Abstract
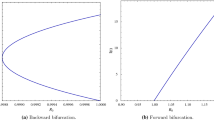
This paper deals with the transmission and spread of the hepatitis A virus in central west Tunisia (the city of Thala). The target of this framework is to determine the global stability of a SEIRD epidemiological model where the infectious compartment is split into symptomatic and asymptomatic compartments. We study the global stability of the equilibrium state using the Lyapunov function which depends on the value of the basic reproduction number \(R_0\). Therefore, we prove that when \(R_0<1\), the disease-free equilibrium (DFE) is globally stable, but when \(R_0>1\), the DFE is unstable, and therefore the the endemic equilibrium is globally stable.












Similar content being viewed by others
Availability of data and materials
In our previous study [6], we developed an age-structured epidemiological model to assess the impact of introducing vaccination. The study was conducted in Thala, a predominantly rural area in west-central Tunisia (Kasserine governorate); covering 34,508 inhabitants; according to the 2014 national census. The study population consisted of 1379 individuals, representing 5% of the target population and selected by a cross-sectional household survey conducted between January and June 2014. Permanent residents aged 5 to 75 were screened for hepatitis A virus antibodies. In [6], we estimated adjusted parameters for seroprevalence, incidence, and strength of infection by a linear compartmental SEIR (Susceptible-Exposed-Infectious-Recovered) model structured by age. Then, a vaccine model was constructed to assess the impact on the epidemiology of hepatitis A virus.
References
Abdal Aziz, A.M., Awad, M.A.M.: Seroprevalence of hepatitis a virus antibodies among a sample of egyptian children. EMHJ-Eastern Mediterranean Health J. 14(5), 1028–1035 (2008)
Alkhalidi, J., Alenezi, B., Al-Mufti, S., al.: Seroepidemiology of hepatitis A virus in kuwait. World J. Gastroenterol.: WJG 15(1), 102 (2009)
Allahamou, A., Azroul, E., Hammouch, Z., Alaoui, A.L.: Modeling and numerical investigation of a conformable co-infection model for describing hantavirus of the european moles. Math. Methods Appl. Sci. (2021)
Almuneef, M., Memish, Z., Balkhy, H., Qahtani, M., Alotaibi, B., Hajeer, A., Qasim, L., Al Knawy, B.: Epidemiologic shift in the prevalence of hepatitis a virus in Saudi Arabia: a case for routine hepatitis a vaccination. Vaccine 24(27–28), 5599–5603 (2006)
Ayouni, K., Kharroubi, G., Mallekh, R., Hammami, W., Marouani, R., Mhamdi, M., Ben Salah, A., Triki, H., Bettaieb, J.: Seroprevalence of hepatitis a virus infection in central-west of Tunisia. J. Med. Virol. 93(6), 3666–3671 (2021)
Ayouni, K., Naffeti, B., Ben Aribi, W., Bettaieb, J., Hammami, W., Ben Salah, A., Ammar, H., Ben Miled, S., Triki, H.: Hepatitis a virus infection in central-west Tunisia: an age structured model of transmission and vaccination impact. BMC Infect. Dis. 20(1), 1–13 (2020)
Babaei, A., Ahmadi, M., Jafari, H., Liya, A.: A mathematical model to examine the effect of quarantine on the spread of coronavirus. Chaos Solitons Fractals 142, 110418 (2021)
Babaei, A., Jafari, H., Ahmadi, M.: A fractional order HIV/AIDS model based on the effect of screening of unaware infectives. Math. Methods Appl. Sci. 42(7), 2334–2343 (2019)
Babaei, A., Jafari, H., Banihashemi, S., Ahmadi, M.: Mathematical analysis of a stochastic model for spread of coronavirus. Chaos Solitons Fractals 145, 110788 (2021)
Babaei, A., Jafari, H., Liya, A.: Mathematical models of HIV/AIDS and drug addiction in prisons. Eur. Phys. J. Plus 135(5), 1–12 (2020)
Bauch, C., Rao, A.S.S., Pham, B., Krahn, M., Gilca, V., Duval, B., Chen, M., Tricco, A.: A dynamic model for assessing universal hepatitis A vaccination in Canada. Vaccine 25(10), 1719–1726 (2007)
Bener, A., Al-Kaabi, S., Derbala, M., Al-Marri, A., Rikabi, A.: The epidemiology of viral hepatitis in qatar. Saudi J. Kidney Diseases Transplant. 20(2), 300 (2009)
Bhatia, N.P., Szegö, G.P.: Dynamical Systems: Stability Theory and Applications. Springer, New York (2006)
Boudaoui, A., El hadj Moussa, Y., Hammouch, Z., Ullah, S.: A fractional-order model describing the dynamics of the novel coronavirus (covid-19) with nonsingular kernel. Chaos, Solitons and Fractals 146, 110859 (2021)
Dhankhar, P., Nwankwo, C., Pillsbury, M., Lauschke, A., Goveia, M.G., Acosta, C.J., Elbasha, E.H.: Public Health Impact and Cost-Effectiveness of Hepatitis A Vaccination in the United States: a disease transmission dynamic modeling approach. Value Health 18(4), 358–367 (2015). https://doi.org/10.1016/j.jval.2015.02.004
Dhankhar, P., Nwankwo, C., Pillsbury, M., Lauschke, A., Goveia, M.G., Acosta, C.J., Elbasha, E.H.: Public health impact and cost-effectiveness of hepatitis a vaccination in the United States: a disease transmission dynamic modeling approach. Value Health 18(4), 358–367 (2015)
Van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2002)
Georgescu, P., Hsieh, Y.H.: Global stability for a virus dynamics model with nonlinear incidence of infection and removal. SIAM J. Appl. Math. 67(2), 337–353 (2006)
Guenifi, W., Laouamri, S., Lacheheb, A.: Changes in prevalence of hepatitis a and associated factors in Setif-Algeria. Rev. Epidemiol. Sante Publique 65(6), 437–442 (2017)
Guo, H., Li, M., Shuai, Z.: A graph-theoretic approach to the method of global Lyapunov functions. Proc. Am. Math. Soc. 136(8), 2793–2802 (2008)
Guo, H., Li, M.Y., Shuai, Z.: Global stability of the endemic equilibrium of multigroup SIR epidemic models. Can. Appl. Math. Q. 14(3), 259–284 (2006)
Hamou, A.A., Azroul, E., Hammouch, Z., Alaoui, A.L.: A fractional multi-order model to predict the Covid-19 outbreak in morocco. Appl. Comput. Math 20(1), 177–203 (2020)
Hens, N., Shkedy, Z., Aerts, M., Faes, C., Van Damme, P., Beutels, P.: Modeling Infectious Disease Parameters Based on Serological and Social Contact Data: A Modern Statistical Perspective. Springer, New York (2012)
Hethcote, H.W.: An age-structured model for pertussis transmission. Math. Biosci. 145(2), 89–136 (1997)
Hethcote, H.W., Van Ark, J.W.: Epidemiological models for heterogeneous populations: proportionate mixing, parameter estimation, and immunization programs. Math. Biosci. 84(1), 85–118 (1987)
Huang, G., Ma, W., Takeuchi, Y.: Global properties for virus dynamics model with bedding ton-deangelis functional response. Appl. Math. Lett. 22(11), 1690–1693 (2009)
Huang, G., Takeuchi, Y.: Global analysis on delay epidemiological dynamic models with nonlinear incidence. J. Math. Biol. 63(1), 125–139 (2011)
Jacobsen, K., Koopman, J.: The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int. J. Epidemiol. 34(3), 600–609 (2005)
Jacobsen, K., Wiersma, S.: Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 28(41), 6653–6657 (2010)
Jacobsen, K.H.: Hepatitis A virus in west Africa: Is an epidemiological transition beginning? Niger. Med. J.: J. Nigeria Med.l Assoc. 55(4), 279 (2014)
Jung, Y., Kim, J.: Epidemiology and clinical features of acute hepatitis A: from the domestic perspective. Korean J. Hepatol. 15(4), 438–445 (2009)
Korobeinikov, A.: Lyapunov functions and global properties for SEIR and SEIS epidemic models. Math. Med. Biol.: J. IMA 21(2), 75–83 (2004)
Korobeinikov, A.: Global properties of SIR and SEIR epidemic models with multiple parallel infectious stages. Bull. Math. Biol. 71(1), 75–83 (2009)
Korobeinikov, A., Maini, P.K.: A Lyapunov function and global properties for SIR and SEIR epidemiological models with nonlinear incidence. Math. Bio Sci. Eng. 1(1), 57–60 (2004)
Korobeinikov, A., Wake, G.C.: Lyapunov functions and global stability for SIR, SIRS, and SIS epidemiological models. Appl. Math. Lett. 15(8), 955–960 (2002)
Letaief, A., Kaabia, N., Gaha, R.: Age-specific seroprevalence of hepatitis a among school children in central Tunisia. Am. J. Trop. Med. Hygiene 73(1), 40–43 (2005)
Li, M.Y., Muldowney, J.S.: Global stability for the SEIR model in epidemiology. Math. Bio Sci. 125(2), 155–164 (1995)
Li, M.Y., Shuai, Z.: Global-stability problem for coupled systems of differential equations on networks. J. Differ. Equ. 248(1), 1–20 (2010)
Li, M.Y., Shuai, Z., Wang, C.: Global stability of multi-group epidemic models with distributed delays. J. Math. Anal. Appl. 361(1), 38–47 (2010)
Melgaco, J.G., Morgado, L.N., Santiago, M.A., de Oliveira, J.M., Lewis-**menez, L.L., Hasselmann, B., Cruz, O.G., Pinto, M.A., Vitral, C.L.: A single dose of inactivated hepatitis a vaccine promotes hav-specific memory cellular response similar to that induced by a natural infection. Vaccine 33(32), 3813–3820 (2015)
Melhem, N., Talhouk, R., Rachidi, H., Ramia, S.: Hepatitis a virus in the middle east and north Africa region: a new challenge. J. Viral Hepatitis 21(9), 605–615 (2014)
Memish, Z., Knawy, B., El-Saed, A.: Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int. J. Infect. Dis. 14(2), e115–e120 (2010)
Organization, W.H.: Who position paper on hepatitis A vaccines-June 2012. Weekly Epidemiological Record 87(28-29), 261–276 (2012)
Rezig, D., Ouneissa, R., Mhiri, L., Mejri, S., Haddad-Boubaker, S., Alaya, N.B., Triki, H.: Seroprevalences of hepatitis A and E infections in Tunisia. Pathol. Biol. (Paris) 56, 148–153 (2008)
Röst, G.: SEIR epidemiological model with varying infectivity and infinite delay. Math. Biosci. Eng. 5(2), 389–402 (2008)
Saberifiroozi, M.: Hepatitis A virus infection: Is it an important hazard to public health? Hepatitis Monthly 2011, 235–237 (2011)
Sacy, R., Haddad, M., Baasiri, G., al.: Hepatitis A in lebanon: a changing epidemiological pattern. Am. J. Trop. Med. Hygiene 73(2), 453–456 (2005)
Sahoo, P., Mondal, H.S., Hammouch, Z., Abdeljawad, T., Mishra, D., Reza, M.: On the necessity of proper quarantine without lock down for 2019-ncov in the absence of vaccine. Results Phys. 25, 104063 (2021)
Sharar, Z., Rajah, J., Parsons, H.: Childhood seroprevalence of hepatitis A in the United Arab Emirates. Trop. Doct. 38(1), 65–66 (2008)
Smith, H.L., Waltman, P.: The Theory of the Chemostat: Dynamics of Microbial Competition. Cambridge University Press, Cambridge (1995)
Tufenkeji, H.: Hepatitis A shifting epidemiology in the middle East and Africa. Vaccine 18, S65–S67 (2000)
Van Effelterre, T., Guignard, A., Marano, C., Rojas, R., Jacobsen, K.H.: Modeling the hepatitis A epidemiological transition in Brazil and Mexico. Human Vaccines Immunotherap. 13(8), 1942–1951 (2017)
Van Effelterre, T., Marano, C., Jacobsen, K.H.: Modeling the hepatitis A epidemiological transition in thailand. Vaccine 34(4), 555–562 (2016)
Wang, J., Huang, G., Takeuchi, Y., Liu, S.: SVEIR epidemiological model with varying infectivity and distributed delays. Math. Biosci. Eng. 8(3), 875 (2011)
Wasley, A., Fiore, A., Bell, B.: Hepatitis A in the era of vaccination. Epidemiol. Rev. 28(1), 101–11 (2006)
Xu, R.: Global stability of a delayed epidemic model with latent period and vaccination strategy. Appl. Math. Model. 36(11), 5293–5300 (2012)
Zhou, L., Wang, Y., **ao, Y., Li, M.Y.: Global dynamics of a discrete age-structured SIR epidemic model with applications to measles vaccination strategies. Math. Biosci. 308, 27–37 (2019)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Formal analysis and investigation, Writing the first and original draft preparation: [Walid BEN ARIBI]; Writing and editing: [Walid BEN ARIBI, Kaouther AYOUNI]. Conceptualization: [Walid BEN ARIBI, Slimane BEN MILED]. Mathematical methodology: [Walid BEN ARIBI, Amira KEBIR]. Numerical analysis: [Bechir NAFFETI]. Critically revised and commented the manuscript: [Amia KEBIR, Hamadi AMMAR, Slimane BEN MILED]. Data curation: [Henda Triki]. Supervision: [Slimane BEN MILED]. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A Computation of the Basic Reproductive Number \(R_0\)
A Computation of the Basic Reproductive Number \(R_0\)
Using the system (2) we denote by X(t) a small perturbation about the equilibrium point \(P_{0}^{*}\), defined by
Then, by linearizing the system (2) around \(P^{*}\) we have:
Where A is the matrix which is given by:
and X(t) is a function of \({\mathbf {R}}_{+}^{4 n},\) where diag(E) is defined by the matrix, where the diagonal is equals to the components of the vector E and \(\forall i \ne j\) is the coefficient equals to 0, and equivalent to
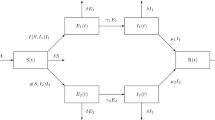
Lets \(F_{a}(y, z, T)\) the rate of appearance of new infected in the infectious compartment, \(F_{t}(y, z, T)\) the transfer rate of the individuals in to and out of the infectious compartment of the system (21) defined by Van Den Driessche [17].
And
Then
And
Consequently
Then, \(R_{0}=\rho (M),\) where \(S_{k}^{0}=\frac{\varLambda _{k}}{e_{k}+\mu _{k}^{S}}\)
Rights and permissions
About this article
Cite this article
Ben Aribi, W., Naffeti, B., Ayouni, K. et al. Global Stability and Numerical Analysis of a Compartmental Model of the Transmission of the Hepatitis A Virus (HAV): A Case Study in Tunisia. Int. J. Appl. Comput. Math 8, 126 (2022). https://doi.org/10.1007/s40819-022-01326-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s40819-022-01326-0