+:ZnAl2O4 spinel nanophosphor. Optik 15:126–132. https://doi.org/10.1016/j.ijleo.2017.10.023
Pereira MFR, Soares SF, Orfao JJM, Figueiredo JL (2003) Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 41(4):811–821. https://doi.org/10.1016/S0008-6223(02)00406-2
Ahangaran F, Hassanzadeh A, Nouri S (2013) Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int Nano Lett 3:23. https://doi.org/10.1186/2228-5326-3-23
Bouzid K, Djelloul A, Bouzid N, Bougdira J (2009) Electrical resistivity and photo-luminescence of zinc oxide films prepared by ultrasonic spray pyrolysis. Phys Status Solidi A 206(1):106–115. https://doi.org/10.1002/pssa.200824403
Ouahdi N, Guillemet S, Durand B, El Ouatib R, Rakho LE, Moussa R, Samdi A (2008) Synthesis of CoAl2O4 by double decomposition reaction between LiAlO2 and molten KCoCl3. J Eur Ceram Soc 28(10):1987–1994. https://doi.org/10.1016/j.jeurceramsoc.2007.12.035
Salleh NFM, Jalil AA, Triwahyono S, Efendi J, Mukti RR, Hameed BH (2015) New insight into electrochemical-induced synthesis of NiAl2O4/Al2O3: synergistic effect of surface hydroxyl groups and magnetism for enhanced adsorptivity of Pd(II). Appl Surf Sci 349:485–495. https://doi.org/10.1016/j.apsusc.2015.05.048
Li X, Bai J, Li J, Li C, Zhong X, Deng S (2020) The effect of n–π* electronic transitions on the N2 photofixation ability of carbon self-doped honeycomb-like g-C3N4 prepared via microwave treatment. RSC Adv 10:7019–7025. https://doi.org/10.1039/D0RA00101E
Trivedi S, Prasad R, Gautam SK (2018) Design of active NiCo2O4-δ spinel catalyst for abatement of CO–CH4 emissions from CNG fueled vehicles. AIChE J 64(7):2632–2646. https://doi.org/10.1002/aic.16162
Sharif SH, Golestani-Fard F, Khatibi E, Sarpoolaky H (2009) Dispersion and stability of carbon black nanoparticles, studied by ultraviolet–visible spectroscopy. J Taiwan Inst Chem Eng 40(5):524–527. https://doi.org/10.1016/j.jtice.2009.03.00
Green DC, McKenzie DR, Lukins PB (1991) The microstructure of carbon thin films. Mater Sci Forum 52–53:103–124. https://doi.org/10.4028/www.scientific.net/msf.52-53.103
Jager C, Henning TH, Schlogl R, Spillecke O (1999) Spectral properties of carbon black. J Non Cryst Solids 258(1–3):161–179. https://doi.org/10.1016/S0022-3093(99)00436-6
Llusar M, Fores A, Badenes JA (2001) Colour analysis of some cobalt-based blue pigments. J Eur Ceram Soc 21(8):1121–1130. https://doi.org/10.1016/S0955-2219(00)00295-8
Cimino A, Lo Jacono M, Schiavello M (1971) Structural, magnetic, and optical properties of nickel oxide supported on .eta.- and .gamma.-aluminas. J Phys Chem 75(8):1044–1050. https://doi.org/10.1021/j100678a005
Karpinska J, Kotowska U (2019) Removal of organic pollution in the water environment. Water 11(10):2017. https://doi.org/10.3390/w11102017
Somraksa W, Suwanboon S, Amornpitoksuk P (2019) Enhanced visible-light driven photocatalytic property of g-C3N4/ZnAl2O4 nanocomposites. J Phys Conf Ser 1380:01021. https://doi.org/10.1088/1742-6596/1380/1/012021
El Jabbar Y, El Hafdi M, Benchikhi M, El Ouatib R, Er-Rakho L, Essadki A (2019) Photocatalytic degradation of navy blue textile dye by nanoscale cobalt aluminate prepared by polymeric precursor method. Environ Nanotechnol Monit Manag 12:100259. https://doi.org/10.1016/j.enmm.2019.100259
Ahmed S, Rasul MG, Martens WN, Brown R, Hashib MA (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water Air Soil Pollut 215:3–29. https://doi.org/10.1007/s11270-010-0456-3
Li L, Li X, Lee JY, Keener TC, Liu Z, Yao X (2012) The effect of surface properties in activated carbon on mercury adsorption. Ind Eng Chem Res 51(26):9136–9144. https://doi.org/10.1021/ie2028156
Pan S, Liu X (2012) CdS–Graphene nanocomposite: synthesis, adsorption kinetics and high photocatalytic performance under visible light irradiation. New J Chem 36:1781–1787. https://doi.org/10.1039/C2NJ40301C
Wu H, Jian W, Dang H, Zhao X, Zhang L, Li J (2017) Hierarchical Ag-ZnO microspheres with enhanced photocatalytic degradation activities. Pol J Environ Stud 26(2):871–880. https://doi.org/10.15244/pjoes/65363
Sridewi N, Tan LT, Sudesh K (2011) Solar photocatalytic decolorization and detoxification of industrial batik dye wastewater using P(3HB)-TiO2 nanocomposite films. Clean: Soil, Air, Water 39(3):265–273. https://doi.org/10.1002/clen.201000344
Markovic D, Jokic B, Saponjic Z, Potkonjak B, Jovancic P, Radetic M (2013) Photocatalytic degradation of dye CI direct blue 78 using TiO2 nanoparticles immobilized on recycled wool-based nonwoven material. Clean: Soil, Air, Water 41(10):1002–1009. https://doi.org/10.1002/clen.201200413
Wanyonyi WC, Onyarib JM, Shiunduc PM, Mulaa FJ (2017) Biodegradation and detoxification of malachite green dye using novel enzymes from Bacillus cereus strain KM201428: kinetic and metabolite analysis. Energy Procedia 119:38–51. https://doi.org/10.1016/j.egypro.2017.07.044
Mostafa EM, Amdeha E (2022) Enhanced photocatalytic degradation of malachite green dye by highly stable visible-light-responsive Fe-based tri-composite photocatalysts. Environ Sci Pollut Res 29:69861–69874. https://doi.org/10.1007/s11356-022-20745-6
Somraksa W, Suwanboon S, Amornpitoksuk P, Randorn C (2020) Physical and photocatalytic properties of CeO2/ZnO/ZnAl2O4 ternary nanocomposite prepared by co-precipitation method. Mater Res 23(1):e20190627. https://doi.org/10.1590/1980-5373-MR-2019-0627
Wilhelm P, Stephen D (2007) Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J Photochem Photobiol A 185(1):19–25. https://doi.org/10.1016/j.jphotochem.2006.05.003
Mills A, Davies RH, Worsley D (1993) Water purification by semiconductor photocatalysis. Chem Soc Rev 22:417–425. https://doi.org/10.1039/CS9932200417
Acknowledgements
The authors thank Karunya Institute of Technology and Sciences (KITS) for promoting nanomaterials chemistry based research activity.
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
Manasai Arunkumar—Resources, design of work, investigation, writing—review & editing; Arputharaj Samson Nesaraj—Conceptualization, Methodology, design of work, writing—original draft; Clementz Edwardraj Freeda Christy—the acquisition, analysis and Chinnappan Joseph Kennady—Interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Yes.
Consent to publish
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arunkumar, M., Nesaraj, A.S., Christy, C.E.F. et al. Improved photocatalytic efficiency of MAl2O4 @ activated carbon based nanocomposites in removing malachite green dye under visible light. Nanotechnol. Environ. Eng. 8, 643–654 (2023). https://doi.org/10.1007/s41204-022-00300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00300-x