Abstract
The manufacturing of bioimplants not only involves selecting proper biomaterials with satisfactory bulk physicochemical properties, but also requires special treatments on surface chemistry or topography to direct a desired host response. The lifespan of a bioimplant is also critically restricted by its surface properties. Therefore, develo** proper surface treatment technologies has become one of the research focuses in biomedical engineering. This paper covers the recent progress of surface treatment of bioimplants from the aspects of coating and topography modification. Pros and cons of various technologies are discussed with the aim of providing the most suitable method to be applied for different biomedical products. Relevant techniques to evaluate wear, corrosion and other surface properties are also reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Addressing health issues such as osteoporosis and osteoarthritis, which are prevalent in an aging population, is a great challenge for modern society. Bioimplants are crucial in this regard. Bioimplants are playing a dominant role in this regard. For example, a study conducted by Kurtz et al. [1] implies that by the end of 2030, the demand for primary total hip and knee arthroplasties in the USA is estimated to grow by 174% to 572 000 and 673% to 3.48 million respectively. Given that such trend may continue [2], it makes sense that the advances in bioimplant manufacturing are required to support the production demand and expected growth. In the past few decades, the manufacturing has essentially become the core of integration domain of the biomedical field. On the other hand, industries are required to master cutting-edge manufacturing techniques that are suitable for commercial production. Such needs, in turn, drive significant levels of research and development in the manufacturing area.
The present progress and development of bioimplants forming technologies were comprehensively reviewed in the first part of this paper. However, untreated bioimplants are prone to wear and corrosion, which are critical factors in the success of possessing optimal service life. The physicochemical interaction between living tissues and biomaterial surfaces is another concern [3]. In other words, a satisfactory biocompatibility after implantation must be guaranteed. The host body normally responds to bioimplants in nanoseconds after initial contact and the environment remains in a state of flux thereafter. It is obvious that an unsatisfactory biocompatibility is likely to result in serious consequences such as immunologic rejection over time. Therefore, a broad range of surface treatment technologies are being developed in order to enable the bioimplants to possess unique surface characteristics [4]. At present, surface modification of coating on biomaterials is normally carried out prior to putting them into practical uses. Typical purposes of surface coating on bioimplants include improving wear and corrosion resistance, achieving high osseointegration and enabling the desired degradation rate.
The surface topography of a bioimplant is an important signalling modality in controlling the cell function and determines the biological reaction to the device [3]. Cell behaviours such as morphology, adhesion, orientation, migration and differentiation have all been observed to be related to the textures or patterns on the surface [5]. As the biocompatibility of an implant is closely related to the response of cells in contact with the surface [3], the surface topography modification aiming to define cells’ reaction has long been a research focus in the field of implantology. Theoretical analysis indicated that the ideal surface roughness (Ra) for hard tissue implants is in a range of 1–10 µm [6]. Numerous in vivo and in vitro studies have supported that the roughness within this range exhibits the best in interlocking implant surface and mineralized bones [7, 8]. Particularly, the osseointegration was stimulated considerably by the microscale roughened surfaces. Therefore, suitable surface modification technologies at microscale are being carried out to achieve a positive influence on protein adsorption, cellular activity and tissue response.
On the other hand, most joint implants are facing tribology issues after long periods of use. For instance, the artificial knee and hip joints would experience a great deal of rolling and sliding contacts under cyclic loading during walking activities. Friction between joint prostheses normally leads to increased energy losses in the biomedical system and ultimately to wear [9]. The wear released debris would, in turn, induce physical pain and adverse immune responses. Instead of rectification by replacing the total joint, surface treatment is believed to be a promising method to reduce the material friction coefficient, and thus prolong the device lifetime [10, 11]. In this regard, surface texturing is favoured for the ability to retain the desired bulk attributes of biomaterials, meanwhile improving the tribological properties required by different clinical applications.
The scope of this paper is to review the typical surface treatment technologies for bioimplants in two aspects, i.e., surface coating and topography modification. A discussion of current challenges and perspectives will be given in the final section.
2 Surface coating technologies
Surface coatings are currently of particular interests in prolonging lifespan and enhancing performances of various bioimplants. Such modification allows both suitable biocompatibility and biofunctionality while preserving the favourable bulk characteristics of the biomaterial. In the past years, a broad range of coating systems has been developed, which generally falls into three categories: physical, chemical and combined physical and chemical methods [4].
2.1 Plasma spraying
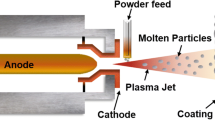
Plasma spraying, which is a subset of thermal spraying, takes advantage of the heat of ionized inert gas (plasma), and sprays molten powders of metal or ceramic onto the objective biomaterials to form a protective layer. As almost all kinds of materials can be melted in the plasma jet, this technique is quite versatile and has been widely applied in the electronic, petrochemical, medical and aerospace industries. Plasma spraying presents many advantages including a rapid deposition rate, thick deposits and also low cost. More attractively, the objects can be kept in low temperature during processing and the gas in the plasma flame can remain chemically inert, which helps to reduce the risk of thermal degradation [12]. Compared to other coating processes, the plasma sprayed layers exhibit relatively better coating properties [13].
Due to the ease of application, plasma spraying is the first method to fabricate calcium phosphate coating on biomaterials [14]. The most commonly used material for spraying is hydroxyapatite (HA), which can promote the osseointegration after implanting an help the biodevices bond directly to the surrounding tissues. Evaluations on the plasma sprayed HA coatings on titanium-based biomaterials showed that the new bone apposed directly on the coatings with satisfactory adhesion [15,16,17], and the overall bone recovery was found to be fairly quick [18]. The poor mechanical properties of HA coatings are likely to cause brittle damages and delamination, hence the alteration of structure is prone to occur. Aiming to address the issue, many parametric studies on the spaying process were carried out and followed by characterizations [19]. It was proved that by using high spraying power, suitable mechanical properties and high bonding strength could be achieved. This is due to a greater extent of coating melting resulted in a denser microstructure. The trade-off, however, is that a higher energy consumption is involved. Yang et al. [20] produced plasma sprayed HA on Ti-6Al-4V with various cooling conditions and substrate temperatures in order to have different residual stress values at the HA/metal interfaces. The evaluation results revealed that the interfacial residual stress played an important role in determining the bonding strength, where coatings with lower residual stress were found to exhibit better adhesion. Apart from temperature effects, the increased coating thickness is believed to be another reason for the rise of residual stress [19]. It is also known from early reports that compared to a smooth substrate, a highly roughened substrate surface is beneficial to achieving a better bond strength [21].
The advantages of low cost and rapid deposition rate make plasma modification process the mainstream for surface modification of biomaterials. The technology provides a flexible and environmentally friendly process that allows manufacturers to tailor the surface properties of the biomaterial to suit specific needs. However, issues cited with the plasma-sprayed coatings include variation in bond strength between the coatings and the substrates, poor adhesion at the interface and alterations in HA structure due to the coating process. In addition, to our best knowledge, there is no evidence showing that plasma sprayed coating would prolong implants’ lifespan or improve the reliability comparing to the uncoated implants. Knowing the problems of coating brought by plasma spraying, numerous alternatives of deposition process were developed.
2.2 Sputter coating
Sputter coating technique is classified as a physical vapour deposition (PVD) method and shows great promises in eliminating the issues associated with the plasma spraying process [22]. During the process, a gas plasma is utilized to eject materials from a negatively charged target. The material would be then deposited as a coating onto the substrate material. From an industrial perspective, the technology is considered as a complex process since it involves many parameters that control sputter deposition. On the other hand, however, the availability of precisely varying parameters allows a large degree of control over the growth and microstructure of the coating. Some early reports showed that the initial sputtering using multi-component ceramic targets such as superconducting oxides, HA and other calcium phosphate materials would produce coatings whose chemistry was different upon deposition than the bulk target [22, 23].
Successful attempts have been made on depositing calcium phosphate layers on metallic biomaterials using radio frequency magnetron sputtering [24, 25]. The sputtered layers were observed to be more homogeneous than the plasma sprayed ones and the surfaces appeared to be very smooth [19]. Meanwhile, the adhesion strength of sputtered HA coating and its reliability have also been found to be higher than most plasma sprayed HA coatings. A comparative study conducted by Ozeki et al. [26] indicated that the adhesion strength of the sputtered coating exceeded that of the plasma sprayed coating by more than 70%, 40%, and 30% after a period of 2 weeks, 4 weeks, and 12 weeks, respectively. In terms of biological responses, improved bond strength and the initial osseointegration rate were observed in sputtered HA coatings. The percent of bone contact length for the as-sputtered calcium phosphate implants was reported to be close to plasma sprayed implants, respectively are (70.4±1.6)% and (78.6±4.9)%, to be specific [22].
One obvious drawback of sputter coated HA layer on the metallic substrate is the poor degree of crystallinity [22, 24, 27], which would increase the dissolution rate of the coating in a human body [19]. Post-treatment of annealing with controlled temperature and processing time was used to crystallize the film. It was found that the thermal process would make a change in the surface morphology and further contribute to the changes in crystal structure [28]. Nevertheless, it should be noted that conventional thermal treatment in the electric furnace increases the potential possibility of cracks formation and may thus degrade the HA films [19]. In addition, since the process involves high energy consumption and large costs, improvement of economic efficiency must be taken into consideration for industrial applications.
Although the sputter coating technique is not currently used as a commercial deposition process by most bioimplant vendors, its capabilities of producing uniform and dense coating with better adhesion strength make it a viable alternative to plasma spraying for the application of HA coatings on bioimplants.
2.3 Ion-beam assisted deposition
The principle of ion implantation was first postulated in 1906, but it was not until the 1990s that the technique was first applied as a coating technology for biomedical implants [29]. In a typical ion implantation process, ions are accelerated through high graded potential difference and directed towards a substrate material. Due to the ion-solid interactions, the energetic ion would get incorporated into the substrate after losing all the energy [29]. Figure 1 illustrates the ion-solid interactions during an ion-beam assisted deposition process [29]. As can be told from the working principle, the penetration of the ion correlates with the level of energy. Therefore, by carefully controlling the ion beam energy to avoid deep penetration inside the substrate, modifications can be confined to the near-surface region, and hence significantly influence the surface characteristics. Except for the ion beam energy, other important parameters involved in ion implantation include ion species, fluence (or the total number of ions that bombard a surface) and beam current density or flux, which can all be adjusted to influence the ultimate effects on the substrate and achieve wide atomic intermixing zone [30].
Illustration of ion-solid interactions in an ion-beam assisted deposition process [29]
An attractive feature of ion-beam assisted deposition is that it offers independent and specific control of the deposition parameters. Such feature enables the manufacturing of gradual transition between the substrate material and the coating, thus a more durable bond can be achieved [31]. Rautray et al. [29] indicated that the adhesion properties of ion-beam implanted and plasma sprayed coatings seemed to be similar, but the atomic intermixing interfacial layer formed by ion dynamic intermixing contributed to a better bonding strength. In the case of fabricating HA coating on a titanium substrate, ion-beam assisted deposition achieved a tensile bonding strength of 70 MPa, which exceeded that of 51 MPa associated with the plasma spraying process. The existence of a transition structure at the HA/Ti interface consisting of amorphous HA, amorphous calcium phosphates and amorphous Ti phosphate compounds were thought to be responsible for such phenomenon. The formation of such a chemical bond was thought to be attributed to the energetic ion bombardment process [29]. It was also reported that the ion-beam treatment was capable of providing HA-coated titanium bioimplants with hardened surfaces, and thus improved the wear resistance [29]. An essential element for the human body, phosphorus, can be implanted on titanium-based biomaterials via ion-beam deposition. In this way, a compact TiP phase could be formed on the titanium surface. The new phase turned out to be helpful for strengthening the corrosion resistance. The satisfactory biocompatibility of phosphorus-ion implanted titanium was confirmed by Krupa et al. [32]. In addition to above, it was also reported that the utilization of ion implantation was advantageous in the aspects of avoiding stress shielding, enhancing fatigue resistance and improving fracture toughness for bioimplants. From the perspectives of biological activities, ion-beam implantation provides the benefits of induction of crystallinity and reduction in apatite dissolution rate [33]. Chen et al. [34] investigated the influence of calcium ion deposition on the apatite-inducing ability of porous titanium in a modified simulated body fluid. The results confirmed the validity of calcium-ion implantation as a pre-treatment to endow the desirable bioactivity on porous Ti for bone tissue engineering applications. Some other reports indicated that the ion implantation of Ca+, N+and F+ was helpful in promoting the anti-bacterial effect of various titanium surfaces [35].
In general, ion implantation technique is useful in improving the mechanical, chemical and biological properties of biomaterials. The process is extremely controllable and can be accurately tailored in order to implant different ions to form ultra-high purity coatings with excellent adhesion. Nevertheless, because the entire process is conducted in a high vacuum and involves costly steps such as beam extraction, beam focusing and beam scanning, the soaring cost has hindered its widespread uses. At present, ion-beam based treatment is mostly applied in high-value-added products and limited in the regular production line. Besides the cost, it is disadvantageous for being inappropriate for components with complex geometries [4].
2.4 Conversion coating
Conversion coating, also referred as in situ grown coating, is formed through specific reactions between materials and environment. This technology is typically used in reactive metallic materials, where an inorganic oxide layer is produced with the help of a chemical or electrochemical process. As the conversion is formed in situ, the adhesion of the coatings to the substrate is relatively strong. Passivation is one typical branch of conversion coating and being used as a simple approach to protect reactive biomaterials, such as magnesium and its alloys. By simply immersing Mg-based biomaterials in a solution with a stable pH of 11 or higher, a passive layer of Mg(OH)2 in nanometric thickness can be formed within a short time period [4]. By means of adding mixtures of oxides or hydroxides in the solution, a film of anti-corrosive metal phosphates could be formed as well. It is noted that although the converted layer provides protection during the initial phases of corrosion in a living body, the protective ability is reported to be inadequate [4]. As a result, researchers tended to develop innovative technologies to produce more stable and powerful conversion coatings.
The anodization process is favoured for its controllability on the coating thickness, and it is mainly used to produce or thicken native oxide layers on metal materials. The coating thickness normally increases with the increased applied voltage and the value is in a range of 5–200 µm [36]. Numerous studies have proved that the anodized layers are more stable and inhibit corrosion better than traditional chemical conversion layers [36,37,38]. When anodizing the metallic material above the breakdown voltage, porous layers can be formed with improved resistance to abrasion and corrosion [38]. Such technology is called plasma electrolytic anodization (PEO), as well as anodic spark deposition (ASD) and micro arc oxidation (MAO) [4]. Commercially, PEO has become the most applied protection method for Mg alloys [4]. Nevertheless, the coating process would result in electric isolation, which makes PEO inappropriate for further processing via electric deposition [4]. Besides the utilizations described above, conversion coating techniques are sometimes performed as a pre-treatment process to promote the expected adhesion of a deposition coating.
2.5 Other methods
In addition to the above-mentioned methods, there are several more techniques being used to create coatings for bioimplants, such as electrophoretic deposition (EPD) [39,40,41,42,54] presented a series of recipes of HA dip-solution, by using which highly porous coatings with over 30 MPa bonding strength could be deposited on the Ti-6Al-4V substrates. Dip coating also introduces its profound advantages in slowing down the corrosion rate of Mg alloys. Gu et al. [55] dipped coated chitosan on a group of Mg-Ca alloys and tested their respective corrosion resistance in a simulated body fluid. The authors implied that this technique showed great promises of future adaptation for Mg substrates in matching the implant corrosion rate with the tissue healing rate.
3 Surface topography generation
To meet the demands from an enhanced interaction between the biomaterials and living body and simultaneously reduce the risk of wearing, various methods were applied to create microstructural surface features for bioimplants. Typical technologies in engineering substrate at the micro and nanoscale will be reviewed in the following sections.
3.1 Blasting
Blasting in biomedical engineering refers to an operation which propels a stream of abrasive particles against the substrate biomaterial under a high pressure. The process is used to remove surface contaminants or roughen the surfaces in order to enhance the biomaterial’s reactivity after implantation [73, 74]. The alteration in the surface topography is attributed to the plastic deformation. Although it is difficult to precisely control the surface texture due to the numerous variables inherent in the blasting process, the size of the particles can be adjusted to meet the roughness requirement. Considering that the particles need to be chemically stable, alumina, titania and hydroxyapatite particles are most commonly employed at the stage [6].
Valverde et al. [75] showed that a wide variety of microtopography, ranging from minimally rough to excessively rough surfaces, could be prepared by regulating the variable factors during the blasting procedure. The effects of blasting parameters on the surface roughness of Ti-6Al-4V were studied by Mohammadi et al. [76]. In their study, two particle materials, i.e., Al2O3 and SiO2, were employed with different sizes using different types of blasting systems. Through optimizing the processing conditions, an equivalent surface roughness of 3.51 µm was achieved. Their follow-up coating experiments confirmed that the substrate surface topography had a significant influence on the coating properties at the interface. Obvious differences were observed between the HA coatings deposited on the substrates with and without blasting treatments in terms of the tensile bonding strength. Arifvianto et al. [77] blasting treated 316L stainless steel using steel slag balls, which were the residues from steelmaking processes and presently regarded as an industrial waste. The authors reported that both surface microhardness and irregularity of the stainless steel were increased after the treatment. It was also found that some bioactive elements such as Ca, Si and Mg were introduced by the slag balls. This study clearly indicated that the steel slag blasting was a promising method for the surface modification of the medical grade 316L stainless steel. It is not only capable of improving the mechanical properties and bioactivity of the biomaterial but also is favoured for its environmental friendliness.
Nevertheless, several studies have reported that blasting with the particle material other than the implant itself bears a potential risk of changing the surface composition [6]. Attention was typically paid to the alumina blasted implants. Some researchers insisted that the remnants of the alumina particles could release aluminium ions into the host body due to dissolution, and further cause inflammatory responses [6]. There are also some concerns that the Al ions would inhibit normal differentiation of the bone marrow stromal cell and normal bone mineralization [78]. Although no statistically significant differences were found between the implants blasted with Al2O3 and other particle materials [74, 79], the application of non-biocompatible particles for blasting remains controversial. As a result, the feasibility of using hydroxyapatite and beta-tricalcium phosphate particles in blasting was investigated. Benefited from the material features of biocompatibility, osteogenesis and resorbability, the bioceramic blasted surfaces exhibited a suitable bone-implant contact after implantation. Meanwhile, other surface properties are reported to be comparable to those treated by conventional blasting procedures [80,81,82].
3.2 Chemical etching
Etching techniques performed on untreated biomaterial surfaces have been used to form micro pits at sizes between 0.5 µm and 2 µm to enhance cell adhesion and osseointegration [6, 83]. In surface etching processes, chemical reagents are selectively applied on specific areas to remove materials and therefore form expected texturing. The etching on specific regions is generally achieved through masking, where the selected masking method determines the resolution of the texture features. Costa et al. [84] proposed the application of drop-on-demand inkjet printing for masking steel surfaces with subsequent chemical etching and ink strip**. It was proven to be a fast, versatile and highly feasible technique for texturing steel surfaces [84]. Figure 2 shows the typical steel surfaces etched via inkjet printing [85].
Inkjet printing of steel surfaces with a parallel gaps and b chevron-like gaps [85]
Strong acids such as HCl, H2SO4 and HNO3 are commonly used in most etching processes. It is believed that higher concentrated acidic solutions normally generate better surface defect distributions while less aggressive mixtures would be conducive to a finer roughening [86]. In etching of titanium-based bioimplants, fluoric acid is regarded as an alternative chemical reagent. Previous reports showed that HF can effectively dissolve the passivation TiO2 layer [6]. In addition, since titanium is very reactive to fluoride ions, the fluoride would be incorporated into the created surface structures and form soluble TiF4. Such incorporation is beneficial for the osseointegration of implants [87]. As a result, HNO3 is usually mixed with HF to produce microscale surface structures on Ti-based implants [88]. However, attention should be paid to fluoride contaminations as they may induce an ambivalent response in the host tissue [6]. The risk of weakening mechanical properties is another concern brought by the chemical etching. In the etching of titanium-based bioimplants, hydrogen embrittlement triggered by the acid environment was reported, which might be the reason for the forming of micro-cracks on the implants’ surfaces and ultimately led to a reduction in the fatigue resistance [83].
Selective infiltration etching (SIE) is a special surface topography modification method, which coats target samples with special infiltration glass [89]. By heating the coated objects above the glass transition temperature, the molten glass would diffuse between the grain boundaries and result in sliding, splitting and rearrangement of the surface grains. After cooling to room temperature, the glass can be dissolved in an acidic bath, thus exposing the newly created surfaces [90]. This technique is now being used in transforming zirconia surfaces into dense, highly retentive and smooth nanoporous surfaces. A significantly higher degree of osseointegration of the selective infiltration etched zirconia implants was claimed by Aboushelib et al. [91].
Presently, attempts are being made regarding the etching process after a blasting step. Such technology integration is designed for removing blasting induced surface damages and simultaneously improving surface roughness characteristics [6]. Many previous investigations have demonstrated that a combined blasting and etching structuring method is of great help in producing superior quality of topographies with different scales at the same surface [6, 92,93,94]. Through incorporating patterning techniques at submicron scale with subsequent sandblasting and etching processes, Zinger et al. [95] achieved desired titanium surface with a combined micrometre and nanometre structures, which showed an improved osteoblast ability. A metallurgic-mechanical analysis conducted by Pazos et al. [94] explained the advantage of blasting + etching surface treatments in improving titanium material properties. The authors suggested that the decrement of fatigue endurance induced by the acid etching could be counteracted by the foregoing blasting process. The formation of a plastically deformed layer and compressive residual stress contributed to the strain-hardening, and therefore resulted in a better fatigue behaviour.
3.3 Laser-based techniques
As reviewed in part I of this paper, the improvement of osseointegration relied on roughening the surfaces of implants. In most cases, the selected surface regions of a biomaterial are blasted to be roughened at microscale. However, one obvious drawback of this technique is that it can only produce randomized surfaces [96]. Such surface features may alter the near-surface mechanical and chemical properties, and thus cause mechanical degradation [97]. Moreover, it gives rise to the local concentrations of toxic elements such as Al after the surface modification [97, 98]. Considering the above-mentioned issues, micro-grooving is now being explored as an alternative surface treatment approach to facilitate the bone-implant integration for bioimplants [96]. Among all known micro-fabrication methods, laser-based technologies showed themselves to be the most advanced way in producing micro-grooves with optimal groove dimensions for cell adhesion.
Previous works have reported on the positive effect of laser-ablated micro-grooves on promoting the contact guidance of cell alignment [96, 98,99,100]. For instance, a comparative experiment conducted by Chen et al. [101] revealed that the laser-irradiated Ti-6Al-4V surfaces with micro-grooves provided the best cell/surface interactions over polished and blasted ones. The migration and alignment of cells would not only enhance the osseointegration but also reduce the extent of scar tissue formation during wound healing [102]. More recently, Hsiao et al. [103] developed an ultraviolet (UV) laser treatment system to texture Ti-6Al-4V biomaterials. Major micro-groove structures and minor porosities were obtained simultaneously. The following in vitro tests proved that the texture effectively offered a favourable environment for the osteogenic cells. Nevertheless, due to the factors such as high energy outputs, top-hat intensity profiles and the high photon energy associated with the deep UV wavelengths, the current UV laser based micro-grooving technologies may introduce micro-cracks and induce heat-affected zones inside the grooves [96, 104]. Such damage would definitely degrade the performances and reduce the lifespan of bioimplants. Considering this, improving laser processing techniques to produce durable laser-textured biomaterial surfaces is crucial [96]. Diode-pumped solid-state (DPSS) laser technologies were developed to address the above issues. Fasasi et al. [96] proposed a nanosecond DPSS UV laser processing technique to optimize the groove geometries. By judiciously adjusting laser processing parameters, such as wavelength, pulse repetition rate and scan speed, satisfactory groove depths and widths of around 11 µm and 14 µm were obtained. Some scanning electron microscopic (SEM) images of micro-grooved surfaces are shown in Fig. 3. In addition, achievements on decreased groove roughness and reduced heat-affected zones on Ti-6Al-4V were claimed by the authors. The absence of micro-cracks is also believed to be beneficial for cell attachment and spreading on the grooved structures.
Typical SEM images of micro-groove surfaces produced by nanosecond DPSS UV laser processing [96]
Apart from creating micro-grooved structures on biomedical materials to improve the integration with surrounding tissues, laser-based texturing technologies are also applied to improve the tribological behaviour [10, 11]. Among various biomaterials, titanium and its alloys are characterized by poor tribological properties, such as high and unstable friction coefficient [11]. The rupture of passive oxide layer would release wear debris to the host body and lead to aseptic loosening of the implant. Therefore, great efforts have been made on introducing specific surface patterns on Ti and Ti alloys to enhance the tribological performances. Laser surface texturing (LST), which utilizes high energy laser pulses to melt and vaporize specific surface regions to fabricate dent arrays, shows a great potential on this aspect. Hu et al. [11] employed this technique to create micro-dimple patterns on Ti-6Al-4V surfaces. Excellent tribological performances of the biomaterial were verified under various loads applied. The effects of texture parameters on tribological behaviours were also investigated in their researches. A higher dimple density was found to result in a lower friction coefficient. This is because the micro-dimples functioned as traps for wear debris. A higher dimple density was more likely to absorb more wear particles and therefore eliminated the potential debris ploughing effect. In addition, it was believed that the micro-dimples might serve as fluid reservoirs in the host body, which would help to retain body fluid as a lubricant and lead to less wear.
The major disadvantage of LST is that the laser ablation may alter surface integrity. Previous reports have indicated that elevated temperatures encountered during ablation may change surface microstructures and form cracks. Such damages would drastically shorten the fatigue life of the material [10, 105]. For this reason, laser shock peening (LSP) process was proposed. LSP is capable of producing micro dent arrays, and at the same time improving surface mechanical properties via inducing deep compressive residual stress in the subsurface. A typical denting schematic of LSP is shown in Fig. 4 [10]. During the process, a short, high-power laser pulse is applied (under a water blanket) to vaporize a sacrificial coating on the objective. Selected surface areas would then undergo plastic deformation by the pressure waves [106]. The improved fatigue performance of Ti alloys offered by LSP processing was claimed by Ruschau et al. [107]. Guo and Caslaru [10] demonstrated that LSP could efficiently manufacture mass microscale dent arrays on Ti-6Al-4V alloy surfaces though adjusting the laser power. Strain hardening and compressive residual stress in the centre area of the peened dents contributed to the increment in microhardness. However, it should be noted that all the laser texturing methods reviewed above have been mainly applied in aeronautical components for their increasing wear-resistance ability, but rarely reported in improving the tribological properties of bioimplants.
Schematic of dent fabrication by laser shock peening [10]
In summary, laser-based surface treatment methods are favoured for the simple processing and effortless operation. Desired surface patterns can be fabricated through varying the laser parameters. The laser micro-grooved metallic biomaterials exhibited better osseointegration and longer lifetime in comparison with blasted or etched ones. More importantly, a potential development tendency on enhancing tribological properties of bioimplant through laser treatment is noteworthy. However, the high energy or elevated temperate involved in the laser treatment process is a major concern as it may alter the surface integrity after treatment. The relatively higher cost is another issue that hinders the wide-spread of laser-based surface treatments. Furthermore, little work has been reported on the laser surface structuring of bioceramics. This is a strong indicator that laser-based texturing on biomedical engineering requires further studies [108].
3.4 Electric discharge machining
Electric discharge machining (EDM) was established to manufacture geometrically complex or hard material parts which are difficult to be machined by conventional processes [109]. More recently, it has become favourable in producing nanostructured biocompatible surfaces [110]. The exploration of the destructive properties of electrical discharges can be traced back to the 1940s, when the first attempt was made on vaporizing material from the difficult-to-machine metal surface [109]. Since then, EDM experienced a successive development in the past decades regarding working efficiency and accuracy. During a typical EDM process, the removal of material is achieved through the high thermal energy generated by a series of high-frequency electrical sparks. Detailed working principles could be found in Refs. [109, 110]. Figure 5 shows the typical EDM experimental setup [110]. In comparison to other surface treatment techniques, this technique does not require any pre-treatments on the objects’ surfaces. Since the electrode and workpiece are not directly contacted in EDM, issues triggered by mechanical stress during conventional contacting machining can be avoided. Another advantage of EDM is that such technology would introduce carbides on the workpiece surface, and hence enhance the surface properties such as hardness and wear and corrosion resistance [109]. Apart from above, what makes EDM attractive in biomaterial manufacturing is that a porous nanostructured oxide layer would be converted on the surface during the process. The layer thickness can be intentionally controlled according to the requirement, so that a suitable biocompatibility could be achieved [111].
Typical representation of the experimental setup for EDM process [110]
Several in vitro and in vivo studies have examined the improved osteoconductivity of metallic biomaterials whose surfaces were modified by EDM. Peng et al. [111] found that a nanophase transition occurred on the titanium surface during EDM, which played a critical role in forming a thick nanoporous TiO2 layer on the titanium surface. The porous structure is believed to be beneficial for enhancing the biocompatibility. In their following work, EDM was applied to produce a rough texture with pores and craters at nanoscale on the surface of Ti-6Al-4V alloys [112]. Again, the formation of nanoporous TiO2 layers was observed. The follow-up evaluations revealed that the EDM-functionalized surfaces significantly increased the activities of surrounding cells in terms of adhesion, differentiation and proliferation. It was confirmed that an improvement of multiple osteoblast functions could be achieved by increasing the pulse durations.
As the present trend of surface treatment has been switched from conventional machining to advanced micro/nano-manufacturing, EDM has become favoured in offering nanoporous surfaces with enhanced mechanical properties and biocompatibility. One major drawback of the EDM fabricated material is the low fatigue performance brought by the recast layer [110]. Post-treatment such as blasting is required to address the issue. In general, the application of EDM in biomanufacturing remains at the initial stage and the fulfilled results are confined to laboratories. More works need to be done before EDM is radically accepted by the biomedical industry.
3.5 Other methods
In the past decade, the emergence of surface texturing technologies on biomaterials goes well beyond the above-mentioned ones. For example, Roy et al. [113] employed the micro-drilling technique to manufacture micro-dimpled surface textures for ceramic-on-ceramic hip prostheses. In the simulated hip joint condition, the dimpled workpieces exhibited obviously improved tribological performances compared to non-dimpled ones. Choudhury et al. [114] revealed that plateau-honed technique was effective in reducing wear rate, friction coefficient and removing wear debris from the contact interface of metal-on-metal hip joints.
Previous studies have proved that the high-frequency tool-work interaction induced by ultrasonic vibration is of great help in the manufacturing of various micro/nanostructures [115,116,117,118]. Some successful attempts of ultrasonic-assisted machining have been made on different materials such as stainless steels [115, 119, 120], silicon carbide [121], glasses [120, 122], polymers [123], etc. Nowadays, an increasing number of studies have shown that the ultrasonic-assisted machining techniques are valuable in achieving high precision textures on biomedical materials. For instance, a novel rotary ultrasonic texturing (RUT) technique was proposed by Xu et al. [124]. In their study, the ultrasonic vibration was integrated into a rotary machining process. The combination of vibration, rotation and feed motion offers high-frequency periodic change. It was suggested that this new technique allowed manufacturers to fabricate various fine surface structures by offering an additional processing freedom.
Electrochemical machining (ECM) is a relatively mature surface modification technique which enables removing metallic materials selectively by an electrochemical reaction at the anode [125]. Its flexible machining rate is attributed to the adjustable electric current [9]. Through years of development, the processing dimension of ECM has reduced to microscale. Electrochemical micromachining (EMM) has replaced traditional ECM in many places and been widely used in producing patterns on stainless steel based hip prosthesis stems. Mask electrochemical micromachining (TMEMM) is typical branch of EMM, which involves photolithography to produce micropatterns on the photoresist-coated substrates [126]. Lu and Leng [125] developed a jet electrochemical micromachining (Jet-EMM) to form micro-holes on the titanium-based bioimplants. The technique exhibits merits of producing patterns on curved surfaces and enables features with a high aspect ratio. Comparing to TMEMM, the equipment required for Jet-EMM is less complicated. The invention of the first rapid, mask-less EMM was claimed by Sjöström and Su [127], where the surface patterns were created via a direct writing manner. During the process, a microscaled single-tip in conjunction with short voltage pulses moves across the substrate and the resolution was kept in the submicron region. This technique is demonstrated to be ideal for the fast fabrication of desired surface patterns on metallic biomaterials. Microscaled grooves and pits (around 50 µm in width and diameter) were successfully produced on titanium surfaces with high manufacturing efficiency. Note that although EMM is favoured for its ability to handle complex geometries, its application is restricted to electrically conductive materials.
4 Characterizations of bioimplant surfaces
The surface properties generally play a dominant role in determining the longevity of bioimplants, among which the major concerns include the products’ wear and corrosion resistance, mechanical properties of hardness and elastic modulus, fabrication caused residual stress and surface composition after surface treatments. Evaluation tests of these aspects are necessary for guaranteeing the final product acceptance.
4.1 Wear tests
Pin-on-disk wear test system has been widely used to measure the friction coefficient and characterize the wear response of the manufactured bioimplants, especially artificial knee and hip joints. Figure 6 shows the schematic of a typical pin-on-disk system. Briefly, the pin moves biaxially with a normal load applied on top. Either cyclical or non-cyclical translating programs can be employed. Parameters such as pin and disk materials, normal load, cycle frequency, sliding speed and lubricant can be judiciously selected to replicate the actual tribological conditions. The friction coefficient is calculated from the applied normal load and the measured friction force. According to a recent pin-on-disk test conducted by Saikko [128], in order to avoid unrealistically low wear and friction values caused by protuberance formation, the contact pressure should be kept below 2 MPa when simulating the wear of ultrahigh molecular weight polyethylene. It should be noted that this technology is a simplified model of normal walking, and simulation of the motion and loading in activity is very limited. In fact, there are three complicated articulation mechanisms that are involved in the motion of a tibiofemoral joint, namely gliding, pure rolling and rolling-slip** [129]. Investigations are required to elucidate the complex environment of the host body and increased patient activities.
Schematic of a pin-on-disk system, modified from Ref. [128]
For a more precise evaluation of the tribological properties of orthopaedic joints, friction and wear experiments are performed on a simulator. Compared to the pin-on-disk test, the simulators are capable of imitating more complex kinetics and kinematics of a human body in a physiological environment. Running in accordance with ISO 14242 and 14243, an array of hip and knee complexities can be evaluated. The simulator testing allows implementing various surface textures on the workpieces as well as a selection of lubricant, which ensures that the simulated condition is as practically similar as possible to the complexities of the human anatomy. In a typical hip simulator, a single joint force was normally applied in one axis, offering a shear stress pattern similar to that of the human body. Bowsher and Shelton [130] added a vertically mounted torque cell on the hip simulator to measure the changes in joint friction. The photographs and schematics of the simulator can be seen in Fig. 7. Such design provided a better understanding of the influence of patient activity level on the tribological performances. In the aspect of knee simulators, since the traditional uniaxial and two-axis simulators resulted in too low wear rate values, Saikko et al. [131] proposed a three-axis wear model which implemented anterior-posterior translation (APT), inward-outward rotation (IOR) and flexion-extension (FE). The principle of the simulator is shown in Fig. 8. Such ball-on-flat contact design has been successfully applied to studying the basic wear and frictions of metal-polymer and ceramic-polymer knee pairs [132, 133]. Again, attention should be paid to the fact that the enhanced walking cycle, such as ascending the stairs, is more aggressive than the standard walking cycle, which may increase approximately 25% in both anterior-posterior shear force and external-internal rotation [134]. Therefore, an enhanced walking cycle program should be applied during the test to improve the wear prediction accuracy.
Photographs and schematics showing a IRC MTS 8-station hip joint simulator, b physiological test setup, c fully constraining socket fixture, d partially constraining socket fixture, e location of horizontal torque cells, and f direction of torque measured [130]
Principle of the ball-on-flat contact knee simulator, proposed by Saikko et al. [131]
Scratch test has long been recognized as a useful tool in emulating an individual deformation or removal event at micro/nanoscale [135]. The feasibility of using this method to estimate the wear debris-induced surface damage was verified by Dearnley [136]. Through adjusting scratching parameters, scratched marks with similar dimensions compared to the abrasion damages produced in vivo were achieved. In their later stage study, scratches were performed on coated/uncoated metallic biomaterials of stainless steel and Co-Cr-Mo alloy. The results proved that the samples with TiN coating contributed to a greater tolerance to the scratch because the hard film hindered the deepening of the scratch.
4.2 Corrosion tests
The most common method to examine the corrosion behaviour of bioimplants is via electrochemical techniques, where the manufactured bioimplants are soaked into a simulated body fluid (SBF). During corrosion testing, the electrochemical corrosion potentials and currents are continuously recorded so that the electrochemical activity of the bioimplants can be obtained. It was suggested that a 0.89% NaCl aqueous solution with a constant temperature of 37 °C could create an environment similar to human body [136]. The SBF is buffered to maintain a physiological pH value slightly above 7 [137, 138]. Hank’spotentiodynamic polarization (Tafel solution is also extensively employed. The detailed information of its composition can be found in Refs. [138, 139]. A three-electrode cell is usually used to carry out the electrochemical studies. Quantitative assessments of corrosion include electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (Tafel analysis), open circuit voltage (OCV) and electrochemical noise (ECN) [140]. Among them, EIS is recognized as one of the most accurate electrochemical methods [140]. This is due to the fact that EIS requires minimum AC signals, hence avoiding the perturbation on the electrochemical system and meanwhile reducing the errors. Additionally, valuable mechanistic information can be offered by this technique as the data are obtained from both electrode capacitance and charge-transfer kinetics.
4.3 Assessment of other properties
In terms of assessing other surface properties, X-ray diffraction (XRD) has been proved as a feasible technique to measure the residual stress by many studies [141, 142]. A recent study conducted by Roy et al. [113] confirmed that XRD was practical on measuring the residual stress on bioceramics after surface treatment process. Due to a potential possibility of introducing foreign materials to the bioimplants during the surface treatment, XRD testing and energy dispersive spectroscopic (EDS) analyses are usually undertaken to detect the elemental composition of the modified surfaces [143]. With respect to measuring the microhardness and elastic modulus of manufactured bioimplants, indentation test has been proved to be a robust technique [113, 144]. To be specific, the indentation process involves penetrating a sharp diamond tip into the surface of workpiece, meanwhile continuously recording the imposed force and corresponding indentation depth. The recorded load-displacement curve is useful in providing insights into the mechanical behaviour of the deformed material. Meanwhile, both elastic modulus and hardness of the workpiece can be extracted. Presently, nanoindentation is recognized as a non-destructive property-investigating method [145], which is well suited to assessing the mechanical properties of bioimplants. As for evaluating the adhesion strength of the coating on biomaterials, both nanoscratching and nanoindentation methods can be applied [66, 146,147,148,149].
5 Conclusions and perspective
Numerous industrial and academic surface treatment technologies have been applied to enrich the functionalities of bioimplants. Previous studies have confirmed that the enhancement of wear and corrosion resistance, improved osseointegration and controllable degradability are achieved after treatment. This paper reviewed both coating and morphology processing techniques, whose advantages and disadvantages were described in comparison with each other. Among various coating methods, plasma spraying is currently the most commonly implemented technique, while the relatively high cost and complexity of process involved simulated researchers to look for alternatives. Laser-based technologies present themselves to be the most advanced way in producing micro-groove structures on bioimplant surfaces. However, few studies have reported the laser-based texturing of bioceramic surfaces, indicating that the development of this technology on biomedical engineering is in its infancy and requires further studies.
In terms of evaluating the manufactured surfaces, although standards are available to assess the wear and corrosion performances of orthopaedic devices, variation always exists in the methodology adopted by different research groups. Thus, it is necessary to develop a unanimous evaluation system. Note that the existing simulators are limited in providing in vitro approximations. Design optimizations are required to guarantee that the complexities of human anatomy are as practically similar as possible.
References
Kurtz S, Ong K, Lau E et al (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. JBJS 89(4):780–785
Prakasam M, Locs J, Salma-Ancane K et al (2017) Biodegradable materials and metallic implants—a review. J Funct Biomater 8(4):44
Roach P, Eglin D, Rohde K et al (2007) Modern biomaterials: a review—bulk properties and implications of surface modifications. J Mater Sci Mater Med 18(7):1263–1277
Hornberger H, Virtanen S, Boccaccini A (2012) Biomedical coatings on magnesium alloys—a review. Acta Biomater 8(7):2442–2455
Curtis A, Wilkinson C (1997) Topographical control of cells. Biomaterials 18(24):1573–1583
Bauer S, Schmuki P, von der Mark K et al (2013) Engineering biocompatible implant surfaces: part I: materials and surfaces. Prog Mater Sci 58(3):261–326
Wennerberg A, Albrektsson T, Andersson B et al (1995) A histomorghometric study of screw-shaped and removal torque titanium implants with three different surface topographies. Clin Oral Implant Res 6(1):24–30
Wennerberg A, Hallgren C, Johansson C et al (1998) A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin Oral Implant Res 9(1):11–19
Ramsden JJ, Allen DM, Stephenson DJ et al (2007) The design and manufacture of biomedical surfaces. CIRP Ann Manuf Technol 56(2):687–711
Guo Y, Caslaru R (2011) Fabrication and characterization of micro dent arrays produced by laser shock peening on titanium Ti-6Al-4V surfaces. J Mater Process Technol 211(4):729–736
Hu T, Hu L, Ding Q (2012) Effective solution for the tribological problems of Ti-6Al-4V: combination of laser surface texturing and solid lubricant film. Surf Coat Technol 206(24):5060–5066
Heimann RB (2008) Plasma-spray coating: principles and applications. Wiley, Weinheim
Mittal M, Nath S, Prakash S (2013) Improvement in mechanical properties of plasma sprayed hydroxyapatite coatings by Al2O3 reinforcement. Mater Sci Eng C 33(5):2838–2845
Mohseni E, Zalnezhad E, Bushroa AR (2014) Comparative investigation on the adhesion of hydroxyapatite coating on Ti-6Al-4V implant: a review paper. Int J Adhes Adhes 48:238–257
Cook SD, Thomas KA, Kay JF et al (1988) Hydroxyapatite-coated porous titanium for use as an orthopedic biologic attachment system. Clin Orthop Relat Res 230:303
Søballe K, Hansen ES, Brockstedt-Rasmussen H et al (1990) Hydroxyapatite coating enhances fixation of porous coated implants: a comparison in dogs between press fit and noninterference fit. Acta Orthop Scand 61(4):299–306
Jansen J, van de Waerden J, Wolke J et al (1991) Histologic evaluation of the osseous adaptation to titanium and hydroxyapatite-coated titanium implants. J Biomed Mater Res Part A 25(8):973–989
Moroni A, Caja V, Sabato C et al (1994) Bone ingrowth analysis and interface evaluation of hydroxyapatite coated versus uncoated titanium porous bone implants. J Mater Sci Mater Med 5(6):411–416
Mohseni E, Zalnezhad E, Bushroa AR (2014) Comparative investigation on the adhesion of hydroxyapatite coating on Ti-6Al-4V implant: a review paper. Int J Adhes Adhes 48:238–257
Yang YC, Chang E (2001) Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V substrate. Biomaterials 22(13):1827–1836
Nimb L, Gotfredsen K, Steen JJ (1993) Mechanical failure of hydroxyapatite-coated titanium and cobalt-chromium-molybdenum alloy implants. An animal study. Acta Orthop Belg 59:333
Yang Y, Kim KH, Ong JL (2005) A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 26(3):327–337
Ong JL, Harris LA, Lucas LC et al (1991) X-ray photoelectron spectroscopy characterization of ion-beam sputter-deposited calcium phosphate coatings. J Am Ceram Soc 74(9):2301–2304
Ozeki K, Yuhta T, Aoki H et al (2000) Crystal chemistry of hydroxyapatite deposited on titanium by sputtering technique. Bio-Med Mater Eng 10(3–4):221–227
Toque JA, Herliansyah M, Hamdi M et al (2010) Adhesion failure behavior of sputtered calcium phosphate thin film coatings evaluated using microscratch testing. J Mech Behav Biomed Mater 3(4):324–330
Ozeki K, Fukui Y, Aoki H (2006) Hydroxyapatite coated dental implants by sputtering technique. Biocybern Biomed Eng 26(1):95–101
Ozeki K, Yuhta T, Fukui Y et al (2002) Phase composition of sputtered films from a hydroxyapatite target. Surf Coat Technol 160(1):54–61
Van Dijk K, Schaeken H, Wolke J et al (1996) Influence of annealing temperature on RF magnetron sputtered calcium phosphate coatings. Biomaterials 17(4):405–410
Rautray TR, Narayanan R, Kim KH (2011) Ion implantation of titanium based biomaterials. Prog Mater Sci 56(8):1137–1177
Sioshansi P, Tobin EJ (1996) Surface treatment of biomaterials by ion beam processes. Surf Coat Technol 83(1–3):175–182
Serekian P (2004) Hydroxyapatite: from plasma spray to electrochemical deposition. In: The fifteen years of clinical experience with hydroxyapatite coatings in joint arthroplasty. Springer, pp 29–33
Krupa D, Baszkiewicz J, Kozubowski J et al (2002) Effect of phosphorus-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 23(16):3329–3340
Choi JM, Kim HE, Lee IS (2000) Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 21(5):469–473
Chen XB, Li YC, Du PJ et al (2009) Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta Biomater 5(5):1808–1820
Yoshinari M, Oda Y, Kato T et al (2001) Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 22(14):2043–2048
Blawert C, Dietzel W, Ghali E et al (2006) Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv Eng Mater 8(6):511–533
Zhang X, Zhao Z, Wu F et al (2007) Corrosion and wear resistance of AZ91D magnesium alloy with and without microarc oxidation coating in Hank’s solution. J Mater Sci 42(20):8523–8528
Jo JH, Hong JY, Shin KS et al (2012) Enhancing biocompatibility and corrosion resistance of Mg implants via surface treatments. J Biomater Appl 27(4):469–476
Sarkar P, Nicholson PS (1996) Electrophoretic deposition (EPD): mechanisms, kinetics, and application to ceramics. J Am Ceram Soc 79(8):1987–2002
Wei M, Ruys A, Milthorpe B et al (2001) Electrophoretic deposition of hydroxyapatite coatings on metal substrates: a nanoparticulate dual-coating approach. J Sol Gel Sci Technol 21(1):39–48
Soares GA, de Sena LÁ, Rossi AM et al (2003) Effect of electrophoretic apatite coating on osseointegration of titanium dental implants. Key Eng Mater 254–256:729–732
Nie X, Leyland A, Matthews A (2000) Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf Coat Technol 125(1):407–414
Zhang Z, Dunn MF, **ao T et al (2002) Nanostructured hydroxyapatite coatings for improved adhesion and corrosion resistance for medical implants. Mater Res Soc Symp Proc 291–296
Larker HT, Larker R (1991) Hot isostatic pressing. In: Cahn RW, Haasen P, Kramer EJ (eds) Materials science and technology. VCH, Weinheim, pp 146–174
Khor K, Yip C, Cheang P (1997) Post-spray hot isostatic pressing of plasma sprayed Ti-6Al-4V/hydroxyapatite composite coatings. J Mater Process Technol 71(2):280–287
Bao Q, Chen C, Wang D et al (2005) Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl Surf Sci 252(5):1538–1544
Cotell CM, Chrisey DB, Grabowski KS et al (1992) Pulsed laser deposition of hydroxylapatite thin films on Ti-6Al-4V. J Appl Biomater 3(2):87–93
Fernández-Pradas J, García-Cuenca M, Clèries L et al (2002) Influence of the interface layer on the adhesion of pulsed laser deposited hydroxyapatite coatings on titanium alloy. Appl Surf Sci 195(1):31–37
Cotell C (1993) Pulsed laser deposition and processing of biocompatible hydroxylapatite thin films. Appl Surf Sci 69(1–4):140–148
Klein LC (2013) Sol-gel optics: processing and applications, vol 259. Springer, New York
Uhlmann D, Suratwala T, Davidson K et al (1997) Sol-gel derived coatings on glass. J Non-Cryst Solids 218:113–122
Wen C, Xu W, Hu W et al (2007) Hydroxyapatite/titania sol-gel coatings on titanium-zirconium alloy for biomedical applications. Acta Biomater 3(3):403–410
Phani A, Gammel F, Hack T et al (2005) Enhanced corrosioon resistance by sol-gel-based ZrO2-CeO2 coatings on magnesium alloys. Mater Corros 56(2):77–82
Mavis B, Taş AC (2000) Dip coating of calcium hydroxyapatite on Ti-6Al-4V substrates. J Am Ceram Soc 83(4):989–991
Gu X, Zheng Y, Lan Q, Cheng Y et al (2009) Surface modification of an Mg-1Ca alloy to slow down its biocorrosion by chitosan. Biomed Mater 4(4):044109
Shadanbaz S, Dias GJ (2012) Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater 8(1):20–30
Wang H, Guan S, Wang X et al (2010) In vitro degradation and mechanical integrity of Mg-Zn-Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater 6(5):1743–1748
Kumar RR, Wang M (2002) Functionally graded bioactive coatings of hydroxyapatite/titanium oxide composite system. Mater Lett 55(3):133–137
Loh N, Sia K (1992) An overview of hot isostatic pressing. J Mater Process Technol 30(1):45–65
Fu Y, Batchelor A (1998) Hot isostatic pressing of hydroxyapatite coating for improved fretting wear resistance. J Mater Sci Lett 17(20):1695–1696
Kameyama T (1999) Hybrid bioceramics with metals and polymers for better biomaterials. Bull Mater Sci 22(3):641–646
Narayanan R, Seshadri S, Kwon T et al (2008) Calcium phosphate-based coatings on titanium and its alloys. J Biomed Mater Res B Appl Biomater 85(1):279–299
Boyd IW (1994) Thin film growth by pulsed laser deposition. In: Laser in der Technik/Laser in Engineering. Springer, pp 349–359
Eason R (2007) Pulsed laser deposition of thin films: applications-led growth of functional materials. Wiley, Southampton
Jelinek M, Olsan V, Jastrabik L et al (1995) Effect of processing parameters on the properties of hydroxylapatite films grown by pulsed laser deposition. Thin Solid Films 257(1):125–129
Arias JL, Mayor MB, Pou J et al (2003) Micro- and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 24(20):3403–3408
Blind O, Klein LH, Dailey B et al (2005) Characterization of hydroxyapatite films obtained by pulsed-laser deposition on Ti and Ti-6AL-4V substrates. Dent Mater 21(11):1017–1024
Mehrotra RC (1990) Chemistry of alkoxide precursors. J Non-Cryst Solids 121(1–3):1–6
Olding T, Sayer M, Barrow D (2001) Ceramic sol-gel composite coatings for electrical insulation. Thin Solid Films 398:581–586
Zhang S, Li Q, Fan J et al (2009) Novel composite films prepared by sol-gel technology for the corrosion protection of AZ91D magnesium alloy. Prog Org Coat 66(3):328–335
Kim HW, Kim HE, Knowles JC (2004) Fluor-hydroxyapatite sol-gel coating on titanium substrate for hard tissue implants. Biomaterials 25(17):3351–3358
Aegerter MA, Mennig M (2013) Sol-gel technologies for glass producers and users. Springer, New York
Kern M, Thompson V (1994) Effects of sandblasting and silica-coating procedures on pure titanium. J Dent 22(5):300–306
Wennerberg A (1998) The importance of surface roughness for implant incorporation. Int J Mach Tools Manuf 38(5–6):657–662
Valverde GB, Jimbo R, Teixeira HS et al (2013) Evaluation of surface roughness as a function of multiple blasting processing variables. Clin Oral Implants Res 24(2):238–242
Mohammadi Z, Ziaei-Moayyed A, Mesgar ASM (2007) Grit blasting of Ti-6Al-4V alloy: optimization and its effect on adhesion strength of plasma-sprayed hydroxyapatite coatings. J Mater Process Technol 194(1):15–23
Arifvianto B, Suyitno K, Mahardika M (2012) Influence of grit blasting treatment using steel slag balls on the subsurface microhardness, surface characteristics and chemical composition of medical grade 316L stainless steel. Surf Coat Technol 210:176–182
Thompson G, Puleo D (1996) Ti-6Al-4V ion solution inhibition of osteogenic cell phenotype as a function of differentiation timecourse in vitro. Biomaterials 17(20):1949–1954
Piattelli A, Degidi M, Paolantonio M et al (2003) Residual aluminum oxide on the surface of titanium implants has no effect on osseointegration. Biomaterials 24(22):4081–4089
Müeller WD, Gross U, Fritz T et al (2003) Evaluation of the interface between bone and titanium surfaces being blasted by aluminium oxide or bioceramic particles. Clin Oral Implants Res 14(3):349–356
Novaes Jr AB, Souza SL, de Oliveira PT et al (2002) Histomorphometric analysis of the bone-implant contact obtained with 4 different implant surface treatments placed side by side in the dog mandible. Int J Oral Maxillofac Implants 17(3):377–383
Piattelli M, Scarano A, Paolantonio M et al (2002) Bone response to machined and resorbable blast material titanium implants: an experimental study in rabbits. J Oral Implantol 28(1):2–8
Le Guéhennec L, Soueidan A, Layrolle P et al (2007) Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater 23(7):844–854
Costa HL, Hutchings IM (2008) Ink-jet printing for patterning engineering surfaces. In: NIP & digital fabrication conference, 2008. vol 1. Society for Imaging Science and Technology, pp 256–259
Bruzzone A, Costa H, Lonardo P et al (2008) Advances in engineered surfaces for functional performance. CIRP Ann Manuf Technol 57(2):750–769
Buser D, Nydegger T, Oxland T et al (1999) Interface shear strength of titanium implants with a sandblasted and acid-etched surface: a biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res Part A 45(2):75–83
Cooper LF, Zhou Y, Takebe J et al (2006) Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 27(6):926–936
Ellingsen JE, Johansson CB, Wennerberg A et al (2004) Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int J Oral Maxillofac Implants 19(5):659–666
Aboushelib M, Feilzer A (2006) New surface treatment for zirconia based materials. European patent application (050773969)
Aboushelib MN, Feilzer AJ, Kleverlaan CJ (2010) Bonding to zirconia using a new surface treatment. J Prosthodont 19(5):340–346
Aboushelib MN, Salem NA, Taleb ALA et al (2013) Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J Oral Implantol 39(5):583–590
Perrin D, Szmukler-Moncler S, Echikou C et al (2002) Bone response to alteration of surface topography and surface composition of sandblasted and acid etched (SLA) implants. Clin Oral Implants Res 13(5):465–469
Zinger O, Zhao G, Schwartz Z et al (2005) Differential regulation of osteoblasts by substrate microstructural features. Biomaterials 26(14):1837–1847
Pazos L, Corengia P, Svoboda H (2010) Effect of surface treatments on the fatigue life of titanium for biomedical applications. J Mech Behav Biomed Mater 3(6):416–424
Zinger O, Anselme K, Denzer A et al (2004) Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 25(14):2695–2711
Fasasi A, Mwenifumbo S, Rahbar N et al (2009) Nano-second UV laser processed micro-grooves on Ti6Al4V for biomedical applications. Mater Sci Eng C 29(1):5–13
Anselme K, Linez P, Bigerelle M et al (2000) The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 21(15):1567–1577
Soboyejo W, Nemetski B, Allameh S et al (2002) Interactions between MC3T3-E1 cells and textured Ti6Al4V surfaces. J Biomed Mater Res Part A 62(1):56–72
Chen J, Ulerich J, Abelev E et al (2009) An investigation of the initial attachment and orientation of osteoblast-like cells on laser grooved Ti-6Al-4V surfaces. Mater Sci Eng C 29(4):1442–1452
Chen J, Bly R, Saad M et al (2011) In-vivo study of adhesion and bone growth around implanted laser groove/RGD-functionalized Ti-6Al-4V pins in rabbit femurs. Mater Sci Eng C 31(5):826–832
Chen J, Mwenifumbo S, Langhammer C et al (2007) Cell/surface interactions and adhesion on Ti-6Al-4V: effects of surface texture. J Biomed Mater Res B Appl Biomater 82(2):360–373
Ricci JL, Alexander H (2001) Laser microtexturing of implant surfaces for enhanced tissue integration. In: Key engineering materials 2001 (pp 179–202). Trans Tech Publ
Hsiao WT, Chang HC, Nanci A et al (2016) Surface microtexturing of Ti-6Al-4V using an ultraviolet laser system. Mater Des 90:891–895
Soboyejo WO, Mercer C, Allameh S (2001) Multi-scale microstructural characterization of micro-textured Ti-6Al-4V surfaces. In: Key engineering materials 2001 (pp 203–230). Trans Tech Publ
Iordanova I, Antonov V, Gurkovsky S (2002) Changes of microstructure and mechanical properties of cold-rolled low carbon steel due to its surface treatment by Nd:glass pulsed laser. Surf Coat Technol 153(2):267–275
Montross CS, Wei T, Ye L et al (2002) Laser shock processing and its effects on microstructure and properties of metal alloys: a review. Int J Fatigue 24(10):1021–1036
Ruschau JJ, John R, Thompson SR et al (1999) Fatigue crack nucleation and growth rate behavior of laser shock peened titanium. Int J Fatigue 21:S199–S209
Vilar R (2016) Laser surface modification of biomaterials: techniques and applications. Woodhead Publishing, Cambridge
Ho K, Newman S (2003) State of the art electrical discharge machining (EDM). Int J Mach Tools Manuf 43(13):1287–1300
Prakash C, Kansal HK, Pabla B et al (2016) Electric discharge machining—a potential choice for surface modification of metallic implants for orthopedic applications: a review. Proc Inst Mech Eng B J Eng Manuf 230(2):331–353
Peng PW, Ou KL, Lin HC et al (2010) Effect of electrical-discharging on formation of nanoporous biocompatible layer on titanium. J Alloy Compd 492(1):625–630
Lee WF, Yang TS, Wu YC et al (2013) Nanoporous biocompatible layer on Ti-6Al-4V alloys enhanced osteoblast-like cell response. J Exp Clin Med 5(3):92–96
Roy T, Choudhury D, Ghosh S et al (2015) Improved friction and wear performance of micro dimpled ceramic-on-ceramic interface for hip joint arthroplasty. Ceram Int 41(1):681–690
Choudhury D, Walker R, Roy T et al (2013) Performance of honed surface profiles to artificial hip joints: an experimental investigation. Int J Precis Eng Manuf 14(10):1847–1853
Brehl D, Dow T (2008) Review of vibration-assisted machining. Precis Eng 32(3):153–172
Thoe T, Aspinwall D, Wise M (1998) Review on ultrasonic machining. Int J Mach Tools Manuf 38(4):239–255
Spur G, Holl SE (1996) Ultrasonic assisted grinding of ceramics. J Mater Process Technol 62(4):287–293
Dambatta YS, Sarhan AA, Sayuti M et al (2017) Ultrasonic assisted grinding of advanced materials for biomedical and aerospace applications—a review. Int J Adv Manuf Technol 92(9–12):3825–3858
Moriwaki T, Shamoto E (1991) Ultraprecision diamond turning of stainless steel by applying ultrasonic vibration. CIRP Ann Manuf Technol 40(1):559–562
Klocke F (2000) Ultrasonic-assisted diamond turning of glass and steel. Ind Diamond Rev 229–239
Negishi N (2003) Elliptical vibration assisted machining with single crystal diamond tools. Dissertation, North Carolina State University
Gan J, Wang X, Zhou M et al (2003) Ultraprecision diamond turning of glass with ultrasonic vibration. Int J Adv Manuf Technol 21(12):952–955
Kim JD, Choi IH (1997) Micro surface phenomenon of ductile cutting in the ultrasonic vibration cutting of optical plastics. J Mater Process Technol 68(1):89–98
Xu S, Kuriyagawa T, Shimada K et al (2017) Recent advances in ultrasonic-assisted machining for the fabrication of micro/nano-textured surfaces. Front Mech Eng 12(1):33–45
Lu X, Leng Y (2005) Electrochemical micromachining of titanium surfaces for biomedical applications. J Mater Process Technol 169(2):173–178
Madore C, Piotrowski O, Landolt D (1999) Through-mask electrochemical micromachining of titanium. J Electrochem Soc 146(7):2526–2532
Sjöström T, Su B (2011) Micropatterning of titanium surfaces using electrochemical micromachining with an ethylene glycol electrolyte. Mater Lett 65(23):3489–3492
Saikko V (2017) Effect of contact area on the wear and friction of UHMWPE in circular translation pin-on-disk tests. J Tribol 139(6):061606
Turger A, Köhler J, Denkena B et al (2013) Manufacturing conditioned roughness and wear of biomedical oxide ceramics for all-ceramic knee implants. Biomed Eng Online 12(1):84
Bowsher J, Shelton J (2001) A hip simulator study of the influence of patient activity level on the wear of crosslinked polyethylene under smooth and roughened femoral conditions. Wear 250(1):167–179
Saikko V, Ahlroos T, Calonius O (2001) A three-axis knee wear simulator with ball-on-flat contact. Wear 249(3):310–315
Wilches L, Uribe J, Toro A (2008) Wear of materials used for artificial joints in total hip replacements. Wear 265(1):143–149
Lee JK, Maruthainar K, Wardle N et al (2009) Increased force simulator wear testing of a zirconium oxide total knee arthroplasty. Knee 16(4):269–274
Walker PS (1987) Biomechanics of total knee replacement. In: Bergmann G, Kölbel R, Rohlmann A (eds) Biomechanics: basic and applied research. Springer, Berlin, pp 19–31
Kang C, Huang H (2016) Mechanical load-induced interfacial failure of a thin film multilayer in nanoscratching and diamond lap**. J Mater Process Technol 229:528–540
Dearnley P (2005) A brief review of test methodologies for surface-engineered biomedical implant alloys. Surf Coat Technol 198(1):483–490
Kannan MB (2012) Enhancing the performance of calcium phosphate coating on a magnesium alloy for bioimplant applications. Mater Lett 76:109–112
Mohan L, Anandan C, Grips VW (2012) Corrosion behavior of titanium alloy Beta-21S coated with diamond like carbon in Hank’s solution. Appl Surf Sci 258(17):6331–6340
Karpagavalli R, Zhou A, Chellamuthu P et al (2007) Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V. J Biomed Mater Res Part A 83(4):1087–1095
Zaveri N, McEwen GD, Karpagavalli R et al (2010) Biocorrosion studies of TiO2 nanoparticle-coated Ti-6Al-4V implant in simulated biofluids. J Nanopart Res 12(5):1609–1623
Khan ZA, Hadfield M (2007) Manufacturing induced residual stress influence on the rolling contact fatigue life performance of lubricated silicon nitride bearing materials. Mater Des 28(10):2688–2693
Arafat M, Haseeb A, Dinan B et al (2013) Stress enhanced TiO2 nanowire growth on Ti-6Al-4V particles by thermal oxidation. Ceram Int 39(6):6517–6526
Roy T, Choudhury D, Mamat AB et al (2014) Fabrication and characterization of micro-dimple array on Al2O3 surfaces by using a micro-tooling. Ceram Int 40(1):2381–2388
Choudhury D, Ay Ching H, Mamat AB et al (2015) Fabrication and characterization of DLC coated microdimples on hip prosthesis heads. J Biomed Mater Res B Appl Biomater 103(5):1002–1012
Taylor CA, Wayne MF, Chiu WK (2003) Residual stress measurement in thin carbon films by Raman spectroscopy and nanoindentation. Thin Solid Films 429(1):190–200
Jun C, Zeng RC, Huang WJ et al (2008) Characterization and wear resistance of macro-arc oxidation coating on magnesium alloy AZ91 in simulated body fluids. Trans Nonferr Met Soc China 18:s361–s364
Kane SR, Ashby PD, Pruitt LA (2010) Characterization and tribology of PEG-like coatings on UHMWPE for total hip replacements. J Biomed Mater Res Part A 92(4):1500–1509
Dey A, Mukhopadhyay AK, Gangadharan S et al (2009) Nanoindentation study of microplasma sprayed hydroxyapatite coating. Ceram Int 35(6):2295–2304
Kang CW, Huang H (2017) Deformation, failure and removal mechanisms of thin film structures in abrasive machining. Adv Manuf 5(1):1–19
Acknowledgements
The authors would like to thank Dr J. Zhang, Dr N. Yu and Dr X. Li at University College Dublin for their valuable discussions. Acknowledgments are also extended to the support of the Science Foundation Ireland (SFI) (Grant No. 15/RP/B3208) and the National Science Foundation of China (Grant Nos. 51320105009 & 61635008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kang, CW., Fang, FZ. State of the art of bioimplants manufacturing: part II. Adv. Manuf. 6, 137–154 (2018). https://doi.org/10.1007/s40436-018-0218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40436-018-0218-9