Abstract
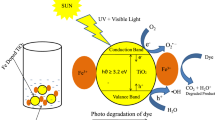
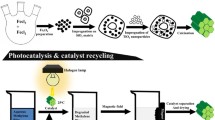
The photocatalytic performance of mechano-thermally synthesized Fe/FeS nanostructures formed from micron-sized starting materials was compared with that of a thermally synthesized nanostructure with nano-sized precursors in this paper. The properties of as-synthesized materials were studied by X-ray diffraction (XRD), transmission electron microscopy (TEM), vibrating sample magnetometry (VSM), diffuse reflectance spectroscopy (DRS), and ultraviolet-visible (UV-Vis) spectroscopy. The effects of irradiation time, methylene blue (MB) concentration, catalyst dosage, and pH value upon the degradation of MB were studied. Magnetic properties of the samples showed that both as-synthesized Fe/FeS photocatalysts are magnetically recoverable, eliminating the need for conventional filtration steps. Degradation of 5 ppm of the MB solution by mechano-thermally synthesized Fe/FeS with a photocatalyst dosage of 1 kg/m3 at pH 11 can reach 96% after 12 ks irradiation under visible light. The photocatalytic efficiency is higher in alkaline solution. The kinetics of photocatalytic degradation in both samples is controlled by a first-order reaction. However, the rate-constant value in the thermally synthesized Fe/FeS photocatalyst sample is only 1.5 times greater than that of the mechano-thermally synthesized one.
Similar content being viewed by others
References
A. Ameta, R. Ameta, and M. Ahuja, Photocatalytic degradation of methylene blue over ferric tungstate, Sci. Revs. Chem. Commun., 3(2013), No. 3, p. 172.
C. Guillard, H. Lachheb, A. Houas, M. Ksibi, E. Elaloui, and J.M. Herrmann, Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2, J. Photochem. Photobiol. A, 158(2003), No. 27-36, p. 27.
S.S. Patil and V.M. Shinde, Biodegradation studies of aniline and nitrobenzene in aniline plant waste water by gas chromatography, Environ. Sci. Technol., 22(1988), No. 10, p. 1160.
A. Houas, I. Bakir, M. Ksibi, and E. Laloui, Removal of a methylene blue from aqueous solution over the commercial activated charcoal CECA40, J. Chim. Phys. Phys. Chim. Biol., 96(1999), No. 3, p. 479.
Y.M. Slokar and A. Majcen Le Marechal, Methods of decoloration of textile wastewaters, Dyes Pigm., 37(1998), No. 4, p. 335.
O. Legrini, E. Oliveros, and A.M. Braun, Photochemical processes for water treatment, Chem. Rev., 93(1993), No. 2, p. 671.
H.T. Gao, Y.Y. Liu, C.H. Ding, D.M. Dai, and G.J. Liu, Synthesis, characterization, and theoretical study of N, S-codoped nano-TiO2 with photocatalytic activities, Int. J. Miner. Metall. Mater., 18(2011), No. 5, p. 606.
R. Yang, J.H. Liu, and S.M. Li, Preparation and characterization of in-site regenerated TiO2-ACFs photocatalyst, Int. J. Miner. Metall. Mater., 18(2011), No. 3, p. 357.
S.H. Xun, Z.Y. Zhang, T.Y. Wang, D.L. Jiang, and H.M. Li, Synthesis of novel metal nanoparticles/SnNb2O6 nanosheets plasmonic nanocomposite photocatalysts with enhanced visible-light photocatalytic activity and mechanism insight, J. Alloys Compd., 685(2016), p. 647.
S. Ahluwalia, N.T. Prakash, R. Prakash, and B. Pal, Improved degradation of methyl orange dye using bio-co-atalyst Se nanoparticles impregnated ZnS phtocatalyst under UV irradiation, Chem. Eng. J., 306(2016), p. 1041.
A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 238(1972), No. 5358, p. 37.
A. Mills and S.K. Lee, A web-based overview of semiconductor photochemistry-based current commercial applications, J. Photochem. Photobiol. A, 152(2002), No. 1-3, p. 233.
P.V. Kamat, Meeting the clean energy demand: Nanostructure architectures for solar energy conversion, J. Phys. Chem. C, 111(2007), No. 7, p. 2834.
V. Etacheri, M.K. Saery, S.J. Hinder, and S.C. Pillai, Oxygen rich titania: A dopant free, high temperature stable and visible light active anatase photocatalyst, Adv. Funct. Mater., 21(2011), No. 19, p. 3744.
A.K. Dutta, S.K. Maji, D.N. Srivastava, A. Mondal, P. Biswas, P. Paul, and B. Adhikary, Synthesis of FeS and FeSe nanoparticles from a single source precursor: a study of their photocatalytic activity, peroxidase-like behavior, and electrochemical sensing of H2O2, ACS Appl. Mater. Interfaces, 4(2012), No. 4, p. 1919.
S.K. Maji, A.K. Dutta, P. Biswas, D.N. Srivastava, D.N. Srivastava, P. Paul, A. Mondala, and B. Adhikary, Synthesis and characterization of FeS nanoparticles obtained from a dithiocarboxylate precursor complex and their photocatalytic, electrocatalytic and biomimic peroxidase behavior, Appl. Catal. B, 419-420(2012), p. 170.
E.J. Kim, J.H. Kim, A.M. Azad, and Y.S. Chang, Facile synthesis and characterization of Fe/FeS nanoparticles for environmental applications, ACS Appl. Mater. Interfaces, 3(2011), No. 5, p. 1457.
M. Harir, A. Gaspar, B. Kanawati, A. Fekete, M. Frommberger, D. Martens, A. Kettrup, M. El Azzouzi, and Ph. Schmitt-Kopplin, Photocatalytic reactions of imazamox at TiO2, H2O2, and TiO2/H2O2 in water interfaces: kinetic and photoproducts study, Appl. Catal. B, 84(2008), No. 3-4, p. 524.
G.N. Nomikos and P. Panagiotopoulou, Kinetic and mechanistic study of the photocatalytic reforming of methanol over Pt/TiO2 catalyst, Appl. Catal. B, 146(2014), p. 249.
H. Feng, P.Z. Si, X.F. **ao, C.H. **, S.J. Yu, Z.F. Li, and H.L. Ge, Large scale synthesis of FeS coated Fe nanoparticles as reusable magnetic photocatalysts, Front. Mater. Sci., 7(2013), No. 3, p. 308.
H. Esmaili, S. Sheibani, and F. Rashchi, Synthesis of nano-structured Fe/FeS powder and photocatalytic activity under visible light irradiation, [in] Proceedings of the 5th International Conference on Materials Engineering and Metallurgy, Shiraz, 2016, p. 235.
A. Kotobi, H. Esmaili, and S. Sheibani, Fe/FeS core/shell nano-particles preparation by thermal method, [in] Proceedings of the 5th International Conference on Materials Engineering and Metallurgy, Shiraz, 2016, p. 120.
B.D. Cullity and S.R. Stock, Elements of X-ray Diffraction, 3rd Ed., Prentice Hall, Upper Saddle River, NJ, 2001, p. 99.
B.D. Cullity and C.D. Graham, Introduction to Magnetic Materials, 2nd Ed., Wiley-IEEE Press, New York, 2008, p. 531.
L. Sagnotti and Iron sulfides, Encyclopedia of Geomagnetism and Paleomagnetism, Edited by D. Gubbins and E. Herrero-Bervera, Springer, The Netherlands, 2007, p. 454.
B.S. Avinash, V.S. Chaturmukha, H.S. Jayanna, C.S. Naveen, M.P. Rajeeva, B.M. Harish, S. Suresh, and A.R. Lamani, Effect of particle size on band gap and DC electrical conductivity of TiO2 nanomaterial, AIP Conf. Proc., 1728(2016), No. 1, art. No. 020426.
H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard, and J.M. Herrmann, Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania, Appl. Catal. B, 39(2002), No. 1, p. 75.
X.Y. Li, C.G. Hu, X. Wang, and Y. **, Photocatalytic activity of CdS nanoparticles synthesized by a facile composite molten salt method, Appl. Surf. Sci., 258(2012), No. 10, p. 4370.
H.L. Zhang and C.G. Hu, Effective solar absorption and radial micr°Channels of SnO2 hierarchical structure for high photocatalytic activity, Catal. Commun., 14(2011), No. 1, p. 32.
Y. Tak, H. Kim, D. Lee, and K. Yong, Type-II CdS nanoparticle-ZnO nanowire heterostructure arrays fabricated by a solution process: enhanced photocatalytic activity, Chem. Commun., 2008, No. 38, p. 4585.
N. Daneshvar, S. Aber, M.S. Seyed Dorraji, A.R. Khataee, and M.H. Rasoulifard, Photocatalytic degradation of the insecticide diazinon in the presence of prepared nanocrystalline ZnO powders under irradiation of UV-C light, Sep. Purif. Technol., 58(2007), No. 1, p. 91.
S. Chakrabarti and B.K. Dutta, Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst, J. Hazard. Mater., 112(2004), No. 3, p. 269.
C. Martinez, M. Canle L, M.I. Fernández, J.A. Santaballa, and J. Faria, Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites, Appl. Catal. B, 102(2011), No. 3-4, p. 563.
A.M. Tayeb and D.S. Hussein, Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue, Am. J. Nanomater., 3(2015), No. 2, p. 57.
S. Mustafa, D. Misbahud, Y.H. Sammad, M.I. Zaman, and K. Sadullah, Sorption mechanism of cadmium from aqueous solution on iron sulphide, Chin. J. Chem., 28(2010), No. 7, p. 1153.
C. Guillard, H. Lechheb, A. Houas, M. Ksibi, E. Elaloui, and J.M. Herrmann, Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2, J. Photochem. Photobiol. A, 158(2003), No. 1, p. 27.
M.A. Fox and M.T. Dulay, Heterogeneous photocatalysis, Chem. Rev., 93(1993), No. 1, p. 341.
S. Sheibani, S. Heshmati-Manesh, and A. Ataie, Influence of Al2O3 nanoparticles on solubility extension of Cr in Cu by mechanical alloying, Acta Mater., 58(2010), No. 20, p. 6828.
Acknowledgements
The authors would like to acknowledge the financial support of University of Tehran for this research. Also, financial support of Iran Nanotechnology Initiative Council is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaili, H., Kotobi, A., Sheibani, S. et al. Photocatalytic degradation of methylene blue by nanostructured Fe/FeS powder under visible light. Int J Miner Metall Mater 25, 244–252 (2018). https://doi.org/10.1007/s12613-018-1567-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-018-1567-x