Highlights
-
A new type of metallacage was successfully fabricated and used as containers for hypocrellin-type photosensitizers, which prevented the self-aggregation of photosensitizers in aqueous solution.
-
The metallacage was also employed as an energy donor to promote the singlet oxygen generation ability via fluorescence resonance energy transfer, thereby achieving highly efficient photodynamic therapy.
Abstract
The development of supramolecular hosts which can efficiently encapsulate photosensitizers to improve the photodynamic efficacy holds great promise for cancer therapy. Here, we report two perylene diimide-based metallacages that can form stable host–guest complexes with planar conjugated molecules including polycyclic aromatic hydrocarbons and photosensitizers (hypocrellin A). Such host–guest complexation not only prevents the aggregation of photosensitizers in aqueous environments, but also offers fluorescence resonance energy transfer (FRET) from the metallacage to the photosensitizers to further improve the singlet oxygen generation (ΦΔ = 0.66). The complexes are further assembled with amphiphilic polymers, forming nanoparticles with improved stability for anticancer study. Both in vitro and in vivo studies indicate that the nanoparticles display excellent anticancer activities upon light irradiation, showing great potential for cancer photodynamic therapy. This study provides a straightforward and effective approach for enhancing the photosensitivity of conventional photosensitizers via host–guest complexation-based FRET, which will open a new avenue for host–guest chemistry-based supramolecular theranostics.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
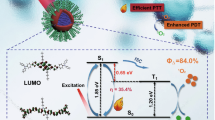
Although chemotherapy is still the most widely used approach in the treatment of tumors, it often suffers from severe side effects, non-targeting capability, and potential drug resistance, which greatly limits its further applications in clinical trials [1,2,3]. In order to solve this problem, combinational therapy is developed via the reasonable integration of other therapeutic methods to increase the efficacy and reduce the side effect of cancer treatment [4,5,6,7]. In this regard, photodynamic therapy (PDT) has been considered to be a promising complementary strategy to chemotherapy because of its negligible drug resistance, minimal invasion, fewer side effects, and less damage to normal tissues [8,9,10,11,S33), which was consistent with the 1O2 signal generated by the complexes. Compared to Cage 4b ⊃ G5, the signal from Cage 4a ⊃ G5 appeared relatively weak. In addition, the 1O2 generation quantum yields of 3a, Cage 4a, 3b, Cage 4b, G5, Cage 4a ⊃ G5, and Cage 4b ⊃ G5 were measured using a reactive 1O2 scavenger, 1,3-diphenylisobenzofuran (DPBF) [67]. Time-dependent UV/vis absorption spectra of a mixture solution of DPBF and different species upon irradiation at 520 nm were recorded (Fig. S34). A gradual decrease in the characteristic absorption band centered at 410 nm for DPBF was observed with increasing exposure time, indicating the accumulation of 1O2. The absorption bands corresponding to Cage 4a, Cage 4b, Cage 4a ⊃ G5, and Cage 4b ⊃ G5 remained almost unchanged, suggesting their good photostability. The 1O2 quantum yields (ΦΔ) of all the compounds were calculated using Rose Bengal (RB) with a known efficiency (ΦΔRB = 0.54) as the reference. The ΦΔ values were determined to be 12%, 9%, 14%, 11%, 45%, 53%, and 66% for 3a, Cage 4a, 3b, Cage 4b, G5, Cage 4a ⊃ G5, and Cage 4b ⊃ G5, respectively (Fig. 3d). Based on these results, both Cage 4a/Cage 4b and G5 exhibited the capacity for 1O2 generation. Additionally, the FRET between metallacages and G5 further enhanced the efficiency of 1O2 generation for complexes Cage 4a ⊃ G5 and Cage 4b ⊃ G5. Notably, Cage 4b ⊃ G5 demonstrated a higher ΦΔ compared with Cage 4a ⊃ G5, attributed to its better host−guest complexation.
Based on the outstanding 1O2 generation capability of Cage 4b ⊃ G5, its application for cancer photodynamic therapy was further explored. 1,2-Distearoyl-phosphatidylethanolamine (DSPE)/polyethylene glycol (PEG) conjugate (mPEG-DSPE2000) [68], a commonly used polymer, was assembled with complex Cage 4b ⊃ G5 to form nanoparticles NPs 5, which were further used for further biological experiments (Fig. 3a). The photo image (Fig. 3e) of NPs 5 presented obvious “Tyndall effect” in solution, which indicated the successful preparation of dispersed colloidal nanoparticles. The size and morphology of NPs 5 were examined using scanning electron microscopy (SEM) and dynamic light scattering (DLS), respectively. The SEM image (Fig. 3f) revealed that NPs 5 exhibited micellar structures with diameters of 140–150 nm. DLS analysis (Fig. 3g) indicated an average hydrodynamic diameter of 142 nm, which agreed well with the SEM results. It is believed that nanoparticles within this size range can exhibit an enhanced permeability and retention effect [69], potentially augmenting their uptake and retention within tumors and increasing their anticancer activity. The size distribution of nanoparticles appeared broad, suggesting that these amphiphilic structures may readily aggregate in aqueous solution. No notable alterations in size (Fig. 3g) or absorption (Fig. S35) were observed for these nanoparticles even after 7 days, demonstrating the exceptional stability of complex-loaded NPs 5, attributed to the protective role of the amphiphilic mPEG-DSPE, which securely houses the complex within the hydrophobic interior.
3.3 Cell Imaging and Anticancer Study
The UV/vis absorption and emission spectra of 3a, Cage 4a, 3b, Cage 4b, G5, Cage 4a ⊃ G5, and Cage 4b ⊃ G5 were recorded to assess their optical characteristics (Fig. S36). Ligand 3a exhibited three absorption bands centered at 466, 496, and 534 nm, which was consistent with the typical absorption of PDI derivatives [70, 71]. The corresponding molar absorption coefficients (ε) were determined to be 1.50 × 104, 3.21 × 104, and 4.08 × 104 M−1 cm−1, respectively. Likewise, ligand 3b, Cage 4a, and Cage 4b, complexes Cage 4a ⊃ G5 and Cage 4b ⊃ G5 all displayed similar absorption bands in the visible region. Furthermore, two distinct emission peaks were observed for 3a at wavelengths of 515 and 551 nm. In contrast, 3b exhibited much weaker emission with peaks centered at 572 and 621 nm, which is probably due to the increased molecular motions in 3b compared with 3a. Cage 4a and Cage 4b exhibited two emission bands centered at 516 and 560 nm, and 556 and 597 nm, respectively. The fluorescence quantum yields (ΦF, Figs. S37–S41) of 3a, 3b, Cage 4a, Cage 4b, and G5 were determined to be 93.91%, 0.96%, 34.06%, 4.93%, and 3.68%, respectively. As for Cage 4a ⊃ G5 and Cage 4b ⊃ G5, the complexation leads to bathochromic shift and increased emission via FRET, with the ΦF values of 42.09% and 27.19%, respectively (Figs. S42 and S43). Therefore, Cage 4b, Cage 4b ⊃ G5, and 5 were further used as contrast agents for bioimaging. Liver cancer MHCC-97L cells and lung cancer NCI-H460 cells were stained with DAPI and treated with above-mentioned compounds simultaneously. After 6 h, the intensity of the fluorescence reached the maximum value which did not increase as time went by, so the images were taken at 6-h post incubation (Figs. 4a and S44) using confocal laser scanning microscopy (CLSM). Based on the merged figures, bright red fluorescence originating from these compounds was observed within the cells. The cellular uptake of complex Cage 4b ⊃ G5 and NPs 5 was assessed through flow cytometry (FCM) analysis. In both MHCC-97L and NCI-H460 cells (Figs. 4b and S45 − S47), stronger emission was observed for NPs 5, suggesting that the cellular uptake of NPs 5 was better than complex Cage 4b ⊃ G5 after incubation. Collectively, these findings confirm the suitability of Cage 4b, Cage 4b ⊃ G5, and NPs 5 as contrast agents for cell imaging.
a CLSM images of MHCC-97L cells after the incubation with DAPI and G5, Cage 4b, Cage 4b ⊃ G5, or NPs 5. b Fluorescence intensity of cells incubated with 4b ⊃ G5 or NPs 5 at different times. c Cell inhibition of MHCC-97L cells incubated with 3b, G5, Cage 4b, Cage 4b ⊃ G5, or NPs 5 without/with light irradiation. d CLSM images of MHCC-97L cells after the incubation with PBS and NPs 5 without/with light irradiation (excitation wavelength: FDA 488 nm, PI 543 nm; emission filter: FDA 500–550 nm, PI 550–650 nm)
Considering the 1O2 generation capacity and good stability of G5 and PDI derivatives, 3b, G5, Cage 4b, Cage 4b ⊃ G5, and NPs 5 were employed to study the intracellular 1O2 production. Flow cytometry (FCM) experiments (Figs. S48 and S49) utilizing 2,7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) as a reactive 1O2 scavenger [72] revealed that only cells treated with light exhibited good fluorescence, indicating that all the compounds were capable of producing 1O2 under light irradiation. Significantly, the fluorescence intensity of Cage 4b ⊃ G5 and NPs 5 was much stronger than that of other compounds, suggesting that these two compounds possessed the highest 1O2 generation capacity among all tested compounds in cells, consistent with their 1O2 generation quantum yields in solution (Fig. 3d). Moreover, due to the higher cellular uptake of NPs 5 compared to Cage 4b ⊃ G5, NPs 5 exhibited better intracellular 1O2 production.
The anticancer efficacy for PDT involving 2, 3b, G5, Cage 4b, Cage 4b ⊃ G5, and NPs 5 against two human cancer cell lines (MHCC-97L and NCI-H460 cells) was assessed using 3-(4’,5’-dimethylthiazol-2’-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Figs. 4c and S50). In order to ensure the comparability of test results, the absolute concentration of all the compounds was set to be 1.25 μM. Compared with compounds without light treatment, all tested substances exhibited significantly enhanced anticancer activities upon light irradiation. Taking NPs 5 as an example, in the absence of irradiation, a small amount of apoptotic and necrotic cells was detected, which may be related to the chemotherapeutic effect of the platinum(II) contained in the metallacage. However, when treated with laser irradiation (50 mW cm−2, white light, 1 min), the inhibition rate increased from 29 to 92% for MHCC-97L cells. For NCI-H460 cells, NPs 5 achieved 92% inhibition rate under irradiation. Notably, NPs 5 showed the highest cytotoxicity among these compounds. This is likely attributed to the occurrence of FRET from Cage 4b to G5, leading to an increase in the efficiency of 1O2 generation in Cage 4b ⊃ G5. The photodynamic therapeutic effect of NPs 5 was further demonstrated by staining live cells and apoptotic cells with fluorescein diacetate (FDA) and propidium iodide (PI), respectively. As depicted in Fig. 4d, the control groups (PBS, PBS with light irradiation, and NPs 5 without light) exhibited green fluorescent live cells. Only the experimental group (NPs 5 with laser irradiation) predominantly showed red fluorescence, indicating apoptotic cells, providing further evidence of effective photodynamic therapy.
In vivo experiments were further conducted to evaluate the theranostic activities of 2, 3b, G5, Cage 4b, Cage 4b ⊃ G5, and NPs 5. In this study, MHCC-97L tumor-bearing nude mice with subcutaneous xenograft tumor models were employed. Initially, in vivo fluorescence imaging of mice was carried out after tail vein injection of these compounds to evaluate their uptake and biodistribution capability (Figs. S51 and S52). A noticeable fluorescence signal was concentrated at the tumor site in mice after the treatment with 3b, G5, Cage 4b, Cage 4b ⊃ G5, or NPs 5. In contrast, negligible fluorescence signal was detected in other organs such as the liver and kidneys, indicating a significant accumulation of compounds in the tumor area 6-h post injection. Notably, the fluorescence signal of NPs 5 was notably higher than that of other compounds, suggesting that these nanoparticles exhibited long-term fluorescence and possessed excellent tumor accumulation and retention capabilities. The strong fluorescence exhibited by NPs 5 facilitated imaging techniques for monitoring the pharmacokinetics in mice with tumors. Following injection, NPs 5 displayed sustained systemic distribution, and the fluorescence of NPs 5 localized in tumor tissues progressively increased over time (Fig. 5a). Fluorescence imaging conducted at 6-h post injection revealed heightened fluorescence at the tumor site compared to normal organs. This signal remained for at least 12-h post injection, indicating obvious biodistribution of NPs 5 within the sample. Conversely, mice administered with Cage 4b ⊃ G5 displayed a more rapid decline in fluorescence signal throughout the body. This can be attributed to the prolonged circulation times of PEGylated NPs 5, which markedly enhanced their permeability and retention within tumors.
a In vivo fluorescence imaging of the tumor-bearing mice after tail vein injection of Cage 4b ⊃ G5 and NPs 5. b Digital photos of the final tumor tissues harvested from the mice treated with different formulations at day 14. c Tumor volume changes, d tumor mass (∗p < 0.1, ∗ ∗p < 0.01, ∗ ∗ ∗p < 0.001), e body weight changes, and f spleen mass after injection of various formulas with 520 nm (50 mW cm−2) irradiation (35 tumor-bearing female nude mice were divided into seven groups, n = 5/group). g H&E and Ki 67 staining of postoperative sections collected from different groups treated tumor tissues after 14 d therapies (The scale bar is 100 μm)
The in vivo antitumor efficacy of the photochemotherapy was further assessed. The mice were randomly divided into seven groups (n = 5/group) when the tumor volume reached ∼100 mm3 and were subcutaneously injected with PBS buffer, 2, 3b, G5, Cage 4b, Cage 4b ⊃ G5, or NPs 5, all at a dose of 2 mg kg−1 in platinum weight, then treated with laser irradiation (50 mW cm−2 for 30 s). The average tumor size of the different groups was observed every 2 days for 2 weeks to evaluate the therapeutic effect. All the test groups, including 3b, G5, Cage 4b, Cage 4b ⊃ G5, and NPs 5, exhibited better antitumor activities compared to the control group administered with PBS and 2, as evidenced by the decreased size of the tumor after treatment (Fig. 5b). As depicted in Fig. 5c, weak therapeutic effects were observed for the mice that only underwent single chemotherapy (2) or single PDT (3b and G5) compared to the PBS group. An exceptional antitumor outcome was achieved for the photochemotherapy group (Cage 4b ⊃ G5 and NPs 5). Notably, the mice treated with NPs 5 exhibited the highest tumor growth inhibition at 14-day post treatment, confirmed by the smallest tumor volume among all the test groups, which aligned with the tumor mass outcome (Fig. 5d). Moreover, the body weights of the mice remained very similar (Fig. 5e). There was no significant reduction in body weight, and no distinct signs of toxic effects such as changes in urination or nervous behavior were observed for all the tested compounds. The spleen mass (Fig. 5f) remained nearly unchanged, indicating that these compounds can be applied as therapeutic agents for cancer treatment.
Hematoxylin and eosin (H&E) staining assay was also conducted to assess the proliferation and apoptosis of cells in the tumor tissue (Fig. 5g). All treatment groups exhibited varying degrees of necrosis compared to the PBS group, indicating that all tested compounds possessed certain antitumor activities. Remarkably, NPs 5 with laser irradiation resulted in noticeable shrinkage and alterations in the tumor cells and the highest level of tumor apoptosis and necrosis, signifying a pronounced inhibitory effect on tumor proliferation. Furthermore, Ki67-positive immunohistochemical staining was utilized, where areas of cell proliferation were marked by brown spots in the captured images in Fig. 5g. Notably, the group treated with NPs 5 under laser irradiation showed the most substantial decrease in the count of Ki67-positive tumor cells compared with other treatment groups. In essence, these results unequivocally confirmed the amplified synergistic therapeutic performance achieved through the combination of chemotherapy and laser irradiation-activated PDT. These results suggested that the host–guest complexation between the metallacages and photosensitizers, which has been demonstrated to increase the 1O2 generation via FRET, played a pivotal role to increase the efficacy of cancer photochemotherapy. The routine blood test analysis and blood biochemical assay were conducted to further assess the potential long-term toxicity of NPs 5 in vivo. All markers remained within normal ranges (Figs. S53 and S54), indicating no significant toxicity or inflammatory response. These findings emphasize the efficacy of combining chemotherapy and PDT in tumor treatment, thereby improving the survival quality of mice and extending their lifespan.
4 Conclusions
In summary, two emissive perylene diimide metallacages with different cavity sizes were prepared and further utilized for complexing hypocrellin-type photosensitizer through host–guest interactions. The aggregation of hypocrellin A in an aqueous solution was suppressed after complexation with the metallacage. Noticeably, the efficient host–guest complexation shortened the distance between the metallacages and hypocrellin A, offering effective FRET from the metallacages to hypocrellin A and increasing the 1O2 generation quantum yields. Thus, the host–guest complexes were further assembled into supramolecular nanoparticles, demonstrating superior photodynamic activities for cancer therapy compared with sole metallacages and photosensitizer, as indicated by both in vitro and in vivo studies. This study offers an efficient strategy to address the photosensitivity limitation of conventional photosensitizers through the host–guest complexation-based FRET, which will promote the development of metallacage-based delivery system for cancer therapy.
References
B.A. Chabner, T.G.J. Roberts, Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer 5, 65–72 (2005). https://doi.org/10.1038/nrc1529
W. Mu, Q. Chu, Y. Liu, N. Zhang, A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett. 12, 142 (2020). https://doi.org/10.1007/s40820-020-00482-6
X. Zhao, R. Zheng, B. Zhang, Y. Zhao, W. Xue et al., Sulfonated perylene as three-in-one STING agonist for cancer chemo-immunotherapy. Angew. Chem. Int. Ed. 63, e202318799 (2024). https://doi.org/10.1002/anie.202318799
K.M. Mahoney, P.D. Rennert, G.J. Freeman, Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 14, 561–584 (2015). https://doi.org/10.1038/nrd4591
W. Fan, B. Yung, P. Huang, X. Chen, Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 117, 13566–13638 (2017). https://doi.org/10.1021/acs.chemrev.7b00258
J. Nam, S. Son, K.S. Park, W. Zou, L.D. Shea et al., Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414 (2019). https://doi.org/10.1038/s41578-019-0108-1
S. Zhang, L. **, J. Liu, Y. Liu, T. Zhang et al., Boosting chemodynamic therapy by the synergistic effect of co-catalyze and photothermal effect triggered by the second near-infrared light. Nano-Micro Lett. 12, 180 (2020). https://doi.org/10.1007/s40820-020-00516-z
S.B. Brown, E.A. Brown, I. Walker, The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 5, 497–508 (2004). https://doi.org/10.1016/S1470-2045(04)01529-3
A.P. Castano, P. Mroz, M.R. Hamblin, Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 6, 535–545 (2006). https://doi.org/10.1038/nrc1894
X. Li, S. Lee, J. Yoon, Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 47, 1174–1188 (2018). https://doi.org/10.1039/C7CS00594F
X. Li, Y. Liu, F. Fu, M. Cheng, Y. Liu et al., Single NIR laser-activated multifunctional nanoparticles for cascaded photothermal and oxygen-independent photodynamic therapy. Nano-Micro Lett. 11, 68 (2019). https://doi.org/10.1007/s40820-019-0298-5
N. Yang, W. **ao, X. Song, W. Wang, X. Dong, Recent advances in tumor microenvironment hydrogen peroxide-responsive materials for cancer photodynamic therapy. Nano-Micro Lett. 12, 15 (2020). https://doi.org/10.1007/s40820-019-0347-0
Y. Cai, D. Ni, W. Cheng, C. Ji, Y. Wang et al., Enzyme-triggered disassembly of perylene monoimide-based nanoclusters for activatable and deep photodynamic therapy. Angew. Chem. Int. Ed. 59, 14014–14018 (2020). https://doi.org/10.1002/anie.202001107
M. Ethirajan, Y. Chen, P. Joshi, R.K. Pandey, The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40, 340–362 (2011). https://doi.org/10.1039/B915149B
X. Zheng, J. Ge, J. Wu, W. Liu, L. Guo et al., Biodegradable hypocrellin derivative nanovesicle as a near-infrared light-driven theranostic for dually photoactive cancer imaging and therapy. Biomaterials 185, 133–141 (2018). https://doi.org/10.1016/j.biomaterials.2018.09.021
Z. Wang, Q. Sun, B. Liu, Y. Kuang, A. Gulzar et al., Recent advances in porphyrin-based MOFs for cancer therapy and diagnosis therapy. Coord. Chem. Rev. 439, 213945 (2021). https://doi.org/10.1016/j.ccr.2021.213945
W. Shao, C. Yang, F. Li, J. Wu, N. Wang et al., Molecular design of conjugated small molecule nanoparticles for synergistically enhanced PTT/PDT. Nano-Micro Lett. 12, 147 (2020). https://doi.org/10.1007/s40820-020-00474-6
L. Tu, C. Li, X. **ong, J. Hyeon Kim, Q. Li et al., Engineered metallacycle-based supramolecular photosensitizers for effective photodynamic therapy. Angew. Chem. Int. Ed. 62, 2301560 (2023). https://doi.org/10.1002/anie.202301560
J. Zhou, G. Yu, F. Huang, Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 46, 7021–7053 (2017). https://doi.org/10.1039/c6cs00898d
H. Zhu, H. Wang, B. Shi, L. Shangguan, W. Tong et al., Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 10, 2412 (2019). https://doi.org/10.1038/s41467-019-10385-9
Y. Inokuma, M. Kawano, M. Fujita, Crystalline molecular flasks. Nat. Chem. 3, 349–358 (2011). https://doi.org/10.1038/nchem.1031
C.J. Brown, F.D. Toste, R.G. Bergman, K.N. Raymond, Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 115, 3012–3035 (2015). https://doi.org/10.1021/cr4001226
T.R. Cook, P.J. Stang, Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 115, 7001–7045 (2015). https://doi.org/10.1021/cr5005666
G.H. Clever, P. Punt, Cation-anion arrangement patterns in self-assembled Pd2L4 and Pd4L8 coordination cages. Acc. Chem. Res. 50, 2233–2243 (2017). https://doi.org/10.1021/acs.accounts.7b00231
S. Chakraborty, G.R. Newkome, Terpyridine-based metallosupramolecular constructs: tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 47, 3991–4016 (2018). https://doi.org/10.1039/c8cs00030a
F.J. Rizzuto, L.K.S. von Krbek, J.R. Nitschke, Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 3, 204–222 (2019). https://doi.org/10.1038/s41570-019-0085-3
M. Yoshizawa, L. Catti, Bent anthracene dimers as versatile building blocks for supramolecular capsules. Acc. Chem. Res. 52, 2392–2404 (2019). https://doi.org/10.1021/acs.accounts.9b00301
M. Pan, K. Wu, J.-H. Zhang, C.-Y. Su, Chiral metal–organic cages/containers (MOCs): from structural and stereochemical design to applications. Coord. Chem. Rev. 378, 333–349 (2019). https://doi.org/10.1016/j.ccr.2017.10.031
H.-Y. Lin, Y.-T. Wang, X. Shi, H.-B. Yang, L. Xu, Switchable metallacycles and metallacages. Chem. Soc. Rev. 52, 1129–1154 (2023). https://doi.org/10.1039/d2cs00779g
L.-J. Chen, G.-Z. Zhao, B. Jiang, B. Sun, M. Wang et al., Smart stimuli-responsive spherical nanostructures constructed from supramolecular metallodendrimers via hierarchical self-assembly. J. Am. Chem. Soc. 136, 5993–6001 (2014). https://doi.org/10.1021/ja500152a
C.-L. Liu, R.-L. Zhang, C.-S. Lin, L.-P. Zhou, L.-X. Cai et al., Intraligand charge transfer sensitization on self-assembled europium tetrahedral cage leads to dual-selective luminescent sensing toward anion and cation. J. Am. Chem. Soc. 139, 12474–12479 (2017). https://doi.org/10.1021/jacs.7b05157
P. Howlader, E. Zangrando, P.S. Mukherjee, Self-assembly of enantiopure Pd12 tetrahedral homochiral nanocages with tetrazole linkers and chiral recognition. J. Am. Chem. Soc. 142, 9070–9078 (2020). https://doi.org/10.1021/jacs.0c03551
K. Yang, B. Hua, S. Qi, B. Bai, C. Yu et al., Suprasomes based on host-guest molecular recognition: an excellent alternative to liposomes in cancer theranostics. Angew. Chem. Int. Ed. 61, e202213572 (2022). https://doi.org/10.1002/anie.202213572
G. Li, T.K. Ronson, R. Lavendomme, Z. Huang, C. Fuertes-Espinosa et al., Enantiopure FeII 4L4 cages bind steroids stereoselectively. Chem 9, 1549–1561 (2023). https://doi.org/10.1016/j.chempr.2023.03.011
J. Zhou, G. Yu, Q. Li, M. Wang, F. Huang, Separation of benzene and cyclohexane by nonporous adaptive crystals of a hybrid[3]arene. J. Am. Chem. Soc. 142, 2228–2232 (2020). https://doi.org/10.1021/jacs.9b13548
L. Ma, C.J.E. Haynes, A.B. Grommet, A. Walczak, C.C. Parkins et al., Coordination cages as permanently porous ionic liquids. Nat. Chem. 12, 270–275 (2020). https://doi.org/10.1038/s41557-020-0419-2
A.B. Sainaba, M. Venkateswarulu, P. Bhandari, K.S.A. Arachchige, J.K. Clegg et al., An adaptable water-soluble molecular boat for selective separation of phenanthrene from isomeric anthracene. J. Am. Chem. Soc. 144, 7504–7513 (2022). https://doi.org/10.1021/jacs.2c02540
S.-C. Li, L.-X. Cai, M. Hong, Q. Chen, Q.-F. Sun, Combinatorial self-assembly of coordination cages with systematically fine-tuned cavities for efficient co-encapsulation and catalysis. Angew. Chem. Int. Ed. 61, e202204732 (2022). https://doi.org/10.1002/anie.202204732
J. Yang, S.-J. Hu, L.-X. Cai, L.-P. Zhou, Q.-F. Sun, Counteranion-mediated efficient iodine capture in a hexacationic imidazolium organic cage enabled by multiple non-covalent interactions. Nat. Commun. 14, 6082 (2023). https://doi.org/10.1038/s41467-023-41866-7
C.F. Espinosa, T.K. Ronson, J.R. Nitschke, Secondary bracing ligands drive heteroleptic cuboctahedral PdII12 cage formation. J. Am. Chem. Soc. 145, 9965–9969 (2023). https://doi.org/10.1021/jacs.3c00661
R. Zhang, D. Hu, Y. Fu, Q. Feng, C. Mu et al., Triazine-based multicomponent metallacages with tunable structures for SO2 selective capture and conversion. Aggregate (2023). https://doi.org/10.1002/agt2.408
L.-X. Cai, S.-C. Li, D.-N. Yan, L.-P. Zhou, F. Guo et al., Water-soluble redox-active cage hosting polyoxometalates for selective desulfurization catalysis. J. Am. Chem. Soc. 140, 4869–4876 (2018). https://doi.org/10.1021/jacs.8b00394
Z. Zhang, L. Ma, F. Fang, Y. Hou, C. Lu et al., Porphyrin-based multicomponent metallacage: host-guest complexation toward photooxidation-triggered reversible encapsulation and release. JACS Au 2, 1479–1487 (2022). https://doi.org/10.1021/jacsau.2c00245
R. Saha, B. Mondal, P.S. Mukherjee, Molecular cavity for catalysis and formation of metal nanoparticles for use in catalysis. Chem. Rev. 122, 12244–12307 (2022). https://doi.org/10.1021/acs.chemrev.1c00811
D.-N. Yan, L.-X. Cai, S.-J. Hu, Y.-F. Zhou, L.-P. Zhou et al., An organo-palladium host built from a dynamic macrocyclic ligand: adaptive self-assembly, induced-fit guest binding, and catalysis. Angew. Chem. Int. Ed. 61, e202209879 (2022). https://doi.org/10.1002/anie.202209879
C. Mu, L. Zhang, G. Li, Y. Hou, H. Liu et al., Isoreticular preparation of tetraphenylethylene-based multicomponent metallacages towards light-driven hydrogen production. Angew. Chem. Int. Ed. 62, e202311137 (2023). https://doi.org/10.1002/anie.202311137
T.R. Cook, V. Vajpayee, M.H. Lee, P.J. Stang, K.W. Chi, Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 46, 2464–2474 (2013). https://doi.org/10.1021/ar400010v
H. Sepehrpour, W. Fu, Y. Sun, P.J. Stang, Biomedically relevant self-assembled metallacycles and metallacages. J. Am. Chem. Soc. 141, 14005–14020 (2019). https://doi.org/10.1021/jacs.9b06222
Q. Feng, R. Li, T. Gao, D. Chu, M. Zhang, Emissive metallacages for biomedical applications. Sci. China Chem. 66, 2447–2459 (2023). https://doi.org/10.1007/s11426-023-1672-4
Y. Xu, W. Tuo, L. Yang, Y. Sun, C. Li et al., Design of a metallacycle-based supramolecular photosensitizer for in vivo image-guided photodynamic inactivation of bacteria. Angew. Chem. Int. Ed. 61, e202110048 (2022). https://doi.org/10.1002/anie.202110048
F. Schmitt, J. Freudenreich, N.P.E. Barry, L. Juillerat-Jeanneret, G. Süss-Fink et al., Organometallic cages as vehicles for intracellular release of photosensitizers. J. Am. Chem. Soc. 134, 754–757 (2012). https://doi.org/10.1021/ja207784t
V. Abdul Rinshad, J. Sahoo, M. Venkateswarulu, N. Hickey, M. De et al., Solvent induced conversion of a self-assembled gyrobifastigium to a barrel and encapsulation of zinc-phthalocyanine within the barrel for enhanced photodynamic therapy. Angew. Chem. Int. Ed. 62, e202218226 (2023). https://doi.org/10.1002/anie.202218226
G. Yu, S. Yu, M.L. Saha, J. Zhou, T.R. Cook et al., A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 9, 4335 (2018). https://doi.org/10.1038/s41467-018-06574-7
C. Li, Y. Pang, Y. Xu, M. Lu, L. Tu et al., Near-infrared metal agents assisting precision medicine: from strategic design to bioimaging and therapeutic applications. Chem. Soc. Rev. 52, 4392–4442 (2023). https://doi.org/10.1039/d3cs00227f
C.-B. Huang, L. Xu, J.-L. Zhu, Y.-X. Wang, B. Sun et al., Real-time monitoring the dynamics of coordination-driven self-assembly by fluorescence-resonance energy transfer. J. Am. Chem. Soc. 139, 9459–9462 (2017). https://doi.org/10.1021/jacs.7b04659
A.J.P. Teunissen, C. Pérez-Medina, A. Meijerink, W.J.M. Mulder, Investigating supramolecular systems using Förster resonance energy transfer. Chem. Soc. Rev. 47, 7027–7044 (2018). https://doi.org/10.1039/C8CS00278A
L. Wu, C. Huang, B.P. Emery, A.C. Sedgwick, S.D. Bull et al., Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 49, 5110–5139 (2020). https://doi.org/10.1039/c9cs00318e
Y. Hou, Z. Zhang, S. Lu, J. Yuan, Q. Zhu et al., Highly emissive perylene diimide-based metallacages and their host–guest chemistry for information encryption. J. Am. Chem. Soc. 142, 18763–18768 (2020). https://doi.org/10.1021/jacs.0c09904
C. Mu, Z. Zhang, Y. Hou, H. Liu, L. Ma et al., Tetraphenylethylene-based multicomponent emissive metallacages as solid-state fluorescent materials. Angew. Chem. Int. Ed. 60, 12293–12297 (2021). https://doi.org/10.1002/anie.202100463
H. Liu, Z. Zhang, C. Mu, L. Ma, H. Yuan et al., Hexaphenylbenzene-based deep blue-emissive metallacages as donors for light-harvesting systems. Angew. Chem. Int. Ed. 61, e202207289 (2022). https://doi.org/10.1002/anie.202207289
Z. Zhang, Z. Zhao, L. Wu, S. Lu, S. Ling et al., Emissive platinum(II) cages with reverse fluorescence resonance energy transfer for multiple sensing. J. Am. Chem. Soc. 142, 2592–2600 (2020). https://doi.org/10.1021/jacs.9b12689
H. Liu, C. Guo, Z. Zhang, C. Mu, Q. Feng et al., Hexaphenyltriphenylene-based multicomponent metallacages: host-guest complexation for white-light emission. Chemistry 29, e202203926 (2023). https://doi.org/10.1002/chem.202203926
F. Biedermann, H.-J. Schneider, Experimental binding energies in supramolecular complexes. Chem. Rev. 116, 5216–5300 (2016). https://doi.org/10.1021/acs.chemrev.5b00583
S. Sarkar, P. Ballester, M. Spektor, E.A. Kataev, Micromolar affinity and higher: synthetic host-guest complexes with high stabilities. Angew. Chem. Int. Ed. 62, e202214705 (2023). https://doi.org/10.1002/anie.202214705
Z. Xu, S. Peng, Y.-Y. Wang, J.-K. Zhang, A.I. Lazar et al., Broad-spectrum tunable photoluminescent nanomaterials constructed from a modular light-harvesting platform based on macrocyclic amphiphiles. Adv. Mater. 28, 7666–7671 (2016). https://doi.org/10.1002/adma.201601719
T. Mirkovic, E.E. Ostroumov, J.M. Anna, R. van Grondelle, Govindjee et al., Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 117, 249–293 (2017). https://doi.org/10.1021/acs.chemrev.6b00002
J. Zou, L. Li, J. Zhu, X. Li, Z. Yang et al., Singlet oxygen “afterglow” therapy with NIR-II fluorescent molecules. Adv. Mater. 33, 2103627 (2021). https://doi.org/10.1002/adma.202103627
F. Andrade, D. Rafael, M. Videira, D. Ferreira, A. Sosnik et al., Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 65, 1816–1827 (2013). https://doi.org/10.1016/j.addr.2013.07.020
A. Gabizon, M. Bradbury, U. Prabhakar, W. Zamboni, S. Libutti et al., Cancer nanomedicines: closing the translational gap. Lancet 384, 2175–2176 (2015). https://doi.org/10.1016/S0140-6736(14)61457-4
A. Nel, E. Ruoslahti, H. Meng, New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano 11, 9567–9569 (2017). https://doi.org/10.1021/acsnano.7b07214
F. Würthner, C.R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat et al., Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 116, 962–1052 (2016). https://doi.org/10.1021/acs.chemrev.5b00188
S. Yan, P. Sun, N. Niu, Z. Zhang, W. Xu et al., “One stone, four birds” ion engineering to fabricate versatile core–shell organosilica nanoparticles for intelligent nanotheranostics. ACS Nano 16, 9785–9798 (2022). https://doi.org/10.1021/acsnano.2c03550
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171219 and 22222112), Innovation Talent Promotion Plan of Shaanxi Province for Science and Technology Innovation Team (2023-CX-TD-51), Key Laboratory Fund for Plasma Physics (6142A04210108), the Interdisciplinary Training Program for Doctoral Candidate of **’an Jiaotong University (IDT2105), and National Natural Science Foundation NSAF Joint Fund (U2230112). We thank Dr. Gang Chang and Dan He at Instrument Analysis Center and Dr. Aqun Zheng and Junjie Zhang at Experimental Chemistry Center of **’an Jiaotong University for NMR and fluorescence measurements. We thank Menghan Sun from Shiyanjia Lab (www.shiyanjia.com) for the X-ray crystal structure analysis and electron spin resonance measurements. We also acknowledge the mass spectrometry characterization provided by the members of Molecular Scale Lab at Shenzhen University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, R., Yang, T., Peng, X. et al. Enhancing the Photosensitivity of Hypocrellin A by Perylene Diimide Metallacage-Based Host–Guest Complexation for Photodynamic Therapy. Nano-Micro Lett. 16, 226 (2024). https://doi.org/10.1007/s40820-024-01438-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-024-01438-w