Abstract
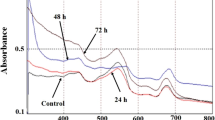
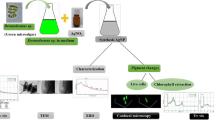
For the first time, microalga Dunaliella salina was successfully applied for synthesis of silver nanoparticles. The process of nanoparticles production was monitored using UV–Vis spectroscopy which exhibited surface plasmon resonance band at 432 nm. Scanning electron microscopy confirmed the presence of spherical silver nanoparticles having diameter in the range of 15–40 nm. Crystalline nature of produced nanoparticles was confirmed by X-ray diffraction, and Fourier transform infrared spectroscopy revealed the involvement of functional groups in nanoparticles formation. The changes in the biomass biochemical composition during silver nanoparticles biosynthesis were assessed as well. Formation of silver nanoparticles was accompanied by the decrease in proteins, β-carotene and lipids content in biomass.







Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon a reasonable request.
References
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534. https://doi.org/10.3390/ijms17091534
Banasiuk R, Frackowiak JE, Krychowiak M, Matuszewska M, Kawiak A, Ziabka M et al (2016) Synthesis of antimicrobial silver nanoparticles through a photomediated reaction in an aqueous environment. Int J Nanomed 11:315–324. https://doi.org/10.2147/IJN.S93611
Hamed S, Emara M, Shawky RM, El-domany RA, Youssef T (2017) Silver nanoparticles: antimicrobial activity, cytotoxicity, and synergism with N-acetyl cysteine. J Basic Microbiol 57:659–668. https://doi.org/10.1002/jobm.201700087
Escárcega-González CE, Garza-Cervantes JA, Vázquez-Rodríguez A, Montelongo-Peralta LZ, Treviño-González MT, Díaz Barriga Castro E et al (2018) In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using acacia rigidula as a reducing and cap** agent. Int J Nanomed 13:2349–2363. https://doi.org/10.2147/IJN.S160605
Amin ME, Azab MM, Hanora AM, Abdalla S (2017) Antifungal activity of silver nanoparticles on Fluconazole resistant Dermatophytes identified by (GACA)4and isolated from primary school children suffering from Tinea Capitis in Ismailia—Egypt. Cell Mol Biol 63:63–67. https://doi.org/10.14715/cmb/2017.63.11.12
**a ZK, Ma QH, Li SY, Zhang DQ, Cong L, Tian YL et al (2016) The antifungal effect of silver nanoparticles on Trichosporon asahii. J Microbiol Immunol Infect 49:182–188. https://doi.org/10.1016/j.jmii.2014.04.013
Mallmann EJJ, Cunha FA, Castro BNMF, Maciel AM, Menezes EA, Fechine PBA (2015) Atividade antifúngica de nanopartículas de prata obtidas por síntese verde. Rev Inst Med Trop Sao Paulo 57:165–167. https://doi.org/10.1590/S0036-46652015000200011
Ogar A, Tylko G, Turnau K (2015) Antifungal properties of silver nanoparticles against indoor mould growth. Sci Total Environ 521–522:305–314. https://doi.org/10.1016/j.scitotenv.2015.03.101
Orlowski P, Tomaszewska E, Gniadek M, Baska P, Nowakowska J, Sokolowska J et al (2014) Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 9:e104113. https://doi.org/10.1371/journal.pone.0104113
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N et al (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed 8:4303–4314. https://doi.org/10.2147/IJN.S50070
Chen N, Zheng Y, Yin J, Li X, Zheng C (2013) Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J Virol Methods 193:470–477. https://doi.org/10.1016/j.jviromet.2013.07.020
Sharma RK, Cwiklinski K, Aalinkeel R, Reynolds JL, Sykes DE, Quaye E et al (2017) Immunomodulatory activities of curcumin-stabilized silver nanoparticles: efficacy as an antiretroviral therapeutic. Immunol Invest 46:833–846. https://doi.org/10.1080/08820139.2017.1371908
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385–406
I. Zinicovscaia I, Cepoi L (eds) (2016) Cyanobacteria for bioremediation of wastewaters. Springer, Switzerland. https://doi.org/10.1007/978-3-319-26751-7
Marques CR (2018) Extremophilic microfactories: applications in metal and radionuclide bioremediation. Front Microbiol 9:1191. https://doi.org/10.3389/fmicb.2018.01191
Pikuta EV, Hoover RB, Tang J (2007) Microbial extremophiles at the limits of life. Crit Rev Microbiol 33:183–209. https://doi.org/10.1080/10408410701451948
Arul D, Balasubramani G, Balasubramanian V, Natarajan T, Perumal P (2017) Antibacterial efficacy of silver nanoparticles and ethyl acetate’s metabolites of the potent halophilic (marine) bacterium, Bacillus cereus A30 on multidrug resistant bacteria. Pathog Glob Health 111:367–382. https://doi.org/10.1080/20477724.2017.1390829
Gan L, Zhang S, Zhang Y, He S, Tian Y (2018) Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep Biochem Biotechnol 2018:1–7. https://doi.org/10.1080/10826068.2018.1476880
Mohite P, Kumar AR, Zinjarde S (2017) Relationship between salt tolerance and nanoparticle synthesis by Williopsis saturnus NCIM 3298. World J Microbiol Biotechnol 33:163. https://doi.org/10.1007/s11274-017-2329-z
Zinicovscaia I, Cepoi L, Chiriac T, Ana Culicov O, Frontasyeva M, Pavlov S et al (2016) Spirulina platensis as biosorbent of chromium and nickel from industrial effluents. Desalin Water Treat 57:11103–11110. https://doi.org/10.1080/19443994.2015.1042061
Zinicovscaia I, Safonov A, Tregubova V, Ilin V, Cepoi L, Chiriac T et al (2016) Uptake of metals from single and multi-component systems by spirulina platensis biomass. Ecol Chem Eng S 3:401–412. https://doi.org/10.1515/eces-2016-0028
Pathak J, Rajneesh, Ahmed H, Singh DK, Pandey A, Singh SP et al (2019) Recent developments in green synthesis of metal nanoparticles utilizing cyanobacterial cell factories. Nanomater. plants, algae microorg. Academic Press, pp 237–265. https://doi.org/10.1016/B978-0-12-811488-9.00012-3
Beardall J, Wangikar PP, Mikulic P, Vonshak A, Varshney P, Mikulic P et al (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372. https://doi.org/10.1016/j.biortech.2014.11.040
Young RA (1993) The Rietveld method. Oxford University Press, New York, p 298
Lowry O, Rosebrough N, Farr A (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mohseniazar M, Barin M, Zarredar H, Alizadeh S, Shanehbandi D (2011) Potential of microalgae and lactobacilli in biosynthesis of silver nanoparticles. BioImpacts 1:149–152. https://doi.org/10.5681/bi.2011.023
Singh AK, Tiwari R, Kumar V, Singh P, Riyazat Khadim SK, Tiwari A et al (2017) Photo-induced biosynthesis of silver nanoparticles from aqueous extract of Dunaliella salina and their anticancer potential. J Photochem Photobiol B Biol 166:202–211. https://doi.org/10.1016/j.jphotobiol.2016.11.020
Desai R, Mankad V, Gupta SK, Jha PK (2012) Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci Nanotechnol Lett 4:30–34. https://doi.org/10.1166/nnl.2012.1278
Singh H, Du J, Yi TH (2017) Kinneretia THG-SQI4 mediated biosynthesis of silver nanoparticles and its antimicrobial efficacy. Artif Cells Nanomed Biotechnol 45:602–608. https://doi.org/10.3109/21691401.2016.1163718
Cepoi L, Rudi L, Chiriac T, Valuta A, Zinicovscaia I, Duca GH et al (2014) Biochemical changes in cyanobacteria during the synthesis of silver nanoparticles. Can J Microbiol 61:13–21. https://doi.org/10.1139/cjm-2014-0450
Mahdieh M, Zolanvari A, Azimee AS, Mahdieh M (2012) Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci Iran 19:926–929. https://doi.org/10.1016/j.scient.2012.01.010
Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC (2016) The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol 44:1150–1157. https://doi.org/10.3109/21691401.2015.1011809
Shu** Z, Yulong W, Mingde Y, Kaleem I, Chun L, Tong J (2010) Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 35:5406–5411. https://doi.org/10.1016/j.energy.2010.07.013
Mishra A, Jha B (2009) Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour Technol 100:3382–3386. https://doi.org/10.1016/j.biortech.2009.02.006
Bramhachari PV, Dubey SK (2006) Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett Appl Microbiol 43:571–577. https://doi.org/10.1111/J.1472-765X.2006.01967.X
Hamouda RA, Hussein MH, Abo-elmagd RA, Bawazir SS (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep 9:1–17. https://doi.org/10.1038/s41598-019-49444-y
Oukarroum A, Bras S, Perreault F, Popovic R (2012) Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–85. https://doi.org/10.1016/j.ecoenv.2011.11.012
Ramasubburayan R, Sumathi S, Prakash S, Ramkumar VS, Titus S, Immanuel G et al (2017) Synthesis of nano silver by a marine epibiotic bacterium Bacillus vallismortis and its potent ecofriendly antifouling properties. Environ Nanotechnol Monit Manag 8:112–120. https://doi.org/10.1016/j.enmm.2017.06.005
Golubev AA, Prilepskii AY, Dykman LA, Khlebtsov NG, Bogatyrev VA (2016) Colorimetric evaluation of the viability of the microalga Dunaliella salina as a test tool for nanomaterial toxicity. Toxicol Sci 151:115–125. https://doi.org/10.1093/toxsci/kfw023
Cepoi L, Rudi L, Zinicovscaia I, Chiriac T, Miscu V, Rudic V (2021) Biochemical changes in microalga Porphyridium cruentum associated with silver nanoparticles biosynthesis. Arch Microbiol 203:1547–1554. https://doi.org/10.1007/s00203-020-02143-z
Elleuch F, Ben HH, Barkallah M, Baril P, Abdelkafi S, Pichon C et al (2019) Carotenoids overproduction in Dunaliella sp.: transcriptional changes and new insights through lycopene cyclase regulation. Appl Sci 9:5389. https://doi.org/10.3390/app9245389
Chavoshi ZZ, Shariati M (2019) Lipid production in Dunaliella salina under autotrophic, heterotrophic, and mixotrophic conditions. Biologia (Bratisl) 74:1579–1590. https://doi.org/10.2478/s11756-019-00336-6
Vanitha A, Narayan MS, Murthy KNC, Ravishankar GA (2007) Comparative study of lipid composition of two halotolerant alga, Dunaliella bardawil and Dunaliella salina. Int J Food Sci Nutr 58:373–382. https://doi.org/10.1080/09637480701252252
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design of the experiment and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cepoi, L., Zinicovscaia, I., Rudi, L. et al. Changes in the Dunaliella salina biomass composition during silver nanoparticles formation. Nanotechnol. Environ. Eng. 7, 235–243 (2022). https://doi.org/10.1007/s41204-022-00218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00218-4