Abstract
Objectives
Antimicrobial resistance (AMR) is a public health threat associated with antibiotic consumption. Community-acquired acute respiratory tract infections (CA-ARTIs) are a major driver of antibiotic consumption in primary care. We aimed to quantify the investments required for a large-scale rollout of point-of care (POC) diagnostic testing in Dutch primary care, and the impact on AMR due to reduced use of antibiotics.
Methods
We developed an individual-based model that simulates consultations for CA-ARTI at GP practices in the Netherlands and compared a scenario where GPs test all CA-ARTI patients with a hypothetical diagnostic strategy to continuing the current standard-of-care for the years 2020–2030. We estimated differences in costs and future AMR rates caused by testing all patients consulting for CA-ARTI with a hypothetical diagnostic strategy, compared to the current standard-of-care in GP practices.
Results
Compared to the current standard-of-care, the diagnostic algorithm increases the total costs of GP consultations for CA-ARTI by 9% and 19%, when priced at €5 and €10, respectively. The forecast increase in Streptococcus pneumoniae resistance against penicillins can be partly restrained by the hypothetical diagnostic strategy from 3.8 to 3.5% in 2030, albeit with considerable uncertainty.
Conclusions
Our results show that implementing a hypothetical diagnostic strategy for all CA-ARTI patients in primary care raises the costs of consultations, while lowering antibiotic consumption and AMR. Novel health-economic methods to assess and communicate the potential benefits related to AMR may be required for interventions with limited gains for individual patients, but considerable potential related to antibiotic consumption and AMR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antimicrobial resistance (AMR) is a major public health treat, mainly caused by unnecessary use of antibiotics. A major driver of antibiotic use is patients consulting for respiratory infections in primary care. Rapid diagnostics at the point of care (POC) have been shown to reduce antibiotic prescribing without negatively impacting patient outcomes. |
This study presents a novel health-economic model, which calculates potential reductions in AMR by implementing POC diagnostics in Dutch primary care. The results show that improved diagnostics may reduce AMR in the next decade, but that there also are major costs associated. |
Novel health-economic methods to assess and communicate the potential benefits of AMR reductions may be required for interventions with limited gains in terms of QALYs, but with a lot of potential related to antibiotic consumption and AMR. The potential to contain, or even reduce, AMR is relevant when deciding to reimburse interventions focussing on reducing antibiotic use. |
1 Introduction
Antimicrobial resistance (AMR) is a major threat to public health; resistant organisms are estimated to account for over 650.000 infections and over 30.000 attributable deaths in Europe each year [1] or 1.27 million deaths globally [2]. The economic case for fighting AMR is increasingly being made [3,4,5]. In light of the evidence that AMR results in considerable societal costs, it has been argued that costs associated with AMR need to be included in health-economic assessments [6,7,8]. This is not straightforward as mechanisms for the development of resistance and the spread of resistant bacteria are not clear [9].
Economic analyses of innovations in healthcare serve as important tools for policy makers in many health systems to inform reimbursement decisions. In these analyses, the incremental cost-effectiveness ratio (ICER) is an often-used outcome and is generally based on estimates of the costs per quality-adjusted life-year (QALY) gained for individuals who benefit from a novel health-related technology. However, as resistant pathogens can spread through the general population and over the longer term, more individuals may benefit from reducing AMR than only those who directly benefit from stewardship interventions. Additionally, the harm caused by AMR is difficult to capture in terms of an ICER: studies assessing the burden of resistant versus susceptible infections rarely report short- and long-term illness duration, AMR effects on hospital length-of-stay (LOS) or productivity losses [10]. Moreover, if AMR levels reach uncontrollable levels and few new effective antibiotics are discovered, we could enter a post-antibiotic era, where simple surgical procedures, such as total hip replacements or caesarean sections, can no longer be safely performed, due to the risk of infections [5]. Despite that this worst-case scenario is highly uncertain, and its costs are difficult to predict, working towards preventing this scenario should be a priority for clinicians and policy makers alike.
AMR has been associated with antibiotic consumption at an individual, regional and country level [11, 12], and as such, appropriate prescribing of antibiotics is key in combating AMR [3]. Even though bacteria are estimated to cause a minority of community-acquired acute respiratory tract infection (CA-ARTI) cases in Europe [13], CA-ARTIs account for around 40% of antibiotic prescriptions by general practitioners (GPs) in the Netherlands [14]. The most commonly prescribed class of antibiotics are broad-spectrum penicillins (BSPs) [15,16,17,18] and the most common bacterial cause of CA-ARTI is Streptococcus pneumoniae [13], with resistance of this bug-drug combination varying between 4% and 33% in Europe, depending on the country [19]. Point-of-care (POC) diagnostics could ensure more people who are likely to benefit from antibiotic treatment are prescribed antibiotics, while those unlikely to benefit are not, and thus enhance the appropriateness of antibiotic prescribing [20, 21]. A commonly used POC test is the C-reactive protein (CRP) test, which can help identify patients presenting with a lower respiratory tract infection who are more likely to benefit from antibiotic treatment [22]. A recent meta-analysis concluded that CRP testing significantly reduced antibiotic use with a risk ratio of 0.79 (95% confidence interval (CI): 0.70–0.90), without negatively impacting on recovery, hospital admissions and mortality [22]. Rapid Streptococcal A antigen detection testing may also help better target antibiotic treatment for another CA-ARTI, namely streptococcal A pharyngitis [23]. In a recent meta-analysis, rapid testing to guide antibiotic treatment for patients consulting with sore throat was estimated to reduce antibiotic prescribing by 25% (95% CI: 18–31) [23]. POC tests for viral infections, such as influenza, for patients consulting for CA-ARTIs can possibly contribute to decreasing antibiotic prescribing [24] and targeting antiviral medication [25].
We aimed to quantify the investments required for a large-scale rollout of POC diagnostic testing in primary care and the effects on AMR, using resistance of S. pneumoniae to BSPs in the Netherlands as an example. We developed a model that simulates consultations for CA-ARTIs at GP practices, and compared a scenario in which GPs test all CA-ARTI patients to a hypothetical diagnostic strategy in which they continue to deliver current standard-of-care unchanged for the years 2020–2030.
2 Methods
2.1 Population and Comparators
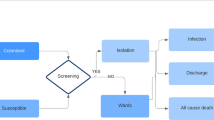
We estimated costs of testing all patients consulting for CA-ARTIs with a hypothetical diagnostic strategy that is effective at reducing antibiotic prescribing, compared to the current standard-of-care in GP practices. To simulate the current standard-of-care in the Netherlands, we used data from a point-prevalence audit survey (PPAS) in primary care for patients of all ages consulting for CA-ARTIs [18], including data on tests performed and antibiotics prescribed. The CRP POC test is currently used in about a third of all patients in the Netherlands. A BSP is prescribed in two-thirds of the 35% of patients who are prescribed an antibiotic for this condition [18]. More information is available in the Online Supplementary Material (OSM).
The efficacy of reducing antibiotic prescriptions of the hypothetical diagnostic strategy is assumed to be as effective as CRP testing, resulting in a 21% decrease in prescriptions (95% CI: 10–30), according to a recent meta-analysis [22]. The sensitivity and specificity of the diagnostic strategy were not considered, as we were interested in comparing the potentially optimal clinical outcomes for patients, and not in the technical performance of the diagnostic strategy. We used two price points in the calculation: €5 and €10 per patient consulting for CA-ARTIs and the model was run separately for both price points. This is assumed to include not only the costs of the machine itself and materials used for the test, but also costs related to the depreciation and quality assurance related to the use of the hypothetical diagnostic strategy. For the price points, we used Dutch reference prices for laboratory diagnostics, which are considered a reasonable approximation for the real costs, which range from €1.89 to €8.44, excluding €1.89 for sample collection [26]. For our purposes, we analysed round figures of €5 and €10 as conservative estimates. We assumed clinical non-inferiority, meaning that the reduction of antibiotic prescriptions did not affect patient outcomes, in line with published literature [22, 27], showing patient outcomes are neither improved nor worsened. As it is unrealistic that the diagnostic strategy would be implemented overnight, we gradually implement the diagnostic strategy in three years (33%, 67% and 100% of consultations).
2.2 Model Structure
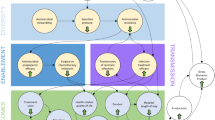
The simulation was run in the Modelling the Economics of Respiratory tract Infections and AMR (MERIAM) model, an individual-based simulation model for CA-ARTIs. The model consists of three modules, all programmed in R [28], which are combined to produce the results presented in this paper. Figure 1 provides a graphical representation of the analysis performed within MERIAM. The model was developed by SvdP; the model structure was validated externally by an expert advisory panel and the technical details internally by MJP and ADIvA. The R code is available online on GitHub [29].
The demographic and AMR modules use annual cycles, while the consultation module uses weekly incidence rates. To assess the long-term impact of large-scale testing using the hypothetical strategy, we assessed the intervention for a time horizon of 10 years: starting in 2020 and ending in 2030. An elaborate explanation of the various modules of MERIAM can be found in the OSM.
2.2.1 Demographic Module
In the simulation, 100,000 individuals were modelled, based on demographic data for the Dutch population [30]. The demographic module of the model was used to create the modelled population and simulate population changes based on Eurostat demographic data and population forecasts [30], including ageing, births, mortality and migration.
2.2.2 Consultation Module
The consultations for CA-ARTIs were simulated using a separate module. This used the incidence of respiratory infections (acute respiratory infections and influenza-like illness) based on consultation data from the European Centre for Disease Prevention and Control (ECDC) [31]. Considering four age categories (0–4 years, 5–15 years, 15–64 years, and ≥ 65) and the individuals from the demographic module, the incidence rates were used to simulate GP consultations. Within these consultations, the number of tests performed and the number of antibiotics were modelled using data from the PPAS, also considering age [18].
2.2.3 Antimicrobial Resistance (AMR) Forecasting Module
An ensemble of three machine-learning models was used to forecast AMR levels in the future for the care-as-usual scenario. Then, using the reduction in antibiotic consumption of implementing the POC test strategy, the reduction in AMR levels in the population was estimated for the diagnostic scenarios compared to the standard-of-care scenario [32]. Specifically, a bacterium-antibiotic-specific elasticity was applied, defining the subsequent percentage reduction in AMR following a 1% reduction in antibiotic consumption.
2.3 Input Parameters
2.3.1 Consultations
We used historic GP consultation data in the Netherlands [31] for acute respiratory infection (seasons 2016–2017, 2017–2018 and 2018–2019) and influenza-like illness (seasons 2016–2017 and 2018–2019) to simulate the number of consultations for the modelled population. Using the incidence package for R [33], two exponential models were fit to the incidence data for each season. Subsequently, these two models were combined to simulate a peak in the middle of the influenza season. For each modelled year, we randomly picked an incidence model from the historical data and predicted a representative number of consultations. This resulted in varying annual incidences over the time horizon, an overview of which is reported in the OSM.
Performed tests and antibiotics prescribed during the initial consultation were modelled using data from the PPAS.
2.3.2 Antibiotic Consumption and AMR
A risk ratio of 0.79 (95% CI: 0.70–0.90), as reported by Martínez-González et al. for POC CRP testing was applied to estimate the reduction of antibiotic consumption in the hypothetical diagnostic strategy [22]. Total antibiotic consumption and AMR data for the period 2005–2018 were provided by the ECDC TESSy database [31], but are also publicly available on the surveillance atlas for infectious disease [19] and the antimicrobial consumption database [16]. Building on methods developed by Hashigushi et al. [34], exponential smoothing was used to forecast consumption of BSPs [35] and an ensemble model was used to forecast future resistance of S. pneumoniae to BSPs, which were assumed to reflect the current standard-of-care. The ensemble model was constructed as a combination of three different statistical forecasting approaches: exponential smoothing [35], random forests [36] and XGBoost models [37]. To estimate the impact of widespread diagnostic testing, the elasticity between reducing antibiotic consumption and reduced AMR was estimated. From this estimation, a 1 percentage point (ppt) decrease in antibiotic consumption would lead to around 0.7 ppt decrease in AMR for S. pneumoniae against BSPs within 1 year. More information can be found in the OSM.
2.3.3 Costs
Dutch reference prices were used in the analysis [26]. List prices for medication were collected from the Dutch National Health Care Institute [38] and diagnostic test costs were collected from a major Dutch laboratory [39]. All costs were converted to Euros at the price level of the year 2019, using the harmonized index of consumer prices [40]. Training GPs is considered highly important to effectively reduce antibiotic prescribing for CA-ARTIs, not only in the use of POC tests, but also in patient communication related to antibiotics [41, 42]. Annual training costs were incorporated into the model for the hypothetical testing strategy by quantifying the time spent by the GP based on a previous trial that included training on the use of CRP tests and patient communication [26, 43]. Results were rounded to the nearest hundred euros. A complete overview of all the included costs can be found in the OSM.
Costs were discounted with 4%, in accordance with Dutch health-economic guidelines [44], no long-term effects, such as QALYs, were included in the analysis, so no discounting rate was applied to effects.
2.4 Sensitivity Analyses
To consider the uncertainty of all parameters simultaneously, a Monte Carlo analysis was run using 2,000 model replications. Uncertainty was incorporated in the antibiotic prescribing reductions related to the hypothetical diagnostic strategy, incidence (consultation rates), PPAS data, antibiotic consumption projections, and AMR projections. For costs the median and 95% Bayesian credible intervals (CrIs) are presented, calculated using the 2.5th and 97.5th percentile of the model replications.
Analogous to the widely applied cost-effectiveness acceptability curve, we used the probabilistic analysis to calculate the probability that the additional investments in a POC testing strategy is cost-effective based on the reduction in AMR, presented against various willingness-to-pay (WTP) thresholds for a 1 ppt reduction in resistance.
3 Results
3.1 Costs
In Table 1, the costs are summarized, aggregated over the years 2020 up to 2030 for the care-as-usual scenario, as well as the hypothetical diagnostic strategy at both price points; Fig. 2 shows an overview of the discounted costs for the 10-year period. On average, the diagnostic strategy increases the total costs with 9% at the €5 price point and with 19% at the €10 price point over 10 years for a population of 100,000 individuals, with the only significant difference being the costs of the diagnostics. In the hypothetical diagnostic scenario fewer antibiotics are prescribed (as can be seen in Fig. 3), but the cost savings are not sufficient to offset all costs of the additional POC tests. The hypothetical diagnostic strategy did not produce overall cost savings in any of the model replications. The total annual costs and details on the antibiotics prescribed are included in the OSM.
3.2 Antibiotic Consumption and AMR
The reduction in antibiotic consumption after implementing the hypothetical diagnostic strategy is shown in Fig. 3. Figure 4 shows the estimated development of resistance of S. pneumoniae against BSPs. Using the AMR forecasting module of MERIAM, we forecast resistance will increase in the coming years, which can be partly restrained by the hypothetical diagnostic strategy, albeit with considerable uncertainty. Figure 5 relates the WTP to reduce AMR to the modelled probability that this is achieved. It shows that at a WTP of €3 per citizen/year for a 1 ppt reduction of S. pneumoniae resistance against BSPs, the probability of the POC testing strategy to be cost-effective is around 80% at a price point of €5, and 40% at a price point of €10.
4 Discussion
Our results show that implementing a hypothetical diagnostic strategy in all patients with respiratory tract infections visiting a GP in the Netherlands would be a costly exercise, raising the total costs of these consultations by about 9% at the price point of €5. However, this strategy would reduce antibiotic prescribing by more than 7,500 defined daily doses (DDDs) annually for BSPs per 100,000 modelled individuals. This reduction in antibiotic consumption can be related to an estimated median reduction of resistance of S. pneumoniae to BSPs of 0.26 ppt in 2030 (3.8% compared in the usual-care group to 3.5% for the hypothetical diagnostic strategy). This is the first study to our knowledge that reports an AMR reduction acceptability curve. No country has a specified WTP threshold for reductions in resistance, but it may aid decision makers in prioritizing interventions aimed at reducing AMR. If the Dutch government would be willing to invest €3 per citizen in reducing the resistance of S. pneumoniae against BSPs, widespread POC testing has an 80% and 40% probability of being a cost-effective option at an increased price per consultation of €5 and €10, respectively.
For this analysis, we combined many publicly available data sources [16, 19, 26, 30] and data prospectively collected in clinical practice [18] to assess the opportunity of increased diagnostic testing in primary care to reduce AMR in the Netherlands. As presented results are based on a model that uses Dutch demographic data, we expect these results are generalisable to the whole of the Netherlands. Compared to other European countries, the Netherlands has relatively low antibiotic consumption and AMR rates [16, 19], which means that the potential reduction in antibiotic prescribing and AMR is expected to be higher in other countries. In some countries resistance of S. pneumoniae to BSPs is ten times higher, for example 32% in France and 39% in Romania [19], so we expect the impact of a POC diagnostic strategy to be greater there.
Previously, few economic analyses in the field of diagnostics for infectious diseases incorporated considerations of AMR [45,46,47]. The relative reduction of AMR in the analysis shows considerable uncertainty and there are some important assumptions to consider when interpreting these results. We assumed that the hypothetical diagnostic strategy was non-inferior, in that prescribing fewer antibiotics would not lead to worse patient outcomes. We also do not incorporate any follow-up in the model. This is supported by the results in a meta-analysis for CRP testing, which found no differences in clinical recovery, hospital admissions and mortality [22]. There might be a difference in re-consultations (within the same disease episode) and future consultations (for similar disease episodes in the future), but further research on patient consultation behaviour following novel POC diagnostics is required to quantify this. Combined, these limitations may result in an underestimation of the total costs of the hypothetical diagnostic strategy. Conservatively, we did not consider any long-term clinical effects or costs arising from AMR, as was done in other studies [1, 48]. For BSP-resistant S. pneumoniae, in-hospital pneumonia mortality was estimated to be increased by 29% compared to non-resistant S. pneumoniae in previous research [49], the length of stay was estimated to be around 2 days longer for children and 3 days longer for adults [50]. Taking these future AMR-related costs in consideration would decrease the incremental costs of the POC diagnostic strategy.
Two papers have been previously published on the cost-effectiveness of POC diagnostics in primary care in the Netherlands, both assessing the use of the CRP test [45]. In 2009, Cals et al. reported an increase of €1.62 per consultation for the CRP group, which they relate to an investment of €5.79 to reduce antibiotic prescribing by 1% [20]. A more recent cost-effectiveness analysis by Oppong et al. reported an incremental cost-effectiveness ratio of €27,186 per QALY for CRP versus usual care [43]. This analysis incorporated AMR by adding a cost to all prescribed antibiotics – however, they did not take future AMR into consideration.
Our analysis has several limitations. We use country-wide data for influenza-like-illness and acute respiratory infections (including common cold, pharyngitis, rhinosinusitis, laryngitis and pneumonia [51, 52]) to estimate the number of consultations for different age groups [31] and a PPAS to estimate testing and prescribing behaviour at this consultation [18]. It is uncertain whether the PPAS is representative for GPs’ prescribing behaviour and of all patients seeking care for respiratory complaints, especially given the limited number of GP practices included. In the model, we assumed all consulting patients to receive a hypothetical POC diagnostic strategy. There may be very limited clinical benefit to performing a test when the clinician has a high degree of certainty that prescribing an antibiotic would be unnecessary on clinical grounds alone, as well as for those cases where the GP is certain that the patient does need an antibiotic. For both these groups, the overall costs within this analysis would be reduced, but it is uncertain how antibiotic prescriptions would be affected.
Finally, we provide future AMR estimates in this paper, based on previously discussed methods [34]. Although some uncertainty was included (e.g., uncertainty in some input parameters and imputation methods), development of AMR is a complex process influenced by many factors [4]. Although the used historical AMR rates are representative for the whole of the Netherlands due to a high coverage of participating laboratories [19, 53], they are based on hospital data and may be different for the community setting. Even though the relation of antibiotic consumption and AMR has been described previously [4, 32, 54], the exact relation (or elasticity) is not known. Hence, we expect the uncertainty around our AMR estimates to be wider than the quantified uncertainty as displayed in Figs. 4 and 5. Still, we believe that figures like these could inform decision makers when making decisions on AMR policies, provided they are well-informed regarding the caveats.
In this study, the reduction in antibiotic prescriptions was based on previous research of CRP POC testing [22]. However, these reductions, as seen in clinical trials [22, 23], may not translate to the reductions achieved in clinical practice [55]. Additionally, the effectiveness of POC testing may wane after implementation, as was the case for a previous study considering CRP POC tests [41]. The currently running PRUDENCE trial will assess the implementation of a diagnostic algorithm in primary care and includes diagnostics for various types of CA-ARTIs: both higher (Strep A) and lower (CRP) respiratory tract infections, influenza and SARS-CoV-2. The results from this trial can be used to add further detail to the analyses described in this paper, model the reduction of antibiotic prescriptions specifically for various countries and subgroups, and investigate potential waning effects over a longer period.
This modelling study investigates the potential AMR reductions if a POC test strategy would be implemented for CA-ARTI in primary care in the Netherlands. Yet, we believe just having these POC tests available would not be sufficient to reach the full potential of this intervention. The right conditions need to be in place, including educating GPs and supportive staff, reimbursement of the additional costs, and updated treatment and diagnostic guidelines. Direct costs of some tests are reimbursed in Dutch primary care, including CRP, but this does not include the additional time spent by the GP or supportive staff [56].
Novel health-economic methods to assess and communicate the potential benefits of AMR reductions may be required for interventions with limited gains in terms of QALYs, but with a lot of potential related to antibiotic consumption and AMR. The potential to contain, or even reduce, AMR is relevant when deciding to reimburse interventions focussing on reducing antibiotic use, as AMR is a priority for policy makers worldwide [57,58,59,60]. The general public also seems to be willing to invest in the containment of AMR, with a recent study estimating the WTP for the UK at ₤8.35 billion for 5 years [61]. Future clinical trials will further investigate the assumption of non-inferiority and provide data to estimate macro-economic effects related to POC diagnostic strategies for CA-ARTI and improve our AMR projections. We expect this will aid decision makers in prioritizing strategies to combat AMR.
In the current analysis, we considered the situation before the COVID-19 pandemic. It is difficult to predict how the management of CA-ARTIs will evolve as the pandemic transforms into an endemic situation. We do not know how it will affect consultation rates for CA-ARTIs, tests performed, and antibiotics prescribed. During the first COVID-19 wave in the Netherlands, antibiotic use for CA-ARTIs reduced compared to the previous year [62], but we do not know whether this effect will last after the pandemic. However, diagnostic tests for COVID-19 have received a lot of attention from clinicians, policy makers and the public, which we expect will change expectations and attitudes regarding diagnostics in the future.
5 Conclusions
Introducing a hypothetical diagnostic strategy for all patients seeking care for CA-ARTIs in the Netherlands would increase the costs related to these consultations by 9% and 19% at the €5 and €10 price points, respectively. We estimate resistance will have an upwards trend in the coming years, which can be ameliorated by such increased use of diagnostics, albeit with considerable uncertainty. Considering the potential detrimental effects of AMR on health, we expect investments in affordable POC diagnostics and other interventions that can reduce antibiotic prescribing in primary care to be valuable and justifiable from a heath-economic point of view.
References
Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66.
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet [Internet]. 2022. https://www.sciencedirect.com/science/article/pii/S0140673621027240. Cited 20 Jan 2022.
Aryee A, Price N. Antimicrobial stewardship – can we afford to do without it? Br J Clin Pharmacol. 2015;79:173–81.
Roope LSJ, Smith RD, Pouwels KB, Buchanan J, Abel L, Eibich P, et al. The challenge of antimicrobial resistance: What economics can contribute. Science. 2019;364:eaau4679.
Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346: f1493.
Coast J, Smith RD, Millar MR. Superbugs: Should antimicrobial resistance be included as a cost in economic evaluation? Health Econ. 1996;5:217–26.
Atkins KE, Lafferty EI, Deeny SR, Davies NG, Robotham JV, Jit M. Use of mathematical modelling to assess the impact of vaccines on antibiotic resistance. Lancet Infect Dis. 2018;18:e204–13.
Mauskopf J, Standaert B, Connolly MP, Culyer AJ, Garrison LP, Hutubessy R, et al. Economic analysis of vaccination programs: an ISPOR good practices for outcomes research task force report. Value Health. 2018;21:1133–49.
Birkegård AC, Halasa T, Toft N, Folkesson A, Græsbøll K. Send more data: a systematic review of mathematical models of antimicrobial resistance. Antimicrob Resist Infect Control. 2018;7:117.
Pezzani MD, Tornimbene B, Pessoa-Silva C, Kraker M de, Rizzardo S, Salerno ND, et al. Methodological quality of studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System target bacteria. Clin Microbiol Infect [Internet]. Elsevier; 2021. https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00006-9/abstract. Cited 12 Apr 2021.
Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13.
Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect. 2009;15:12–5.
Ieven M, Coenen S, Loens K, Lammens C, Coenjaerts F, Vanderstraeten A, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24:1158–63.
van den Broek DJ, Verheij TJM, Numans ME, van der Velden AW. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother. 2014;69:1701–7.
Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73:ii2–10.
European Centre for Disease Prevention and Control. Antimicrobial consumption database (ESAC-Net) [Internet]. Eur. Cent. Dis. Prev. Control. https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database. Cited 15 Apr 2021.
Butler CC, Hood K, Verheij T, Little P, Melbye H, Nuttall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338: b2242.
Velden A van der, Pol AC van de, Bongard E, Cianci D, Aabenhus R, Balan A, et al. Point of care testing, antibiotic prescribing and prescribing confidence for respiratory tract infections in primary care: Prospective audit in 18 European countries. BJGP Open [Internet]. Royal College of General Practitioners; 2021. https://bjgpopen.org/content/early/2022/01/19/BJGPO.2021.0212. Cited 2 Feb 2022.
European Centre for Disease Prevention and Control. Antimicrobial resistance [Internet]. Surveill. Atlas Infect Dis. 2021. https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=4. Cited 19 Mar 2021.
Cals JWL, Butler CC, Hopstaken RM, Hood K, Dinant G-J. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ. 2009;338: b1374.
Anthierens S, Tonkin-Crine S, Cals JW, Coenen S, Yardley L, Brookes-Howell L, et al. Clinicians’ views and experiences of interventions to enhance the quality of antibiotic prescribing for acute respiratory tract infections. J Gen Intern Med. 2015;30:408–16.
Martínez-González NA, Keizer E, Plate A, Coenen S, Valeri F, Verbakel JYJ, et al. Point-of-care C-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: systematic review and meta-analysis of randomised controlled trials. Antibiotics. 2020;9:610.
Cohen JF, Pauchard J-Y, Hjelm N, Cohen R, Chalumeau M. Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat. Cochrane Database Syst Rev [Internet]. Wiley; 2020. https://doi.org/10.1002/14651858.CD012431.pub2/full. Cited 24 Feb 2022.
Bruning AHL, de Kruijf WB, van Weert HCPM, Willems WLM, de Jong MD, Pajkrt D, et al. Diagnostic performance and clinical feasibility of a point-of-care test for respiratory viral infections in primary health care. Fam Pract. 2017;34:558–63.
Rothberg MB, Bellantonio S, Rose DN. Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy. Ann Intern Med. 2003;139:321.
Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan S. Kostenhandleiding: methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg [Internet]. Diemen: Zorginstituut Nederland; 2015. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+(verdie**smodules).pdf. Acessed 15 Apr 2021.
Little P, Hobbs FDR, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ. 2013;347:f5806.
R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria; 2020. https://www.R-project.org/. Accessed 03 June 2020.
van der Pol S. MERIAM [Internet]. Groningen, the Netherlands: Global Health Hub Groningen; 2022. https://github.com/UMCG-Global-Health/MERIAM/releases. Cited 24 May 2022.
European Commission. Eurostat [Internet]. https://ec.europa.eu/eurostat. Cited 12 Apr 2021.
European Centre for Disease Prevention and Control. Data from The European Surveillance System - TESSy, provided by Belgium, Germany, the Netherlands, Austria, Sweden, Spain, Norway, Italy, France, Poland, Bulgaria, Czech Republic, Denmark, United Kingdom, Greece, Hungary, Ireland, Lithuania, Romania, Slovakia [Internet]. Eur. Cent. Dis. Prev. Control. https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy. Cited 12 Apr 2021.
Cecchini M, Lee S. Low-value health care with high stakes: Promoting the rational use of antimicrobials. OECD; 2017;115–58.
Jombart T, Kamvar ZN, FitzJohn R, Taylor T, Cai J, Bhatia S, et al. incidence: Compute, Handle, Plot and Model Incidence of Dated Events [Internet]. 2020. https://CRAN.R-project.org/package=incidence. Cited 15 Apr 2021.
Hashiguchi TCO, Ouakrim DA, Padget M, Cassini A, Cecchini M. Resistance proportions for eight priority antibiotic-bacterium combinations in OECD, EU/EEA and G20 countries 2000 to 2030: a modelling study. Eurosurveillance. 2019;24:1800445.
Hyndman RJ, Athanasopoulos G. Forecasting: principles and practice (3rd ed) [Internet]. 3rd edition. Melbourne, Australia: OTexts; 2021. https://Otexts.com/fpp3/. Cited 19 Mar 2021.
Wright MN, Ziegler A. ranger: a fast implementation of random forests for high dimensional data in C++ and R. J Stat Softw. 2017;77:1–17.
Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of 22nd ACM SIGKDD international conference knowledge discovery data mining. 2016. pp. 785–94.
Zorginstituut Nederland. Medicijnkosten [Internet]. Medicijnkosten. https://www.medicijnkosten.nl/. Cited 15 Apr 2021.
Certe. Price list [Internet]. Certe. https://www.certe.nl/over-certe/organisatie/tarieven-certe/de-nza-tarieven. Cited 19 Apr 2021.
European Commission. Harmonised Indices of Consumer Prices [Internet]. Eurostat. https://ec.europa.eu/eurostat/web/hicp. Cited 15 Apr 2021.
Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Antibiotic prescribing for acute respiratory tract infections 12 months after communication and CRP training: a randomized trial. Ann Fam Med. 2019;17:125–32.
Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet. 2013;382:1175–82.
Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost-effectiveness of internet-based training for primary care clinicians on antibiotic prescribing for acute respiratory tract infections in Europe. J Antimicrob Chemother. 2018;73:3189–98.
Zorginstituut Nederland. Guideline for economic evaluations in healthcare [Internet]. Diemen; 2016. Report No.: 2016077622. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare. Accessed 03 Jan 2018.
van der Pol S, Rojas García P, Postma MJ, Villar FA, van Asselt ADI. Economic analyses of respiratory tract infection diagnostics: a systematic review. Pharmacoeconomics. 2021;39:1411–27.
Rojas García P, van der Pol S, van Asselt ADI, Postma M, Rodríguez-Ibeas R, Juárez-Castelló CA, et al. Efficiency of diagnostic testing for Helicobacter pylori infections—a systematic review. Antibiotics. 2021;10:55.
Rojas García P, van der Pol S, van Asselt ADI, Postma MJ, Rodríguez-Ibeas R, Juárez-Castelló CA, et al. Diagnostic testing for sepsis: a systematic review of economic evaluations. Antibiotics. 2022;11:27.
OECD. Stemming the superbug tide: just a few dollars more [Internet]. OECD; 2018. https://www.oecd-ilibrary.org/social-issues-migration-health/stemming-the-superbug-tide_9789264307599-en. Cited 15 Mar 2019.
Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2006;42:788–97.
Schrag SJ, McGee L, Whitney CG, Beall B, Craig AS, Choate ME, et al. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob Agents Chemother. 2004;48:3016–23.
European Centre for Disease Prevention and Control, World Health Organization. Influenza Surveillance Country, Territory and Area Profiles 2019 [Internet]. 2019. https://www.euro.who.int/__data/assets/pdf_file/0016/402082/InfluenzaSurveillanceProfiles_2019_en.pdf. Cited 17 Nov 2021.
Nederlands Huisartsen Genootschap (NHG). ICPC-online [Internet]. 2021. https://www.nhg.org/themas/artikelen/icpc-online. Cited 17 Nov 2021.
European Centre for Disease Prevention and Control. Country summaries—antimicrobial resistance in the EU/EEA 2019 [Internet]. 2020. https://www.ecdc.europa.eu/sites/default/files/documents/Country%20summaries-AER-EARS-Net%20202019.pdf. Accessed 17 Nov 2021.
Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87.
Minnaard MC, van de Pol AC, Hopstaken RM, van Delft S, Broekhuizen BDL, Verheij TJM, et al. C-reactive protein point-of-care testing and associated antibiotic prescribing. Fam Pract. 2016;33:408–13.
Prestatie-en tariefbeschikking huisartsenzorg en multidisciplinaire zorg 2022—TB/REG-22622-04 - Nederlandse Zorgautoriteit [Internet]. https://puc.overheid.nl/nza/doc/PUC_694745_22/. Cited 4 May 2022.
European Commission. A European one health action plan against antimicrobial resistance [Internet]. Brussels, Belgium; 2017. https://ec.europa.eu/health/sites/health/files/antimicrobial_resistance/docs/amr_2017_action-plan.pdf. Accessed 27 Apr 2021.
Department of Health and Social Care. Contained and controlled: the UK’s 20-year vision for antimicrobial resistance [Internet]. Brittish Government; 2019. p. 19. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/773065/uk-20-year-vision-for-antimicrobial-resistance.pdf. Accessed 27 Apr 2021.
Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019 [Internet]. Centers for Disease Control and Prevention (U.S.). 2019. https://stacks.cdc.gov/view/cdc/82532. Accessed 27 Apr 2021.
World Health Organization. Global action plan on antimicrobial resistance [Internet]. 2016. https://www.who.int/publications-detail-redirect/9789241509763. Cited 27 Apr 2021.
Dorgali MV, Longo A, Vass C, Shields G, Harrison R, Scarpa R, et al. A general public study on preferences and welfare impacts of antimicrobial resistance in the United Kingdom. Pharmacoeconomics. 2022;40:65–76.
van de Pol AC, Boeijen JA, Venekamp RP, Platteel T, Damoiseaux RAMJ, Kortekaas MF, et al. Impact of the COVID-19 pandemic on antibiotic prescribing for common infections in The Netherlands: a primary care-based observational cohort study. Antibiot Basel Switz. 2021;10(2):196.
Acknowledgements
We thank our collaborators within the VALUE-Dx project for their valuable input. This paper reflects only the authors’ view, not that of the funder or supporting organizations. The views and opinions of the authors expressed herein do not necessarily state or reflect those of ECDC. The accuracy of the authors’ statistical analysis and the findings they report are not the responsibility of ECDC. ECDC is not responsible for conclusions or opinions drawn from the data provided. ECDC is not responsible for the correctness of the data and for data management, data merging and data collation after provision of the data. ECDC shall not be held liable for improper or incorrect use of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 820755. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and bioMérieux SA, Janssen Pharmaceutica NV, Accelerate Diagnostics S.L., Abbott, Bio-Rad Laboratories, BD Switzerland Sàrl, and The Wellcome Trust Limited.
Conflict of interest
Maarten J. Postma reports grants and personal fees from various pharmaceutical industries, all outside the submitted work. He holds stocks in Pharmacoeconomics Advice Groningen (PAG Ltd) and is advisor to Asc Academics, all pharmacoeconomic consultancy companies. AvdV reports educational fees from Reckitt Benckiser. The other authors have nothing to disclose.
Availability of data and material
The R code of the model is available from: https://github.com/UMCG-Global-Health/MERIAM (version 1.1.0).
Authors’ contributions
Concept and design: SvdP, CB, AWF, MJP, ADIvA. Acquisition of data: SvdP, AvdV, CCB, TJMV. Analysis and interpretation of data: SvdP, DEMCJ, CCB, TJMV, AWF, MJP, ADIvA. Drafting of the manuscript: SvdP, CCB, TJMV, ADIvA. Critical revision of paper for important intellectual content: SvdP, DEMCJ, AvdV, CCB, TJMV, AWF, MJP, ADIvA. Statistical analysis: SvdP. Obtaining funding: AvdV, CCB, TJMV, MJP. Administrative, technical or logistic support: SvdP. Supervision: DEMCJ, AWF, MJP, ADIvA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van der Pol, S., Jansen, D.E.M.C., van der Velden, A.W. et al. The Opportunity of Point-of-Care Diagnostics in General Practice: Modelling the Effects on Antimicrobial Resistance. PharmacoEconomics 40, 823–833 (2022). https://doi.org/10.1007/s40273-022-01165-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01165-3